Back to Journals » Clinical Epidemiology » Volume 13
Risk of Severe Covid-19 in Patients with Celiac Disease: A Population-Based Cohort Study
Authors Lebwohl B, Larsson E, Söderling J, Roelstraete B, Murray JA, Green PHR, Ludvigsson JF
Received 2 December 2020
Accepted for publication 21 January 2021
Published 18 February 2021 Volume 2021:13 Pages 121—130
DOI https://doi.org/10.2147/CLEP.S294391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eyal Cohen
Benjamin Lebwohl,1,2 Emma Larsson,3 Jonas Söderling,4 Bjorn Roelstraete,4 Joseph A Murray,5 Peter HR Green,1 Jonas F Ludvigsson1,4,6
1Celiac Disease Center, Department of Medicine, Columbia University Medical Center, New York, NY, USA; 2Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA; 3Department of Physiology and Pharmacology, Karolinska Institute, Stockholm, Sweden; 4Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden; 5Department of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA; 6Department of Pediatrics, Örebro University Hospital, Örebro University, Örebro, Sweden
Correspondence: Jonas F Ludvigsson
Department of Medical Epidemiology and Biostatistics, Karolinska Institute, PO Box 281, Stockholm, SE-17177, Sweden
Tel +46-19-6021000
Email [email protected]
Background: Patients with celiac disease (CeD) are at increased risk of certain viral infections and of pneumococcal pneumonia, raising concerns that they may be susceptible to severe coronavirus disease 2019 (Covid-19). We aimed to quantify the association between CeD and severe outcomes related to Covid-19.
Methods: We performed a population-based cohort study, identifying individuals with CeD in Sweden, as defined by small intestinal villus atrophy diagnosed at all (n=28) Swedish pathology departments during the years spanning 1969– 2017, and alive on February 1, 2020. We compared these patients to controls matched by sex, age, county, and calendar period. We performed Cox proportional hazards with follow-up through July 31, 2020, assessing risk of 1) hospital admission with a primary diagnosis of laboratory-confirmed Covid-19 (co-primary outcome); and 2) severe disease as defined by admission to intensive care unit and/or death attributed to Covid-19 (co-primary outcome).
Results: Among patients with CeD (n=40,963) and controls (n=183,892), the risk of hospital admission for Covid-19 was 2.9 and 2.2 per 1000 person-years respectively. After adjusting for comorbidities, the risk of hospitalization for Covid-19 was not significantly increased in patients with CeD (HR 1.10; 95% CI 0.80– 1.50), nor was the risk of severe Covid-19 increased (HR 0.97; 95% CI 0.59– 1.59). Results were similarly null when we compared CeD patients to their non-CeD siblings with regard to these outcomes. Among all patients with CeD and controls hospitalized with a diagnosis of Covid-19 (n=58 and n=202, respectively), there was no significant difference in mortality (HR for CeD compared to controls 0.96; 95% CI 0.46– 2.02).
Conclusion: In this population-based study, CeD was not associated with an increased risk of hospitalization for Covid-19 or intensive care unit and/or death attributed to Covid-19.
Keywords: Covid-19, celiac disease, epidemiology, infection, SARS-CoV-2
Introduction
Celiac disease (CeD) is characterized by the activation of innate and adaptive immunity in the small bowel in response to the ingestion of gluten.1 CeD is paradoxically a condition of both increased and defective immune activity. Though it is characterized by an immune hyper-responsiveness to ingested gluten, multiple studies have shown that patients with CeD have an increased susceptibility to, and/or worse outcomes in, infections such as invasive pneumococcal disease2–4 and viral illnesses including influenza5 and varicella zoster.6 Though the magnitude of risk increase is modest, these associations have been found for multiple forms of infection.7 Unlike inflammatory bowel disease, in which infection risk is attributed to immunosuppression brought about by medical therapy,8 the infection risks associated with CeD (in which such therapy is offered to only a small minority of patients with refractory CeD) appear to be intrinsic to the disease.
