Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Risperidone long-acting injection in the treatment of schizophrenia: 24-month results from the electronic Schizophrenia Treatment Adherence Registry in Canada
Authors Williams R, Chandrasena R, Beauclair L, Luong D, Lam A
Received 20 September 2013
Accepted for publication 3 December 2013
Published 27 February 2014 Volume 2014:10 Pages 417—425
DOI https://doi.org/10.2147/NDT.S54740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Richard Williams,1 Ranjith Chandrasena,2 Linda Beauclair,3 Doanh Luong,4 Annette Lam4
On behalf of the e-STAR study group
1Vancouver Island Health Authority, Victoria, BC, Canada; 2Chatham-Kent Health Alliance, Chatham, ON, Canada; 3Allan Memorial Institute, Montreal, QC, Canada; 4Janssen Inc., Toronto, ON, Canada
Objective: To assess outcomes over 24 months in Canadian patients with schizophrenia initiated on risperidone long-acting injection (RLAI) and participating in the electronic Schizophrenia Treatment Adherence Registry (e-STAR).
Materials and methods: Patients with schizophrenia or schizoaffective disorder were enrolled from 24 sites after an independent decision to initiate RLAI. Subsequent patient management was based on usual clinical practice at each site and was not protocol-driven. Relevant data were collected retrospectively by chart review for 12 months prior to RLAI and prospectively for 24 months following RLAI initiation.
Results: Patients (n=188) had a mean age of 39.2 years, were 66.3% male, and 27.7% were inpatients at baseline. Twenty-four months after initiating therapy (initial dose =28.7 mg), 34.1% (95% confidence interval 27.2%–42.2%) of patients had discontinued RLAI with a mean time to discontinuation of 273.4±196 days. Over the treatment period, there were significant (P<0.001) changes from baseline in Clinical Global Impression-Severity (CGI-S; 3.48 versus [vs] 4.31 at baseline), Global Assessment of Functioning (GAF; 56.1 vs 48.1), and Personal and Social Performance (PSP; 59.1 vs 46.9) scale scores. In addition, after 12 months, there were significant (P<0.001) decreases in the percentage of patients hospitalized (23.9% vs 58.5% pre-RLAI), mean length of stay (11.4 vs 30.4 days), and number of hospitalizations (0.32 vs 0.87) compared to the 12-month pre-RLAI period. Reductions in hospitalization continued into the second 12 months of therapy, when only 9% of patients were hospitalized and mean length of stay was 2.0 days.
Conclusion: In a routine clinical practice setting, patients switched to RLAI showed significant improvements in clinical outcomes and in global and social functioning, and hospitalization was significantly reduced. The data confirm that RLAI provides effective long-term management of schizophrenia in Canada.
Keywords: schizophrenia, Canada, risperidone long-acting injection, e-STAR
Introduction
Antipsychotic depot formulations offer a potential solution to poor oral medication adherence in patients with schizophrenia.1 Nonadherence and partial adherence to therapy are common in patients on oral antipsychotic therapy, resulting in poor symptom control and increased rates of relapse and hospitalization.2–4 In addition to the negative clinical patient outcomes, increased hospitalizations due to relapse are a major contributing factor in the overall treatment costs of schizophrenia.4–6 In Canada, the annual direct health care cost of schizophrenia was estimated at CAD$1,868 million in 2004, with 66% of this cost due to hospitalization, a major component of which can be attributed to disease relapse.7 Depot formulations, administered through periodic injections, offer several clinical and therapeutic advantages over oral antipsychotics. They avoid potential problems associated with reliance on patients taking daily oral therapy, allow monitoring of patient compliance through regular clinic contact, provide more consistent plasma levels of antipsychotic drug between injections, and improve adherence to therapy.8,9
First-generation or typical depot antipsychotic formulations have been available since the 1960s, and have been used extensively in the maintenance treatment of schizophrenia.10 Although these medications may offer a benefit to patients in terms of increased adherence to therapy, the evidence for reduction in relapse rates and hospitalization compared to oral drugs is inconsistent.8,10 One of the limiting factors of first-generation oral and depot drugs is the relatively high incidence of extrapyramidal symptoms, including tardive dyskinesia.8,11 These unpleasant side effects reduce the effective capacity of these depot and oral agents to control schizophrenia.11
Second-generation or atypical antipsychotics can be more efficacious, and are generally better tolerated than first-generation drugs.12,13 However, long-term therapy with oral atypicals is still compromised by poor adherence.3,13,14 Risperidone long-acting injection (RLAI) was the first atypical antipsychotic available in an injectable formulation, and its efficacy and tolerability have been demonstrated in clinical trials.15–17 RLAI therefore offers the tolerability of a second-generation antipsychotic with the improved adherence of an injectable therapy. However, stringent patient-inclusion criteria and short study durations in clinical trials limit the ability of investigators to apply observations from such studies to antipsychotic use in real-world clinical practice.18,19 Observational studies are frequently used to assess the impact of therapies in clinical practice, where recruited patients and their treatment are more reflective of the routine management of schizophrenia.19,20 The electronic Schizophrenia Treatment Adherence Registry (e-STAR) was established to assess long-term outcomes in patients with schizophrenia initiating treatment with RLAI under real-world clinical practice conditions. Initial e-STAR data from numerous countries indicate that a switch to RLAI leads to significant improvements in clinical and functional outcomes, reduction in hospitalizations, and high levels of adherence to therapy in schizophrenia patients.2,21–24 The present paper describes the results of the e-STAR study in Canada.
