Back to Journals » OncoTargets and Therapy » Volume 8
Risks on N-acetyltransferase 2 and bladder cancer: a meta-analysis
Authors Zhu Z, Zhang J, Jiang W, Zhang X, Li Y, Xu X
Received 15 February 2015
Accepted for publication 5 May 2015
Published 11 December 2015 Volume 2015:8 Pages 3715—3720
DOI https://doi.org/10.2147/OTT.S82927
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jianmin Xu
Zongheng Zhu,1 Jinshan Zhang,2 Wei Jiang,3 Xianjue Zhang,4 Youkong Li,4 Xiaoming Xu5
1Department of General Surgery, Huangshi Love & Health Hospital, Huangshi, 2Department of Tumor surgery, Huangshi Central Hospital, Huangshi, 3Department of Urinary Surgery, Huangshi No 5 Hospital, Huangshi, 4Department of Urinary Surgery Jingzhou Central Hospital, Jingzhou, 5Department of Bone Surgery, Jingzhou Central Hospital, Jingzhou, People’s Republic of China
Background: It is known that bladder cancer disease is closely related to aromatic amine compounds, which could cause cancer by regulating of N-acetylation and N-acetyltransferase 1 and 2 (NAT1 and NAT2). The NAT2 slowed acetylation and would increase the risk of bladder cancer, with tobacco smoke being regarded as a risk factor for this increased risk. However, the relationship between NAT2 slow acetylation and bladder cancer is still debatable at present. This study aims to explore preliminarily correlation of NAT2 slow acetylation and the risk of bladder cancer.
Methods: The articles were searched from PubMed, Cochran, McGrane English databases, CBM, CNKI, and other databases. The extraction of bladder cancer patients and a control group related with the NAT2 gene were detected by the state, and the referenced articles and publications were also used for data retrieval. Using a random effects model, the model assumes that the studies included in the analysis cases belong to the overall population in the study of random sampling, and considering the variables within and between studies. Data were analyzed using STATA Version 6.0 software, using the META module. According to the inclusion and exclusion criteria of the literature study, 20 independent studies are included in this meta-analysis.
Results: The results showed that the individual differences of bladder cancer susceptibility might be part of the metabolism of carcinogens. Slow acetylation status of bladder cancer associated with the pooled odds ratio was 1.31 (95% confidence interval: 1.11–1.55).
Conclusion: The status of NAT2 slow N-acetylation is associated with bladder cancer risks, and may increase the risk of bladder cancer.
Keywords: N-acetyltransferase 2, NAT2 slow acetylation status, bladder cancer, meta-analysis
Introduction
The morbidity rate and mortality rate of bladder tumor rank as the first one in the urinary system tumors, and tend to increase year by year. Clinically, bladder tumor is divided into superficial bladder cancer and invasive bladder cancer. At present, the main treatment of the bladder cancer is operation and supplemented by radiotherapy and chemotherapy. However, the recurrence rate is relatively high and 5-year survival rate is approximately 50%.1–4 How to find a more effective biomedical treatment to reduce the recurrence rate and metastatic rate of bladder cancer has become a critical problem in the world?
The risk of bladder cancer relates to NAT2 slow acetylation was confirmed by domestic and foreign scholars.5–11 However, this relationship was not approved in some researches. Part of the reasons can be attributed to the differences in statistical sample size. Only a small amount of research shows significant difference. The purpose of this meta-analysis explores the risk of NAT2 slow acetylation and the bladder cancer.
Materials and methods
Retrieval of articles
By retrieving electronic database, like PubMed, Cochran, McGrane English databases, CBM, CNKI, and other databases, the author tested the status of NAT2 that was taken from patients with bladder cancer and control group. At the same time, also using reference articles and publications for data retrieval that were published from January 1980 to February 2014. The English key words include NAT2 or N-acetyl and bladder cancer. Using the combined form of key words and free words, all of the above searching strategies are obtained from the pre-searching methods. Three doctors operated the article retrieval independently. In order to reduce the leakage of literature, we refined search for reference documentation that were brought into literature.
