Back to Journals » Cancer Management and Research » Volume 12
Risk Stratification Based on Metastatic Pelvic Lymph Node Status in Stage IIIC1p Cervical Cancer
Authors Li A, Wang L, Jiang Q, Wu W, Huang B, Zhu H
Received 19 March 2020
Accepted for publication 12 June 2020
Published 28 July 2020 Volume 2020:12 Pages 6431—6439
DOI https://doi.org/10.2147/CMAR.S253522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Anyang Li,1,2 Luhui Wang,2 Qi Jiang,2 Wenlie Wu,2 Baoyou Huang,2 Haiyan Zhu1,2
1Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai 200126, People’s Republic of China; 2Department of Gynecology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325027, People’s Republic of China
Correspondence: Haiyan Zhu
Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai 200126, People’s Republic of China
Tel +86 57755069162
Email [email protected]
Purpose: Stage IIIC1 cervical cancer showed heterogeneous in oncologic outcomes with highly variable survival rates. Our objective was to determine the prognostic significance of removed and metastatic pelvic lymph node status and further perform risk stratification in patients with stage IIIC1p cervical cancer.
Patients and Methods: Patients with stage IIIC1p cervical cancer and undergoing radical hysterectomy with lymphadenectomy in 2008– 2018 were retrospectively analyzed. Patients’ stage was classified using the revised 2018 International Federation of Gynecology and Obstetrics (FIGO) staging schema. Univariate and multivariable models were used to examine the association between removed and metastatic lymph node status and recurrence-free survival/overall survival.
Results: During a median follow-up of 34 months, 73 relapses and 44 deaths were observed among 273 patients with stage IIIC1p cervical cancer. Parametrial involvement and metastatic lymph node ratio (mLNR) were identified as independent predictors for recurrence-free survival. Parametrial involvement and mLNR were independent predictors for overall survival. A stratification system was then created based on parametrial involvement and mLNR. A total of 123 (45.1%), 127 (46.5%) and 23 (8.4%) patients were classified into the low-risk, intermediate-risk, and high-risk groups, with as a 5-year recurrence-free survival of 81.7%, 51.1%, 38%, respectively. Compared to the low-risk group, the intermediate- and high-risk groups had a significantly greater risk of recurrence and death.
Conclusion: The prognosis of stage IIIC1p patients varied significantly. A risk stratification system based on parametrial involvement and mLNR successfully separated patients into low, intermediate, and high-risk group. Our findings could facilitate the practical use of further stratification in Stage IIIC1p cervical cancer.
Keywords: cervical cancer, stage IIIC1p, pelvic lymph node, prognosis
Introduction
Cervical cancer is the most common gynecologic malignancy worldwide and represents a major global health challenge.1 In 2018, approximately 596,847 women were diagnosed with cervical cancer and the disease resulted in 311,365 deaths worldwide.1 Lymph node metastasis is one of the most important factors for relapse and poor clinical outcomes.2 Following radical hysterectomy and lymphadenectomy, the 5-year survival rates for stage IB cervical cancer patients with or without lymph node metastasis, were 73.1% and 87.0%, respectively.3 More recently, one study involving 17,173 patients also reported that lymph node metastasis negatively affected prognosis in cervical cancer.4 In 2018, the status of lymph node was included in the revised 2018 International Federation of Gynecology and Obstetrics (FIGO) staging system,5 further supporting the important prognostic role in cervical cancer.
In the new revised staging schema, patients with positive pelvic lymph nodes and para-aortic lymph nodes are classified as stage IIIC1 and IIIC2, respectively, in which imaging diagnosis was defined as “r” and pathologically detected was defined as “p”.5 Thus, patients with positive pelvic lymph nodes diagnosed by pathological evaluation were classified into stage IIIC1p and by imaging diagnosis were defined as stage IIIC1r. Subsequently, several studies examining the prognostic performance of the 2018FIGO cervical cancer staging schema found dividing all women with positive lymph nodes into one stage would lead to a heterogeneous group of patients with very different survival rates.6,7 Therefore, further stratification in patients with stage IIIC1p cervical cancer will help accurately evaluate prognosis and tailor adjuvant therapy.