Materials and methods
Study design
The e-STAR study was an international, multicenter, noninterventional, observational registry designed to collect clinical outcomes in patients with schizophrenia initiating treatment with RLAI. The objective was to assess the effectiveness of RLAI on control of schizophrenia symptoms and to quantify the impact of treatment on hospitalization. The e-STAR methodology has been described by Olivares et al.25 In brief, patients with schizophrenia were recruited across Canada and enrolled in e-STAR after the decision to initiate treatment with RLAI, or switch to RLAI from their current oral or depot antipsychotic regimen, was made by their physicians. At baseline, prior to initiation of RLAI, data on hospitalization history and medication usage were collected by retrospective chart review for a minimum period of 12 months. Following initiation of RLAI, prospective data were collected for 24 months at approximately 3-month intervals. This study reports on the outcomes for all patients participating in the e-STAR study in Canada after 24 months.
Study population
The study was conducted at 24 community mental health centers across Canada, and all participating psychiatrists were actively involved in the treatment of schizophrenia. Any male or female inpatient or outpatient who was being initiated or switched to RLAI, based on an independent decision by the treating physician, was eligible for inclusion in the e-STAR registry, with the exception of chronically hospitalized patients who had no possibility of being discharged over the 24-month observation period, patients with treatment- resistant schizophrenia, or patients who were pregnant or currently breastfeeding. In addition, patients with a contraindication to RLAI or those currently participating in a clinical trial were excluded. There were no study-mandated treatment choices once a patient was enrolled, and clinical management was determined solely by the treating psychiatrist. Participating investigators were instructed to treat patients with RLAI in accordance with the manufacturer’s prescribing information, but initial drug doses and use of concomitant psychiatric medications were based on the physician’s judgment. All recruited patients or their authorized legal representative provided written informed consent, and the study was approved by appropriate local institutional review boards/independent ethics committees.
Data collection
As in other countries, e-STAR data were collected mainly through a secure web-based system that maintained patient and data confidentiality.24 However, traditional paper-based data collection was also available. Data for patients enrolled in e-STAR were recorded at baseline and every 3 months for a total of 24 months following RLAI initiation, even if patients had discontinued RLAI and switched to an alternative therapy. At baseline, patient-demographic and disease-history data were collected, as well as the reason for switching to RLAI. In addition, Clinical Global Impression-Severity (CGI-S),26 Global Assessment of Functioning (GAF),27 and Personal and Social Performance (PSP)28 scale scores were assessed by investigators. Retrospective chart review was used to collect data on hospitalization and overall medication use over a 12-month period prior to the initiation of RLAI.
Following initiation of RLAI, the patient returned to the clinic every 3 months (±2 weeks), when data on hospitalization, CGI-S, GAF, PSP, RLAI dosing changes, concomitant medication utilization, treatment discontinuation, and reasons for discontinuation were collected. In addition, at each visit, psychiatrists were asked to assess the patient’s adherence to RLAI using a 5-point Likert scale, with scores ranging from 0 (never) to 4 (always). Every attempt was made to follow all patients for the full 24 months, even those who discontinued RLAI.