Inclusion criteria and exclusion criteria
The following are inclusion criteria. 1) English literature; 2) randomized controlled trial, prospective or retrospective control study, and cohort study; 3) data should be complete and creditable; and 4) accord with retrieval condition and requirement.
The following are exclusion criteria. 1) Language is non-English literature; 2) no summary; 3) no specific statistical data of NAT2; and 4) no control group treatment, a review of the literature, comments, and in vitro study of tumor.
Statistical analysis
By comparing these specific values and their corresponding 95% confidence interval (CI) with the number of research object in research, the risk of being infected with bladder cancer was found associate with slow NAT2 acetylation by some researches, and check whether there are any corresponding sample bias. At the same time, considering the variable within and between the researches according to the previous studies.12–15 Data were analyzed using STATA Version 6.0 software (StataCorp LP, College Station, TX, USA), using the META module.
Results
Figure 1 shows the flow chart of study selection. Through the screening of the database, 20 studies that analyzing the risks of being infected by NAT2 and bladder cancer in the controlled study were brought into analysis (Figure 2 and Table 1),7–26 including 2,463 patients with bladder cancer cases and 3,451 cases of control group which are all without randomness.
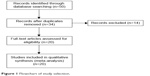  | Figure 1 Flowchart of study selection. |
  | Figure 2 Forest plot for the risk of bladder cancer influenced by NAT2. |
  | Table 1 Comparative study of NAT2 and risk of bladder cancer patients |
Excluding the NAT2 phenotype and to prevent bias, only patients group and the control group were considered for subgroup analysis research. There were account 12 articles cited by phenotypic description of NAT2 gene and account eight by genotype. However, according to the aim of this meta-analysis, the present only cited the studies that described the genetic data. In some researches, healthy people are treated as controlled group, and in some of the researches, hospitalized patients with malignant tumor are concerned. The age of control group is matched up with cases group. Smoking history and the patient’s occupational exposure in some studies are analyzed.
Relationship between feature genes of NAT2 and bladder cancer
We made a forest plot for 3,451 cases and 3,451 control groups (Figure 2). Figure 2 shows 20 studies that include forest odds ratio analysis and 95% CI. This suggests the possibility of the man with changeable NAT2 gene become a bladder cancer patient. In the overall sample, the slow acetylator’s odds ratio is 1.31 (95% CI: 1.11–1.55). The heterogeneity was studied by statistical analysis (Q=35.6, df=21, P=0.024). There is some evidence that may be due in part of the differences in the allocation method of the state of NAT2.27–31 By layer approaching, the study focuses on phenotypic, the obtained odds ratio is 1.34 (95% CI: 1.08–1.69; test for heterogeneity: Q=17.24, df=12, P=0.14). Genotyping studies and pooled odds ratio are 1.27 (95% CI: 0.97–1.67; test for heterogeneity Q=18.03, df=8, P=0.021).
The result was drawn from the analysis of pooled data of research based on many factors. The risk of the bladder cancer that defined the phenotype and genotype of NAT2 state is 1:3 (95% CI: 1.18–1.64; heterogeneity inspection: Q=28.09, df=17, P=0.04).32,33 Only in the study of pooled phenotype, the odds ratio is 1.36 (95% CI: 1.08–1.70; heterogeneity inspection: Q=16.86, df=11, P=0.12), and only in the study of aggregate genotype, the odds ratio is 1.44 (95% CI: 1.10–1.89; heterogeneity inspection: Q=11.21, df=5, P=0.05). It is concluded from three Asian studies (Figure 3A) that the ratio is 0.75 (95% CI: 0.45–1.28).33–35
Relationship between slow acetylation and bladder cancer
In this research, we investigate and summarize from smoking (Figure 3A) or the influence of occupational exposure (Figure 3B). Odds ratio of smoking group is 1.21 (95% CI: 0.826–0.827; heterogeneity inspection: Q=0.6435, P=0.842; the figure of area under the curve is 0.6881). Odds ratio of occupational exposure group is 1.9 (95% CI: 0.589–0.656; heterogeneity inspection: Q=0.6433, P=0.623; the figure of area under the curve is 0.6879). Table 1 shows the risk research on patients with slow acetylation and bladder cancer, and the interaction between these risks.23 Excess slow acetylation in patients with bladder cancer cases had exposed to more carcinogens or the smokers.