Several studies explored various ways for assessing the prognostic value of pelvic lymph nodal status, including the number of involved metastatic nodes,8 lymph node ratio,9,10 and localization of the metastatic nodes in the pelvic.11 The number of metastatic lymph nodes (mLNs) is the most simple, intuitive and widely used predictor in cervical cancer.8,12 Recently, metastatic lymph node ratio (mLNR), the ratio of positive nodes to the total number of nodes harvested, has been found to be a more significant and consistent prognostic indicator than the absolute lymph node number in various gynecological malignancies, including cancer of cervix.9,10 However, it is not clear which parameter is superior in prognostic evaluation for patients with lymph node-positive cervical cancer. Whether these variables would help further stratify patients with stage IIIC1p cervical cancer is unknown.
In the current study, at first, we comprehensively examined the impact of pelvic lymph node status, including the absolute number of removed and metastatic pelvic lymph nodes, the metastatic lymph node ratio, and unilateral/bilateral pelvic lymph node metastases, on survival for patients with stage IIIC1p cervical cancer, then, identified the independent prognostic parameter using Cox proportional hazards regression, finally, stratified this group of patients based on these prognostic factors.
Materials and Methods
Study Cohort
Clinical data were reviewed from a database of 1713 patients pathologically confirmed uterine cervical carcinoma and underwent radical hysterectomy and lymphadenectomy between January 2008 and December 2018 at the First Affiliated Hospital of Wenzhou Medical University, China. This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and informed consent was signed by the patients before surgery. The eligibility criteria were as follows: 1) those pathologically confirmed cervical cancer and pelvic lymph node metastases; 2) those confirmed FIGO stage IIIC1p based on the 2018 revised FIGO staging system; 3) those received radical hysterectomy and bilateral pelvic lymphadenectomy.
The deadline for follow-up time was February 19, 2020. The endpoints of this study were overall survival and recurrence-free survival. Overall survival was determined from the date of surgery to death or the last follow-up. Recurrence-free survival was calculated from the date of surgery to the tumor recurrence or distant metastasis.
Statistical Analysis
Continuous data were presented as median (P25-P75) or mean ± standard deviation based on their distribution. In this study, both mLNR and mLNs are non-normal distribution data and discrete variables, therefore, Receiver Operating Characteristic (ROC) curve is not suitable to determine the cut-off. In the present study, we first selected multiple cutoff values based on previous reports. The evaluated cutoffs of the number of mLNs were 2, 3, 4, and 5; the evaluated cutoffs of mLNRs were 0.08, 0.1, 0.2, and 0.3. Then, we determined the optimal cutoffs for high and low mLNs or mLNRs by predicting their roles in survival. Finally, we determined the median number of mLNs (n=2) and mLNR (LNR=0.08) as optimal cutoffs. Survival parameters were estimated by the Kaplan–Meier method and compared with the Log rank test. A Cox proportional hazards model was used for multivariate survival analysis. A two-tailed P < 0.05 was considered statistically significant. SPSS Statistics version 19.0 (IBM Corp., Somers, NY) was used for all statistical analyses.
Results
Characteristics of Cervical Cancer
In all, 273 patients with stage IIIC1p were enrolled in this study. The clinicopathologic characteristics are shown in Table 1. Of these, squamous cell carcinoma remained the most common histological subtypes accounting for 87.2% of all cervical cancers. The majority cases showed poor differentiation (53.5%), tumors <4 cm (52.4%), and stromal infiltration depth ≥1/2 (90.5%). Besides, 122 cases (44.7%) showed lymphovascular space invasion, 30 cases (11%) showed parametrial invasion, and 12 cases (4.4%) showed positive surgical margins (Table 1). The mean number of removed LNs was 22.7 (median 22.0; range 5–50), the mean number of mLNs was 2.97 (median 2; range 1–27), and the mean mLNR was 0.145 (median 0.083; range 0.022–1.000).