This increased risk of infection has led to concern that patients with CeD may be at risk of contracting SARS-CoV-2 and developing severe outcomes in the coronavirus disease 2019 (Covid-19). As the Covid-19 pandemic has evolved, there has been an urgent need to identify risk factors for severe outcomes so as to risk-stratify patients for monitoring and therapeutic interventions.9 An analysis of a questionnaire administered to patients in Italy in March, 2020 (during the height of the pandemic in that country to date) found that approximately one third of CeD patients responded that they do not know if their condition places them at increased risk of severe complications of Covid-19 infection.10 In this study, we performed a population-based analysis, aiming to quantify the association between CeD and severe outcomes related to Covid-19.
Methods
We performed an analysis of the ESPRESSO cohort, a nation-wide histology-based population of patients with gastrointestinal disease in Sweden; a subset of this cohort, consisting of patients with CeD, was analyzed in the present study. Full details of the ESPRESSO cohort have been reported previously.11 In brief, between 2015 and 2017, data from all (n=28) pathology departments in Sweden were queried for histopathology records pertaining to all gastrointestinal specimens in the years spanning 1969–2017. These specimens were linked to the patient’s personal identity number, topography within the gastrointestinal tract, and morphology.
Identification of CeD Subjects and Controls
Within this cohort, we identified all patients with CeD using the Systematized Nomenclature of Medicine (SNOMED) codes corresponding to villus atrophy (see Supplementary Table S1). Each patient was then matched by Statistics Sweden to up to 5 control subjects using the following matching parameters: age, gender, county, and calendar period.
Outcomes
We had two primary outcomes: 1) hospitalization for Covid-19, defined as the first date of a hospital admission with aprimary discharge diagnosis ICD-10 code U07.1 within the Swedish National Patient Registry (Supplementary Table S2); and 2) severe Covid-19 as defined by a composite of either admission to intensive care unit with a Covid-19 diagnosis code, death with Covid-19 listed as the underlying cause, or death within 30 days of Covid-19 being listed as a primary diagnosis in the patient register. Data on intensive care admissions were derived from the Swedish Intensive Care Registry (SIR), which collects individual patient data from all Swedish intensive care units (n=83). In co-operation with the Public Health Agency of Sweden, mandatory surveillance data of COVID-19 is routinely reported.
We analyzed the following secondary outcomes: 1) a composite of the above outcomes (hospitalizations or severe Covid-19); 2) all-cause mortality during the observation period; 3) the development of Covid-19 (regardless of severity), defined as any mention of a Covid-19 diagnosis code (see Supplementary Table S2) in the patient register, the cause of death register, the Swedish Public Health Agency, or any inclusion criteria in the outcomes listed above.
Finally, we also examined death after diagnosis of Covid-19, an all-cause mortality analysis restricted to those CeD patients and controls who had a diagnosis of Covid-19.
Statistical Analysis
We used stratified Cox proportional hazards, with follow-up time commencing on February 1, 2020, and continuing until death, the development of a Covid-19-related outcome, or July 31, 2020. Hazard Ratios (HR) and corresponding 95% confidence intervals (CI) were conditioned on matching parameters to estimate the association between CeD and the Covid-19-related outcomes listed above. In addition to these conditioned HR’s, we also reported HR’s with further adjustment for educational attainment, country of birth (Nordic versus other), and the following medical comorbidities documented prior to the index date (date of CeD diagnosis and corresponding date in controls): cardiovascular disease, diabetes, chronic obstructive pulmonary disease (COPD), end-stage renal disease, alcohol liver disease/alcohol use disorder, obesity/dyslipidemia, obstructive sleep apnea, cancer, and psychiatric disease. The diagnosis codes used to ascertain these medical comorbidities are listed in Supplementary Table S3.