Statistical analysis
A sample-size analysis for the Canadian study was based on the difference in the number of days spent in hospital during the first year with RLAI compared to the 12 months prior to RLAI. In a previous study, the 12-month reduction in hospitalization with RLAI was 8.8±37.1 days.29 Based on this estimate, a sample size of 189 patients (237 assuming a 20% dropout) would be required to show, with 90% power, a significant difference at α=0.05 using a two-sided paired t-test.
Kaplan–Meier survival curves were used to estimate time to all-cause RLAI discontinuation; patients who were still using RLAI at 24 months were censored. All models were fitted using a stepwise selection procedure, with inclusion and exclusion significance of 5%.21 CGI-S, GAF, and PSP scores at baseline and each visit were expressed as mean scores, and the statistical significance of change in score from baseline was calculated using a paired t-test.
Hospitalization parameters over the retrospective period (pre-RLAI) were compared with the first and second 12-month RLAI treatment periods. Statistical significance of change in percentage of patients hospitalized, length of stay, and number of stays over 12 months pre- and post-RLAI were assessed using McNemar’s test, paired t-test and signed-rank test, respectively. For those who were inpatients at the time of initiation of RLAI, the baseline date was assumed to be the date of discharge, ie, hospitalization was assumed to apply to the retrospective period, since this was related to prior antipsychotic therapy. All statistical analyses were carried out using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
A total of 188 patients were recruited for the study from 24 sites across Canada. Patients had a mean age of 39.2 years, the majority were male (66.3%), and 27.7% were inpatients at initiation of RLAI (Table 1). At baseline, mean CGI-S score was 4.31, and 36.9% of the patients were categorized as having marked or severe disease. Mean scores for GAF and PSP were 48.1 and 46.9, respectively. The mean GAF baseline score indicated moderate-to-severe functional impairment,27 and the PSP score categorized patients as having marked to very severe difficulties in two or more of the four PSP domains.30
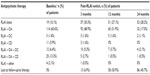
Three months prior to the initiation of RLAI, the majority of patients (n=130 [69.1%]) were receiving an oral atypical (51.5% on risperidone, 41.5% on olanzapine) either alone (n=74 [39.4%]) or in combination with a conventional depot (n=44 [23.4%]) or a conventional oral antipsychotic (n=7 [3.7%]) or both (n=5 [2.7%]), while 31 patients (16.5%) were receiving no treatment (Table 1). In addition, before the switch to RLAI, 80.7% of the patients were receiving concomitant psychiatric medication (Table 1). The most common reasons for initiation of RLAI were poor compliance (28.7% of patients), insufficient response (28.2%) with prior antipsychotic medication, and unacceptable tolerability/adverse events (21.2%) (Table 1).
The mean RLAI dose at initiation of therapy was 28.7 mg, with 82.4%, 4.3%, and 12.8% of patients initiated on 25 mg, 37.5 mg, and 50 mg, respectively. RLAI dose increased over the treatment period, and at 24 months, mean dose was 39.9 mg (44.6% were receiving 50 mg RLAI versus [vs] 31.5% and 21.7% on 25 mg and 37.5 mg, respectively; data not shown). The mean time on RLAI for the 188 patients in the study was 15.92 months. Table 2 provides additional details on the utilization of RLAI and concomitant antipsychotics over the treatment period, and shows that at baseline 89.9% of patients initiated RLAI in combination with another antipsychotic. Over the treatment period, the proportion of patients receiving concomitant oral atypicals decreased (17% of patients were receiving RLAI and oral atypicals at 24 months, vs 60.6% at baseline) while the number receiving RLAI alone increased. At 24 months, of the 92 patients still using RLAI, 57.6% were on monotherapy.
Investigator-assessed levels of treatment adherence indicated that the proportion of patients always adherent increased from 88.5% at 3 months post-RLAI initiation (n=131) to 100% at 24 months (n=72). There were no significant differences in the proportion of patients receiving other psychiatric medications following initiation of RLAI, and the levels observed at baseline (Table 1) were similar to those seen at 24 months (data not shown).
As shown in Figure 1, the Kaplan–Meier analysis indicated that after 24 months, 34.1% (95% confidence interval [CI] 27.2%–42.2%) of the patients had discontinued RLAI therapy. The mean time to discontinuation was 273.4±196 days, with a median, minimum, and maximum of 229, 15, and 682 days, respectively. The major reasons for discontinuation were loss to follow-up (35.2%), insufficient response (22.2%), and patient/family choice (13.0%) (data not shown). Adverse events and unacceptable tolerability led to discontinuation of only five patients (9.3%).