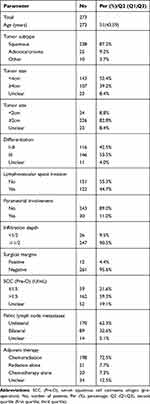 |
Table 1 Patients’ Characteristics |
All of the 273 patients underwent radical hysterectomy and pelvic lymphadenectomy, in which 239 were followed by adjuvant therapy. One hundred and ninety-eight patients (72.5%) received chemo-radiotherapy, 21 patients (7.7%) received radiotherapy alone, 20 patients (7.3%) received chemotherapy alone, and the other 34 patients (12.5%) missed the adjuvant treatment information.
Univariate and Multivariate Analyses for Recurrence-Free Survival and Overall Survival
Median follow-up time was 34 months. There were 73 relapses and 44 deaths during follow-up. At first, we comprehensively analyzed the prognostic signification of removed and metastatic pelvic lymph node status among patients with stage IIIC1p cervical cancer. As shown in Table 2, there is no association between the number of removed pelvic lymph, bilateral pelvic lymph node metastases, and recurrence-free survival/overall survival of patients with cervical cancer. Interestingly, patients with a high mLNs and mLNR were significantly correlated with worse overall survival and recurrence-free survival for all cutoffs. Finally, we selected the median number of mLNs (n=2) and mLNR (INR=0.08) as the optimal cutoffs for further analysis.
 |
Table 2 The Correlation Between Removed and Metastatic Lymph Node Status and Survival of Cervical Cancers by Cox Regression Test |
Next, we analyzed the prognostic predictor among patients with stage IIIC1p cervical cancer. Univariate analyses with Log rank test identified three prognostic factors associated with recurrence-free survival: parametrial involvement, the number of mLNs and mLNR. Multivariate analysis with Cox regression identified two factors as independent prognostic predictors of recurrence-free survival: parametrial involvement, and mLNR (HR=2.456, 95% CI 1.393–4.330, P=0.002; HR=2.357, 95% CI 1.396–3.981, P=0.001, respectively). (Table 3, Figure 1A and B).
 |
Table 3 Univariate Cox Regression Analysis and Multivariate Cox Regression Regarding Recurrence-Free Survival |
As for overall survival, univariate survival analysis indicated that parametrial involvement and mLNR were potential prognostic factors that correlated with poor overall survival (all P<0.05; Table 4). Further multivariate analysis showed that parametrial involvement, and mLNR were independent adverse prognostic factors for overall survival (HR=2.477, 95% CI 1.212–5.059, P=0.013; HR=2.014, 95% CI 1.046–3.875, P=0.036). (Table 4, Figure 1C and D).
 |
Table 4 Univariate Cox Regression Analysis and Multivariate Cox Regression Regarding Overall Survival |
Risk Stratification
Based on the above results, parametrial involvement and metastatic lymph node status were identified as the prognostic predictors among patients with stage IIIC1p cervical cancer. Therefore, a scoring system was performed based on parametrial involvement and mLNR. Accordingly, parametrial involvement and mLNR≥0.08 were counted independently as 1 point with a total score ranging from 0 to 2. As a result, patients were stratified into three groups: 123 patients had 0 risk factor (low-risk), 127 patients had 1 risk factor (intermediate risk), and 23 patients had 2 risk factors (high-risk). Five-year recurrence-free survival was 81.7%, 51.1% and 38.0%, respectively, and 5-year overall survival was 86%, 70.2%, and 55.3%, respectively. There was a significant difference regarding recurrence-free survival and overall survival among different risk groups (both P≤0.001) (Figure 2A and B). Compared to the low-risk group, the intermediate-risk and high-risk groups showed a significantly greater risk of recurrent disease (HR=2.660, 95% CI 1.517–4.664, P=0.001; and HR=5.676, 95% CI 2.749–11.720, P=0.000, respectively), and higher risk of death (HR=2.131, 95% CI 1.048–4.332; P=0.037; and HR =4.944, 95% CI 1.986–12.311, P=0.001, respectively).