After calculating risk estimates for the overall cohort, we performed stratified analyses by the following parameters: follow-up time (per month), age group at diagnosis and at start of follow-up (<18, 18-<40, 40-<60, and ≥60 years), gender, year of diagnosis (1969–1999, 2000–2009, 2010–2017), country of birth (Nordic or other), and educational attainment (≤9 years, 10–12 years, >12 years).
Sensitivity Analyses
Given the association between medical comorbidities and Covid-19 outcomes,12 we repeated the analyses, now by matching CeD patients to controls by means of propensity scores. Patients with CeD were matched 1:5 on birth year, sex, county and a nearest neighbor propensity score algorithm including age, sex, education, Nordic country of birth and the above-mentioned list of comorbidities. For this analysis we were limited to comorbidities up until 2016. In the spring of 2020, the Swedish Ethical Review Authority created a fast-track for Covid-19 research that allowed researchers to update existing cohorts with data on deaths and hospital care for Covid-19 (until July 31, 2020) as well as data on death dates (until July 31, 2020), to allow researchers to examine cohort members still alive in 2020. The ESPRESSO cohort was updated accordingly, but the extra ethical permit did not include update of non-Covid-19-related information. For that reason, our data on comorbidity were limited to the original data retrieval for the ESPRESSO cohort (up until Dec 31, 2016, but not for years 2017–2020).
In addition, we considered that the development of Covid-19 is often a function of social factors, such as socioeconomic status and living arrangement.13 We therefore performed an analysis comparing patients with CeD to their non-CeD siblings, using the Total Population Register, and further adjusting for all of the previous-mentioned covariates as well as age and gender.
Ethics Approval
This cohort was approved by the Stockholm Ethics Review Board (No. 2014/1287-31/4) on August 27, 2014, with a Covid-19-specific amendment: 2020–02307. The Ethics Review Board waived the requirement for informed consent; this was a register-based study with large-scale pseudonymized data. Swedish Ethics review boards typically waive patient consent for such studies.14 (We included safeguards to ensure patient data confidentiality, and the research adhered to the Declaration of Helsinki.)
Results
Of the 50,000 individuals in Sweden with small intestinal villus atrophy during the years spanning 1969–2017, 9037 were excluded due to not being identified in other health care registers (n=130), death before February 1, 2020 (n=7506), emigration prior to February 1, 2020 (n=1205), or lack of a matched comparator (n=196). The remaining 40,963 individuals were matched to 204,453 controls (Figure 1).
 |
Figure 1 Flow chart of patients with celiac disease and their matched comparators. |
Baseline characteristics of the cohort are shown in Table 1. Females comprised nearly 65% of patients with CeD. The mean age of CeD diagnosis was 26.5 years and the mean age at the start of follow-up (February 2020) was 42.8 years. Cardiovascular disease was present in 13.3% of patients with CeD, compared to 9.8% of controls. Diabetes was present in 7.3% and 3.1% of patients with CeD and controls, respectively.
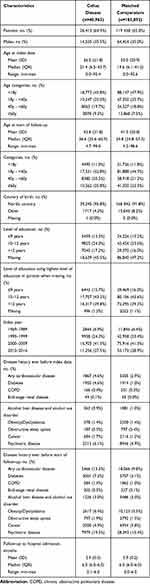 |
Table 1 Baseline Characteristics of Study Cohort |
Among 40,963 individuals with CeD, 58 (0.14%) were hospitalized with Covid-19 during the six-month follow-up period, yielding an incidence of 2.9/1000 person-years. The corresponding rate for controls was 0.11% and 2.2/1000 person-years (see Table 2). There was no significant increased risk of hospitalization for Covid-19 in CeD patients compared to controls (HR 1.10; 95% CI 0.80–1.50).
 |
Table 2 Risk of Covid-19 in Patients with Celiac Disease and Matched General Population Comparators from February 1 to July 31, 2020 |
Severe Covid-19 developed in 24 individuals with celiac disease (0.06%) and 78 controls (0.04%), with corresponding incidences of 1.2 and 0.9 per 1000 person-years, respectively. There was no significant association between CeD and the development of severe Covid-19 (HR 0.97; 95% CI 0.59–1.59). This null relationship was also found when considering hospitalizations and severe Covid-19 as a composite outcome (1.09; 95% CI 0.82–1.46), all-cause mortality during this period (HR 0.97; 95% CI 0.81–1.15), or the development of any Covid-19 (HR 1.00; 95% 0.89–1.11, see Table 2).