Over the treatment period there were statistically significant changes in all effectiveness parameters assessed (Figure 2). After only 3 months, the mean CGI-S score decreased significantly from 4.31 at baseline to 3.95 (P=0.001), and this decrease continued over 24 months to 3.48 (P<0.001 compared to baseline), giving a reduction of 0.82 points. In addition, only 13.0% of patients were categorized as having marked or severe disease at 24 months (36.9% at baseline), while 50.7% had very mild/mild disease (20.9% at baseline) (results not shown). The rapidity of RLAI action on effectiveness parameters was also apparent in the GAF and PSP scores (Figure 2). GAF scores at 3 months increased significantly from baseline (53.0 versus 48.1, P<0.001), and showed further improvement at 24 months (56.1 at 24 months, P<0.001). For the PSP, the baseline score of 46.9 increased to 54.2 (P<0.001) and 59.1 (P<0.001) at 3 and 24 months, respectively, indicating rapid and sustained improvement in social functioning (Figure 2). There were no significant differences in baseline parameters (age, sex, CGI-S, GAF, and PSP scores, hospitalization at baseline, and diagnosis) between patients who did not discontinue RLAI and those who were lost to follow-up or discontinued. However, post hoc analysis indicated that with RLAI therapy, the latter patients were not improving as quickly as patients who continued RLAI therapy. For example, when change in CGI-S scores from baseline to 24 months was assessed separately for patients who continued RLAI and those who did not, change for the latter patients was 0.6 (CGI-S scores were 4.3 at baseline [n=52] vs 3.7 at 24 months [n=7]), compared to 1.0 for patients who continued (4.3 at baseline [n=136] vs 3.3 at 24 months [n=48]); results were similar for the two populations in GAF and PSP score changes from baseline.
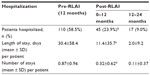
RLAI also had a significant impact on hospitalization parameters (Table 3). Following initiation of RLAI, there was a significant decrease in the percentage of patients hospitalized from 58.5% over the pre-RLAI period to 23.9% over the 12-month post-RLAI period (P<0.001). In addition, average length of stay decreased by 62.5% (30.4 to 11.4 days per patient, P<0.001) and the average number of stays decreased by 63.2% (0.87 to 0.32 per patient, P<0.001) (Table 3). The impact of RLAI continued into the second 12-month period, with further decreases in patients hospitalized (9.0%) and in the duration (2.0 days) and number of stays per patient (0.11 stays) (Table 3).
Discussion
The e-STAR study has consistently shown that RLAI is associated with high medication retention rates and clinical and functional improvements in schizophrenia patients in several countries, despite structural differences in health care-delivery systems and in the management of schizophrenia.2,21–25 In addition, in all countries, RLAI therapy led to significant reductions in psychiatric-related hospitalization, a major contributing factor in the overall treatment costs of schizophrenia.2,21–24 The results from the present study indicate a similar impact of RLAI on the treatment of patients with schizophrenia in Canada after a switch from prior therapy, which included oral first- and second-generation antipsychotics and typical depot antipsychotics. Over the 24 months of treatment, 65.9% of patients remained on RLAI therapy, and there were rapid and maintained significant improvements in clinical effectiveness and patient functioning, which were accompanied by significant decreases in hospitalization.