Discussion
Although the application of the FIGO 2018 staging schema will provide improved discriminatory ability for women with stage IB tumors, classification of all women with positive lymph nodes into one stage will result in a very heterogeneous group of patients with highly variable survival rates.6,13 The objective of this study was to explore the prognostic factors in patients with cervical cancer in stage IIIC1p and further stratify this group.
At first, we comprehensively explored the prognostic signification of removed and metastatic pelvic lymph node status among patients with stage IIIC1p cervical cancer. Our results showed that while the number of removed pelvic lymph and bilateral pelvic lymph node metastases showed no effect on survival, the number of metastatic pelvic lymph and mLNR was associated with clinical outcomes for patients with stage IIIC1p cervical cancer. Furthermore, mLNR was identified as an independent prognostic predictor of recurrence-free survival and overall survival.
The effect of the number of removed LNs on the survival of patients with node-positive cervical cancer remains controversial. Theoretically, extensive evaluation of LNs in patients with cervical cancer can result in more accurate staging and clinical outcomes, as residual lymph nodes after treatment could increase the risk of distant metastasis.14,15 Indeed, previous studies have found that a higher number of removed LNs was associated with better survival in patients with node-positive disease. In an analysis of 11,830 patients with cervical cancer from the SEER database, Zhou et al reported that a higher number of examined LNs were associated with better survival in patients with early-stage cervical cancer, especially in patients with a node-positive disease.16 Pieterse et al reported that the number of removed nodes was not significantly associated with the cancer-specific survival but it was for the disease-free survival in patients with node-positive cervical cancer.17 However, several studies have shown that extensive lymphadenectomy had no effect on the survival of women with positive lymph nodes.18 Our results showed the number of removed LNs was not a prognostic indicator for patients with stage IIIC1p cervical cancer. A potential explanation is that the number of removed LNs (the median number of removed LNs = 22) was more than previous reports,16,17 suggesting an adequacy of surgical resection in our cohort. Another feasible explanation is that the extent of lymphadenectomy and the number of total examined LNs are not important in a node-positive patient, because the presence of nodal metastasis is of great importance in determining the prognosis of patients with cervical cancer.
The number of metastatic LNs is the most simple, intuitive and widely used predictor in cervical cancer.8,19 Increasing number of metastatic pelvic lymph nodes was associated with worse prognosis of cervical cancer in most previous studies, with a variable cut-off rang 1–5.11,12,20 In this study, our results showed an increased number of metastatic lymph nodes elevated the risk of recurrence and deaths of patients with stage IIIC1p cervical cancer in univariate analysis. However, the number of mLNs showed no effect on recurrence-free survival and overall survival using multivariate analysis. Interestingly, when we set the cutoff values at 3, we got the same result (data not shown), further supporting the conclusion that the number of metastatic LNs is not an independent prognostic factor.
The metastatic LN ratio (mLNR), which has been developed recently to comprehensively reflect the extent of LN resection and the burden of nodal disease, is suggested as a prognostic variable related to LN status. Li et al reported metastatic LNR ≥0.2 correlated with a poor disease-free survival and overall survival, and stated metastatic LNR as an independent factor in patients with squamous cervical cancer.21 Aslan et al reported that LNR more than 0.05 seemed to be an independent prognostic factor for decreased disease-free survival and overall survival in stage IIIC cervical carcinoma.22 Similar results were reported by Polterauer et al,10 and Fleming et al9 with a cut-off of 0.1 and 0.066, respectively. We showed here that a significant decrease in survival as the mLNR increased using several previous reported cut-off values (0.1–0.3) as well as the median mLNR in the current study.
Although several studies have explored various methods to assess pelvic lymph node status and showed promising results. However, it is not clear which parameter is superior in prognostic evaluation for patients with lymph node-positive cervical cancer. Polterauer et al10 showed that mLNR was an independent prognostic parameter in patients with lymph node metastasis cervical cancer and superior to the number of metastatic lymph node in the evaluation of overall survival. In agreement with previous data, our results showed mLNR but not the number of mLNs exerted an independent effect on survival. Accordingly, we suggested mLNR was superior to the absolute number of mLNs in prognostic evaluation for patients with cervical cancer, and this parameter was selected for further stratification.