Stratified analyses are shown in Table 3. The risk of hospitalization for Covid-19 was highest among those ≥60 years (8.3/1000 person years among patients with CeD and 6.3/1000 person-years among controls). There was no significant association between CeD and hospitalization for Covid-19 when stratified by follow-up time, gender, age, year of diagnosis or educational attainment.
 |
Table 3 Risk of Covid-19 (Hospital Admission) Overall and by Subgroups in Patients with Celiac Disease and Matched General Population Comparators from February 1 to July 31, 2020 |
When considering those patients with CeD and controls who were diagnosed with Covid-19, among those who were hospitalized (CeD n=58, controls n=202), the absolute risk of death was 19.0% in those with CeD and 17.3% in controls. There was no association between CeD and the risk of mortality among those hospitalized with Covid-19 (HR 0.96; 95% CI 0.46–2.02). Among all patients with CeD who were diagnosed with Covid-19 (n=414) and all controls diagnosed with Covid-19 (n=1793), the overall mortality rate was 5.3% in both CeD patients and 4.1% in controls. There was no association between the presence of CeD and mortality among those diagnosed with Covid-19 (HR 0.80; 95% CI 0.48–1.31, see Table 4 and Figure 2).
 |
Table 4 Risk of All-Cause Mortality in Individuals with Covid-19 and Celiac Disease and Matched General Population Comparators with Covid-19 from February 1 to July 31, 2020 |
Sensitivity Analyses
We repeated the analyses, now matching patients with CeD (n= 40,755) to controls, (n=202,028) using propensity score matching including comorbidities up until 2016 (see Supplementary Table S4). There was no association between CeD and the primary outcome of hospital admission for Covid-19 (HR 1.13; 95% CI 0.85–1.51), or the development of severe Covid-19 (HR 1.02; 95% CI 0.66–1.58). Stratified analyses likewise showed no significant associations (see Supplementary Tables S5 and S6).
When we compared CeD patients (n= 28,168) to their non-CeD siblings (n= 45,669), there was likewise no association between CeD and hospitalization for Covid-19 (HR 1.08; 95% CI 0.64–1.85), or the development of severe Covid-19 (HR 0.62; 95% CI 0.22–1.75, see Supplementary Tables S7 and S8.
Discussion
In this population-based cohort study set in Sweden during its first six months of the Covid-19 pandemic, we found that the incidence of hospitalization for Covid-19 or severe Covid-19 was not increased among people with CeD compared to a control group matched by age, gender, county, and calendar period of cohort entry. Though the risk of hospitalization for Covid-19 was approximately 1 in 1000 over the six-month period, and the risk of being diagnosed with Covid-19 was approximately 1% (see Table 2), there was no difference in these outcomes when comparing CeD patients to controls. This finding was robust to sensitivity analyses that employed the use of two alternative methods for selection of controls, including propensity-score matching, and an analysis of siblings.