Publication bias
Publication bias was investigated by Begg’s funnel plot, and the funnel plot’s asymmetry was further assessed by Egger’s test. As shown in Figure 4, there was low possibility of asymmetry in the dominant model of this meta-analysis. Besides, finding from the Egger’s test further suggested that there was no significant risk of publication bias (P=0.183).
  | Figure 4 Funnel plot for the detection of the publication bias in this meta-analysis. |
Discussion
In the late 1970s and early 1980s, many experts started to study the NAT2 slow acetylator status which is a risk factor for bladder cancer, and is put forward by many experts and scholars.10–14 This meta-analysis aimed to clarify whether NAT2 slow acetylator status can increase the risk of bladder cancer.
The bladder cancer is one of the few cancers that have been found to be involved directly with environmental carcinogens. Exposure to industrial chemicals, aromatic amines, is one of the environmental factors that can cause bladder cancer. People who engage in rubber, dyestuff, and printing industry will be more easily affected by chemical composition. In addition, the factors of inhalation of diesel exhaust and smoke are also included. These factories bring aromatic amine that mainly include high polymer aromatic amines, such as naphthylamine, 4-aminobiphenyl, benzidine, and their N-hydroxylated derivative. All of these are known or potential NAT2 substrates.36,37 Currently, the view, exposure to carcinogens is a risk factor for bladder cancer, has been a broad consensus. That is the reason why NAT2 slow acetylator has been recognized as a risk factor for assessing bladder cancer risk. At present, some studies failed to prove the relationship between NAT2 slow acetylator status and bladder cancer, and the main reason can be attributed to the differences in statistical sample size. Only a few studies in the bilateral 5% statistical level have significant sexual differences. In our study, we also cannot discover the significant sexual differences (data not shown). The advantage of this study is that the statistical data can be combined and analyzed by different methods.
Influence factors of the disease, we will follow contrast principles of single variable and adopt the allele frequency research, including racial and geographic origin. However, in some previous studies, considering race and other reasons, we do the mixed study of contrasted race and geographical origin. Thus, unmatched cases and contrast are the sources of bias. In addition, as previously mentioned, in view of any polymorphism, carcinogen metabolism enzyme is impossible to increase the risk of bladder cancer twice or 1.5 times than the associated smaller risk, there are many biases of NAT2 status in most published studies.25,38,39 Many published reports are based on the comparison of cases and control groups. By adopting this, comparison can show the susceptibility to NAT2 slow acetylator status. By this meta-analysis, we can get that although heterogenicity exists in the relationship between NAT2 slow acetylator status and bladder cancer, slow acetylator status still can increase the risk of bladder cancer. Because of gene frequency of occurrence in population, area and other factors are pretty high. It means that the status of NAT2 will increase the morbidity of bladder cancer obviously. Although NAT2 is present in the bladder epithelium and its expression is not high, the status of fast acetylation will not increase the morbidity of bladder cancer.40 If diversity of genetic susceptibility exists in bladder cancer, then maybe the correspondent gene positions are not the same. However, the expressive of some genotypic combination are higher. For example, NAT2 slow acetylation status combined Glutathione S-transferase M1 defects. The possibility of such inconclusive studies have been checked so far because of a shortage of convincing evidence.40,41 Maybe future work will be influenced by the decrease of match detection. By adding sampling rate and specific groups to prove gene, polymorphism and environmental factor will have an influence on morbidity of bladder cancer.