Although lymph node metastases negatively affect prognosis in cervical cancer, the survival of patients with lymph nodes metastasis is also strongly influenced by parametrial involvement.8,23,24 Along similar lines, our results showed parametrial involvement was an independent predictor both for recurrence-free survival and overall survival among patients with stages IIIC1p. Accordingly, we rank parametrial involvement as a risk factor for further stratification.
Risk stratification of patients with stage IIIC1p using these two parameters separated patients into low-risk (45.1%), intermediate-risk (46.5%), and high-risk (8.4% of patients), for which rates of recurrence-free survival at 5 years were 81.7% vs 51.1% vs.38%, respectively. In other words, while about a half of stage IIIC1p patients had a ~50% risk of relapse after primary therapy, the remainder of stage IIIC1p patients faced a much lower risk of the same even. This simple classification system may be clinically useful for patient prognostication and guiding post-adjuvant therapy.
More recently, Liu et al investigated the heterogeneity of patients with stage IIIC1 cervical cancer and stratified patients into three subgroups based on tumor size and number of pelvic lymph node metastasis.7 The most difference between our study and theirs is the difference in the patients included. We included stage IIIC1p, and they included stage IIIC1r. Second, their study used mLNs to evaluate the status of lymph nodes directly. Our study chose the most suitable method by comparing various assessment methods. The best LNR is used as the evaluation status. Accordingly, we believe that the stratification based on pelvic metastatic lymph node status is a promising risk stratification among patients with IIIC1, especially in stage IIIC1p cervical cancer.
This work has several limitations. First, this investigation is a retrospective study using a single-institution Chinese database. The validation of this system is needed at multiple medical centers in the future. Second, it is well known that the total number of removed and metastatic LNs is dependent on how extensively a pathologist examines the surgical specimen. In our center, all surgeries were performed by experienced surgeons, and all pathology specimens were evaluated by cancer specializes, which may deduce the bias.
Conclusions
Parametrial involvement and mLNR were independently prognostic predictors for patients with stage IIIC1p cervical cancer. A stratification system based on parametrial involvement and mLNR could stratify the risk groups of recurrence and survival in the patients with stageIIIC1p. Our findings could facilitate the practical use of further stratification in Stage IIIC1p cervical cancer.
Abbreviations
FIGO, Federation of Gynecology and Obstetrics; LNs, lymph nodes; mLNs, metastatic lymph nodes; mLNR, metastatic lymph node ratio; SCC-Ag, serum squamous cell carcinoma antigen.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and informed consent was signed by the patients before taking part in this study. All procedures were in accordance with the Declaration of Helsinki.
Acknowledgments
We would like to thank all doctors, nurses, patients, and their family members for their kindness to support our study.
Author Contributions
HZ main conception and design the study; AL, LW, WW main acquisition of data, AL, and QJ analysis of data; AL drafting the manuscript; HZ and BH revising this manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi:10.1016/S0140-6736(18)32470-X
3. Lee YN, Wang KL, Lin MH, et al. Radical hysterectomy with pelvic lymph node dissection for treatment of cervical cancer: a clinical review of 954 cases. Gynecol Oncol. 1989;32:135–142. doi:10.1016/S0090-8258(89)80024-1
4. McComas KN, Torgeson AM, Ager BJ, et al. The variable impact of positive lymph nodes in cervical cancer: implications of the new FIGO staging system. Gynecol Oncol. 2020;156:85–92. doi:10.1016/j.ygyno.2019.10.025
5. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. doi:10.1002/ijgo.12611
6. Wright JD, Matsuo K, Huang Y, et al. Prognostic performance of the 2018 international federation of gynecology and obstetrics cervical cancer staging guidelines. Obstet Gynecol. 2019;134:49–57. doi:10.1097/AOG.0000000000003311
7. Liu X, Wang W, Hu K, et al. A risk stratification for patients with cervical cancer in stage IIIC1 of the 2018 FIGO staging system. Sci Rep. 2020;10:362. doi:10.1038/s41598-019-57202-3
8. Aoki Y, Sasaki M, Watanabe M, et al. High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol. 2000;77:305–309. doi:10.1006/gyno.2000.5788
9. Fleming ND, Frumovitz M, Schmeler KM, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol. 2015;136:48–53. doi:10.1016/j.ygyno.2014.11.010
10. Polterauer S, Hefler L, Seebacher V, et al. The impact of lymph node density on survival of cervical cancer patients. Br J Cancer. 2010;103:613–616. doi:10.1038/sj.bjc.6605801
11. Horn LC, Hentschel B, Galle D, Bilek K. Extracapsular extension of pelvic lymph node metastases is of prognostic value in carcinoma of the cervix uteri. Gynecol Oncol. 2008;108:63–67. doi:10.1016/j.ygyno.2007.08.086
12. Kasuya G, Ogawa K, Iraha S, et al. Postoperative radiotherapy for uterine cervical cancer: impact of lymph node and histological type on survival. Anticancer Res. 2013;33:2199–2204.
13. Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152:87–93. doi:10.1016/j.ygyno.2018.10.026
14. Kim HS, Park NH, Wu HG, et al. Matched-case comparison for the role of surgery in FIGO stage Ib1-IIa squamous cell carcinoma of cervix and suspicious para-aortic lymph node metastasis. Ann Surg Oncol. 2009;16:133–139. doi:10.1245/s10434-008-0197-3
15. Ferrandina G, Distefano M, Ludovisi M, et al. Lymph node involvement in locally advanced cervical cancer patients administered preoperative chemoradiation versus chemotherapy. Ann Surg Oncol. 2007;14:1129–1135. doi:10.1245/s10434-006-9252-0
16. Zhou J, Zhang WW, Wu SG, et al. The impact of examined lymph node count on survival in squamous cell carcinoma and adenocarcinoma of the uterine cervix. Cancer Manag Res. 2017;9:315–322. doi:10.2147/CMAR.S141335
17. Pieterse QD, Kenter GG, Gaarenstroom KN, et al. The number of pelvic lymph nodes in the quality control and prognosis of radical hysterectomy for the treatment of cervical cancer. Eur J Surg Oncol. 2007;33:216–221. doi:10.1016/j.ejso.2006.09.037
18. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a gynecologic oncology group study. Gynecol Oncol. 1990;38:352–357. doi:10.1016/0090-8258(90)90072-S
19. Chen Y, Zhang L, Tian J, Fu X, Ren X, Hao Q. Significance of the absolute number and ratio of metastatic lymph nodes in predicting postoperative survival for the International Federation of Gynecology and Obstetrics stage IA2 to IIA cervical cancer. Int J Gynecol Cancer. 2013;23:157–163. doi:10.1097/IGC.0b013e3182778bcf
20. Kim HS, Kim JH, Chung HH, et al. Significance of numbers of metastatic and removed lymph nodes in FIGO stage IB1 to IIA cervical cancer: primary surgical treatment versus neoadjuvant chemotherapy before surgery. Gynecol Oncol. 2011;121:551–557. doi:10.1016/j.ygyno.2011.01.024
21. Li C, Liu W, Cheng Y. Prognostic significance of metastatic lymph node ratio in squamous cell carcinoma of the cervix. Onco Targets Ther. 2016;9:3791–3797. doi:10.2147/OTT.S97702
22. Aslan K, Meydanli MM, Oz M, Tohma YA, Haberal A, Ayhan A. The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy. J Gynecol Oncol. 2020;31:e1. doi:10.3802/jgo.2020.31.e1
23. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Further stratification of risk groups in patients with lymph node metastasis after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol. 2010;117:53–58. doi:10.1016/j.ygyno.2009.12.006
24. Chen Z, Huang K, Lu Z, et al. Risk model in stage IB1-IIB cervical cancer with positive node after radical hysterectomy. Onco Targets Ther. 2016;9:3171–3179. doi:10.2147/OTT.S94151
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