The observed RLAI 24-month discontinuation rate in Canada (34.1%) was within the range of rates reported in other countries. The e-STAR study has reported variable 24-month discontinuation rates of 2.1%–49% in six European countries, with an overall rate of 15%.22 Variation in RLAI discontinuation rates across countries likely reflects differences in clinical practice patterns, variable patient disease severity, and RLAI dosing strategies.22 One of the major reasons for RLAI discontinuation in the present study was loss to follow-up; 19 patients (35.2% of discontinued patients) discontinued therapy for this reason, likely a reflection of the observational nature of the study design. In the absence of this extensive loss to follow-up, Canadian discontinuation rates may have been much lower. Although oral atypicals were not assessed in the present study, previous naturalistic studies have suggested that retention rates with RLAI in patient populations with similar disease severity are superior to rates in patients receiving oral therapy.21,31 In a prior Canadian retrospective chart-review study, over 3 years 50.5% (95% CI 41.9%–62.1%) of patients initiated on an oral atypical switched medication, compared to only 39.1% (95% CI 28.8%–51.7%) initiated on RLAI.31 These naturalistic studies provide consistent real-world evidence that long-term adherence to RLAI therapy is high, and that this in turn is associated with significant impacts on symptom control in schizophrenia and reductions in disease-related hospitalization.21,31
RLAI treatment also led to significant changes in CGI-S scores, which decreased by 0.82 points over 24 months, and there was a 2.4-fold increase from baseline in the proportion of patients with very mild or mild disease after the switch to RLAI. These changes are of a similar magnitude to those reported in previous studies (decreases ranged from 0.6 to 0.87 points)21 after schizophrenia patients were switched to RLAI, and reflect the effectiveness of this second-generation injectable antipsychotic in controlling symptoms of schizophrenia.2,21,25 The CGI-S improvements were not due solely to poor-outcome patients discontinuing or being lost to follow-up. All patients showed CGI-S improvement with RLAI therapy, but patients who did not discontinue had a faster and greater CGI-S decrease than patients who discontinued or were lost to follow-up. A similar difference in CGI-S score changes in RLAI continuers and discontinuers has been reported by Peuskens et al.22 There were also significant changes in measures of global and social functioning, with increases in mean scores of 8.0 and 12.2 points on the GAF and PSP scales, respectively, after the switch to RLAI. There is increasing interest in social functioning in schizophrenia and a recognition that in addition to symptom control, treatment goals should include improvement in functional outcomes to facilitate reintegration of patients into society.32,33 In schizophrenia patients, a change of ≥7 points on the PSP scale has been defined as a clinically meaningful change in social functioning.30,32 On this basis, the mean change of 12.2 points on the PSP 24 months after initiation of RLAI would indicate a clinically meaningful change in social functioning in patients treated in this study. Furthermore, this increase occurred rapidly after initiation of RLAI; of the total change in PSP score, 59.8% was apparent 3 months after the medication switch. The positive impact on patient functioning was also apparent in the significant change in GAF score over 24 months, 61.3% of which was apparent at 3 months. Apiquian et al23 have also recently reported a rapid and significant change in PSP after schizophrenia patients were switched to RLAI. These data suggest that in addition to rapid improvement in schizophrenia symptoms, RLAI may also be effective in improving social functioning, evidence of a broad impact on both clinical and psychosocial aspects of schizophrenia.
Consistent with evidence from earlier observational studies,2,21–23 the e-STAR data from Canada showed that over 12 months post-RLAI, 59.1% fewer patients were hospitalized compared to the pre-RLAI period (P<0.001), and there were significant (P<0.001) reductions in mean length of stay (62.5% reduction) and the number of hospitalizations (63.2% reduction). As in previous studies,21,22 the decreases in hospitalization parameters were stable and continued into the second year of therapy. These data also confirm an earlier retrospective chart-review study of schizophrenia patients in Canada, which showed significant decreases in hospitalization when patients were switched to RLAI; in contrast, similar patients maintained on oral atypicals had an increased risk of hospitalization (95% CI defining risk of hospitalization was 54.7%–76.4% for oral atypicals over 3 years vs 1.8%–16.5% for RLAI).31 These data indicate that in Canada, as in other countries,21–23 a switch to RLAI from current antipsychotic therapy is associated with significant decreases in patient hospitalization, which could lead to considerable schizophrenia-related cost savings.5,7,31 Potential cost savings of such a strategy have been quantified in Spain, where total per-patient monthly schizophrenia-treatment costs (including drug costs) over 24 months decreased by 22% after a switch to RLAI compared to a similar pre-RLAI period.25 Similarly, in 2005, Chue et al5 used a discrete-event simulation model to show that over 5 years, treatment of high-risk noncompliant schizophrenia patients in Canada with RLAI would generate assumed discounted savings of $13,130 per patient compared to treatment with oral risperidone. The present study demonstrates that in Canada, the long-term impact of RLAI on hospitalization is dramatic, and potentially provides Canadian-specific data for a more accurate assessment of the overall economic impact of RLAI utilization in this country.