In this study, we analyzed the correlation between NAT2 slow acetylation and the risk of bladder cancer. The result showed that the individual differences of bladder cancer susceptibility might be part of the metabolism of carcinogens. Slow acetylation status of bladder cancer is associated with the bladder cancer risks.
In conclusion, the status of NAT2 slow N-acetylation is associated with bladder cancer risks, and may increase the risk of bladder cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
Cai T, Li Y, Jiang Q, Wang D, Huang Y. Paraganglioma of the vagina: a case report and review of the literature. Onco Targets Ther. 2014;7:965–968. | ||
Vineis P, Pirastu R. Aromatic amines and cancer. Cancer Causes Control. 1997;8(3):346–355. | ||
Wolf H, Lower GM, Bryan GT. Role of N-acetyltransferase phenotype in human susceptibility to bladder carcinogenic arylamines. Scand J Urol Nephrol. 1980;14(2):161–165. | ||
Brockmöller J, Cascorbi I, Kerb R, Roots I. Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1, microsomal epoxide hydrolase, and cytochrome P450 enzymes as modulators of bladder cancer risk. Cancer Res. 1996;56(17):3915–3925. | ||
Office of National Statistics (ONS). Cancer Incidence in England and Wales. London: HMSO, Office of National Statistics; 1996. | ||
Cartwright RA, Glashan RW, Rogers HJ, et al. Role of N-acetyltransferase phenotypes in bladder carcinogenesis: a pharmacogenetic epidemiological approach to bladder cancer. Lancet. 1982;2(8303):842–845. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;4:154–176. | ||
Evans DA, Eze LC, Whibley EJ. The association of the slow acetylator phenotype with bladder cancer. J Med Genet. 1983;20(5):330–333. | ||
Filiadis IF, Georgiou I, Alamanos Y, Kranas V, Giannakopoulos X, Lolis D. Genotypes of N-acetyltransferase-2 and risk of bladder cancer: a case-control study. J Urol. 1999;161(1):1672–1675. | ||
Risch A, Wallace DM, Bathers S, Sim E. Slow N-acetylation genotype is a susceptibility factor in occupational and smoking related bladder cancer. Hum Mol Genet. 1995;4(2):231–236. | ||
Fleiss JL, Tytun A, Uray HK. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. 1980;36(5):343–346. | ||
Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140(3):290–296. | ||
Hanke J, Krajewska B. Acetylation phenotypes and bladder cancer. J Occup Med. 1990;32(9):917–918. | ||
Stanley LA, Coroneos E, Cuff R, Hickman D, Ward A, Sim E. Immunochemical detection of arylamine N-acetyltransferase in normal and neoplastic bladder. J Histochem Cytochem. 1996;44(9):1059–1067. | ||
Hanssen HP, Agarwal DP, Goedde HW, et al. Association of N-acetyltransferase polymorphism and environmental factors with bladder carcinogenesis: study in a north German population. Eur Urol. 1985;11(4):263–266. | ||
Hayes RB, Bi W, Rothman N, et al. N-acetylation phenotype and genotype and risk of bladder cancer in benzidine-exposed workers. Carcinogenesis. 1993;14(4):675–678. | ||
Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86(2):289–294. | ||
Hivonen A. Polymorphic NATs and cancer predisposition. IARC Sci Publ. 1999;148(2):251–270. | ||
Schlesselman JJ. Case-Control Studies. Design, Conduct and Analysis. New York: Oxford University Press; 1998:220–226. | ||
Houlston RS, Peto J. Genetics of common cancers. In: Eeles RA, 1996. | ||