There are a number of limitations associated with the present study design, which have been discussed in previous e-STAR publications.2,21,22,24,25 An important limitation is that this was an observational study with loosely defined inclusion criteria and no comparator group, and thus lacks the validity of a randomized controlled trial. However, the value of the latter type of study on schizophrenia has been questioned because of selective patient recruitment, the short duration of studies, and protocol-driven procedures that may artificially enhance medication adherence to levels higher than those observed in clinical practice.19,21 Therefore, large-scale, long-term, observational studies such as e-STAR can provide valuable information on schizophrenia outcomes.19–21 An additional limitation is that part of the collection of data on hospitalization relied on retrospective chart review, and was dependent on the consistent recording of data on charts at all sites. Variation in the quality of data recorded may have affected the validity of the retrospective data. However, this is unlikely to have specifically impacted the Canadian data, since virtually all mirror-image-type studies to date have shown significant reductions in hospitalization after a switch to RLAI using similar retrospective chart-review procedures.2,21–23
Finally, the PSP used in this study required psychiatrists to rate patients’ functioning status.30 Although psychiatrists were trained to use the PSP, there was no information provided as to how they were to assess relevant patient-related parameters, which formed the basis of their ratings. Therefore, source data for the PSP ratings may have varied from site to site, potentially limiting the validity of the overall PSP scores.
At the time the e-STAR study was initiated, RLAI was the only atypical injectable antipsychotic available in Canada. Another second-generation antipsychotic, paliperidone palmitate, has since been approved for the treatment of schizophrenia in Canada, expanding therapeutic options for depot therapy.34 This depot antipsychotic is the palmitate ester of paliperidone, the major metabolite of risperidone, and requires once-monthly injection.35 While the e-STAR study has demonstrated the effectiveness of RLAI in real clinical practice, the long-term comparative effectiveness of paliperidone palmitate and RLAI in a similar clinical environment has not been addressed. However, a short-term (13 weeks) randomized, double-blind clinical trial has demonstrated equivalent efficacy and safety of RLAI and paliperidone palmitate in the treatment of schizophrenia.35 The present study design lays the groundwork for potentially similar studies with paliperidone palmitate, or any future depot antipsychotic, providing an effective way to address the use of these important drugs in clinical practice.
Conclusion
The present study demonstrates that in a real-world clinical practice setting in Canada, patients with schizophrenia switched from their current antipsychotic therapy to RLAI showed significant, rapid, and sustained improvements in clinical outcomes and in global and social functioning over 24 months. In addition, there were significant decreases in hospitalization over the same period. The data indicate that RLAI is an effective, injectable, second-generation antipsychotic, and that in addition to clinical and functional improvement, its use could result in considerable cost savings through reduced schizophrenia-related hospitalization.
Acknowledgments
Funding for this study was provided by Janssen Inc. The authors would like to acknowledge the e-STAR investigators in Canada who contributed patient data for this study. The authors would also like to thank John I McCormick, PhD, McKesson Specialty, Toronto, Canada who provided medical writing services, which were funded by Janssen Inc., Canada.
Disclosure
RW and RC have received speaking honoraria from Janssen Inc. and RC has also participated in Advisory Board meetings for Janssen Inc. RW, RC, and LB received grants to carry out the e-STAR study from Janssen Inc. DL and AL are employees of Janssen Inc. The authors report no other conflicts of interest in this work.
References
Goff DC, Hill M, Freudenreich O. Strategies for improving treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2010;71 Suppl 2:20–26. | |
Lambert T, Olivares JM, Peuskens J, et al. Effectiveness of injectable risperidone long-acting therapy for schizophrenia: data from the US, Spain, Australia, and Belgium. Ann Gen Psychiat. 2011;10:10. | |
Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. | |
Law MR, Soumerai SB, Ross-Degnan D, Adams AS. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008;69(1):47–53. | |
Chue PS, Heeg B, Buskens E, van Hout BA. Modelling the impact of compliance on the costs and effects of long-acting risperidone in Canada. Pharmacoeconomics. 2005;23 Suppl 1:62–74. | |
Knapp M, King D, Pugner K, Lapuerta P. Non-adherence to antipsychotic medication regimens: associations with resource use and costs. Br J Psychiatry. 2004;184:509–516. | |
Goeree R, Farahati F, Burke N, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opin. 2005;21(12):2017–2028. | |
Adams CE, Fenton MK, Quraishi S, David AS. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br J Psychiatry. 2001;179:290–299. | |
Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;195:S63–S67. | |
Haddad PM, Taylor M, Niaz OS. First-generation antipsychotic long-acting injections v oral antipsychotics in schizophrenia: systematic review of randomized controlled trials and observational studies. Br J Psychiatry Suppl. 2009;52:S20–S28. | |
Taylor D. Psychopharmacology and adverse effects of antipsychotic long-acting injections: a review. Br J Psychiatry Suppl. 2009;52:S13–S19. | |
Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414–425. | |
Leucht S, Kissling W, Davis JM. Second-generation antipsychotics for schizophrenia: can we resolve the conflict? Psychol Med. 2009;39(10):1591–1602. | |
Valenstein M, Blow FC, Copeland LA, et al. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull. 2004;30(2):255–264. | |
Kane JM, Eerdekens M, Lindenmayer JP, Keith ST, Lesem M, Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160(6):1125–1132. | |
Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250–1257. | |
Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol. 2005;15(1):111–117. | |
Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia – a critical systematic review and meta-analysis of randomized long-term trials. Schizophr Res. 2011;127(1–3):83–92. | |
Haddad PM, Tiihonen J, Haukka J, Taylor M, Patel MX, Korhonen P. The place of observational studies in assessing the effectiveness of depot antipsychotics. Schizophr Res. 2011;131(1–3):260–261. | |
National Institute for Health and Clinical Excellence. Schizophrenia: The NICE Guideline on Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care. Leicester/London: British Psychological Society and Royal College of Psychiatrists; 2010. Available from: http://www.nice.org.uk/nicemedia/live/11786/43607/43607.pdf. Accessed December 5, 2013. | |
Olivares JM, Rodriguez-Morales A, Diels J, et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry. 2009;24(5):287–296. | |
Peuskens J, Olivares JM, Pecenak J, et al. Treatment retention with risperidone long-acting injection: 24-month results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR) in six countries. Curr Med Res Opin. 2010;26(3):501–509. | |
Apiquian R, Córdoba R, Louzã M. Clinical outcomes of long-acting injectable risperidone in patients with schizophrenia: six-month follow-up from the Electronic Schizophrenia Treatment Adherence Registry in Latin America. Neuropsychiatr Dis Treat. 2010;7(1):19–26. | |
Olivares JM, Peuskens J, Pecenak J, Resseler S, Jacobs A, Akhras KS. Clinical and resource-use outcomes of risperidone long-acting injection in recent and long-term diagnosed schizophrenia patients: results from a multinational electronic registry. Curr Med Res Opin. 2009;25(9):2197–2206. | |
Olivares JM, Rodriguez-Martinez A, Burón JA, Alonso-Escolano D, Rodriguez-Morales A. Cost-effectiveness analysis of switching antipsychotic medication to long-acting injectable risperidone in patients with schizophrenia: a 12- and 24-month follow-up from the e-STAR database in Spain. Appl Health Econ Health Policy. 2008;6(1):41–53. | |
Guy W. Clinical global impression. In: Rush AJ, editor. Handbook of Psychiatric Measures. Washington: American Psychiatric Association; 2000:100–102. | |
Endicott J, Spitzer J, Fleiss J, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. | |
Morosini PL, Magliano L, Branbilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329. | |
Eriksson L, Almqvist A, Mehnert, A, et al. Long-acting risperidone significantly reduces the need for institutional psychiatric care. Poster presented at: 42nd Annual Meeting of the American College of Neuropsychopharmacology; December 7–11, 2003; San Juan, Puerto Rico. | |
Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the personal and social performance scale in patients with stable schizophrenia. Psychiat Res. 2008;161(2):213–224. | |
Beauclair L, Chue P, McCormick J, Camacho F, Lam A, Luong D. Impact of risperidone long-acting injectable on hospitalization and medication use in Canadian patients with schizophrenia. J Med Econ. 2007;10(4):427–442. | |
Patrick DL, Burns T, Morosini P, Gagnon DD, Rothman M, Adriaenssen I. Measuring social functioning with the Personal and Social Performance Scale in patients with acute symptoms of schizophrenia: interpretation of results of a pooled analysis of three Phase III trials of paliperidone extended-release tablets. Clin Ther. 2010;32(2):275–292. | |
Nasrallah HA, Lasser R. Improving patient outcomes in schizophrenia: achieving remission. J Psychopharmacol. 2006;20 (Suppl 6):57–61. | |
Health Canada. Summary basis of decision: Invega, Sustenna. 2010. Available from: http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/prodpharma/sbd-smd/phase1-decision/drug-med/sbd_smd_2010_invega_sustenna_127385-eng.pdf. Accessed August 1, 2013. | |
Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218–226. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.