Ponder RB, Easton DE, Horwich A, editors. Inherited Predisposition to Cancer. London: Chapman & Hall; 2005:208–226. | ||
Smith G, Stanley LA, Sim E, Strange RC, Wolf CR. Metabolic polymorphisms and cancer susceptibility. Cancer Surv. 1995;25(1):27–65. | ||
Horai Y, Fujita K, Ishizaki T. Genetically determined N-acetylation and oxidation capacities in Japanese patients with non-occupational urinary bladder cancer. Eur J Clin Pharmacol. 1989;37(6):581–587. | ||
Peluso M, Airoldi L, Armelle M, et al. White blood cell DNA adducts, smoking, and NAT2 and GSTM1 genotypes in bladder cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1998;7(4):341–346. | ||
Hsieh FI, Pu YS, Chern HD, Chiou HY, Chen CJ. Genetic polymorphisms of N-acetyltransferase 1 and 2 and risk of cigarette smoking related bladder cancer. Br J Cancer. 1999;81(3):537–541. | ||
Kaisary A, Smith P, Jaczq E, et al. Genetic predisposition to bladder cancer: ability to hydroxylate debrisoquine and mephenytoin as risk factors. Cancer Res. 1987;47(20):5488–5493. | ||
Woodhouse KW, Adams PC, Clothier A, Mucklow JC, Rawlins MD. N-acetylation phenotype in bladder cancer. Hum Toxicol. 1982;1(4):443–445. | ||
Mommsen S, Barfod NM, Aagaard J. N-acetyltransferase phenotypes in the urinary bladder carcinogenesis of a low-risk population. Carcinogenesis. 1985;6(2):199–201. | ||
Karakaya AE, Cok I, Sardas S, Gogus O, Sardas OS. N-acetyltransferase phenotype of patients with bladder cancer. Hum Toxicol. 1986;5(5):333–335. | ||
Ladero JM, Kwok CK, Jara C, et al. Hepatic acetylator phenotype in bladder cancer patients. Ann Clin Res. 1985;17(3):96–99. | ||
Lower GM Jr, Nilsson T, Nelson CE, Wolf H, Gamsky TE, Bryan GT. N-acetyltransferase phenotype and risk in urinary bladder cancer: approaches in molecular epidemiology. Preliminary results in Sweden and Denmark. Environ Health Perspect. 1979;29(1):71–79. | ||
Mesrobian HG, Kelalis PP, Kramer SA. Long-term followup of 103 patients with bladder exstrophy. J Urol. 1988;139(4):719–722. | ||
Miller ME, Cosgriff JM. Acetylator phenotype in human bladder cancer. J Urol. 1983;130(1):65–66. | ||
Windmill KF, Gaedigk A, Hall PM, Samaratunga H, Grant DM, McManus ME. Localization of N-acetyltransferases NAT1 and NAT2 in human tissues. Toxicol Sci. 2000;54(1):19–29. | ||
Mostafa MH, Sheweita SA, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12(1):97–111. | ||
Okkels H, Sigsgaard T, Wolf H, Autrup H. Arylamine N-acetyl-transferase 1 (NAT1) and 2 (NAT2) polymorphisms in susceptibility to bladder cancer: the influence of smoking. Cancer Epidemiol Biomarkers Prev. 1997;6(4):225–231. | ||
Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987;316(8):450–455. | ||
Schnakenberg E, Ehlers C, Feyerabend W, et al. Genotyping of the polymorphic N-acetyltransferase (NAT2) and loss of heterozygosity in bladder cancer patients. Clin Genet. 1998;53(5):396–402. | ||
Sharpe S, Sterne J. Fixed and Random-Effects Meta-Analysis, With Graphics. STATA Technical Bulletin. College Station (TX): STATA Corp.; 1998; 38 and 42. | ||
Wang S, Wang Y, Liu J, et al. Silencing B7-H1 enhances the anti-tumor effect of bladder cancer antigen-loaded dendritic cell vaccine in vitro. Onco Targets Ther. 2014;7:1389–1396. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