The rationale for studying CeD as a risk factor for adverse outcomes related to Covid-19 stems from an extensive literature on morbidity in CeD related to respiratory disease and viruses. A recently-published analysis of the ESPRESSO cohort found that overall mortality in patients with CeD was increased (HR 1.21; 95% CI 1.17–1.25), and that this increase was also seen when for deaths attributed to respiratory disease (HR 1.21; 95% CI, 1.08–1.37).15 This may be related to the previously-documented increased risk of invasive pneumococcal disease; another population-based study found a 1.46-fold increased risk of that outcome.4 This susceptibility, which has been found in multiple settings3 may be due to the well-recognized functional hyposplenism that is present in some patients with CeD.16 This mechanistic explanation is not applicable to other respiratory pathogens that are not dependent on splenic function such as tuberculosis and influenza, both of which have been found to be increased in patients with CeD.5,17 An intrinsic susceptibility to viruses is also suggested by a prior study linking CeD to an increased risk of herpes zoster infection.6 Multiple studies suggesting that CeD may be triggered by viruses such as reovirus,18 rotavirus,19 and enterovirus20 likewise point to a susceptibility of this population to viral infections. Despite these concerns, it is important for health care providers and patients to recognize that the absolute risk increases are small. In the study of pneumococcal disease, the absolute increase was only 0.04% (0.15% vs 0.11%) over a median follow-up of 10.5 years.4 Likewise, in the analysis of hospitalization for influenza, CeD was associated with an excess risk of only 16 events per 100,000 person-years.5
The results of the current study results suggest that CeD does not confer additional risk related to Covid-19. This is consistent with a recent survey study of 138 patients enrolled in a CeD clinic in Padua, Italy; upon contact by telephone in April 2020, none had reported being diagnosed with Covid-19.21 A subsequent international survey of CeD patients based on self-reported CeD and Covid-19 diagnoses likewise found no increased risk of developing Covid-19 compared to controls.22 An international registry for healthcare professionals, SECURE-Celiac (www.covidceliac.org), is tracking patients with CeD who develop Covid-19, so as to identify disease-specific risk factors for severe outcomes. The null results also suggest that the previously-described associations between CeD and cardiovascular disease23 and diabetes24 are not sufficiently strong so as to mediate an increased risk of Covid-19, even though they are established risk factors for severe outcomes in Covid-19;25 when examining HR’s that do not adjust for these and other comorbidities, there remained no significant association between CeD and Covid-19 in our analysis. These null results regarding severe outcomes are concordant with earlier data on other immune-mediated diseases.26,27
This study has a number of limitations. The cohort is based in Sweden, where regulations regarding social distancing and school and restaurant closures were distinct from that of other countries, which may impact the generalizability of these findings.28 This is particularly relevant in light of the fact that many risk factors for Covid-19 have social components, such as living arrangements and occupation,29 which may interact with regional regulatory conditions with regard to risk. At the same time, the lack of a generalized lockdown is likely to have increased the number of individuals exposed to Covid-19, and indeed the current study was able to identify more than 400 CeD patients who developed Covid-19. Our analysis of severe Covid-19 warranting intensive care was limited to those patients who were accepted into an intensive care unit; however, this potential for selection bias is balanced by the fact that we included death related to Covid-19 in this composite outcome. Taking advantage of our statistical power, this study was able to rule out even small excess risks for severe Covid-19 (the upper 95% CI for hospital admission was 1.50 and for severe Covid-19 1.59). We did not have data on human leukocyte antigen (HLA) haplotype in this study. While genetic risk factors for Covid-19 infection or outcomes are not yet well understood, it is possible that the genetics underpinning CeD, especially HLA haplotype DQ2 that is present in >90% of individuals with CeD, might impact the immune responses to Covid-19, though the lack of difference in infection rates makes that unlikely.30
In addition, given that Covid-19 carries a wide range of symptom severity, it is likely that a substantial proportion of individuals in this cohort were infected with Covid-19 but were undiagnosed; nevertheless, our primary outcomes, hospitalization with Covid-19 and severe Covid-19, renders under-ascertainment of individuals with mild infection less relevant. This study evaluated patients diagnosed with CeD; as a substantial proportion of patients with CeD are undiagnosed,31 we are unable to extrapolate these findings to patients with undiagnosed (and untreated) CeD. Our primary and secondary outcomes are clinically relevant, but do not include other outcomes of Covid-19 that are increasingly being reported, including prolonged disability.32 It is possible that there is an association between CeD and Covid-19 that our cohort did not detect due to our sample size; in a post-hoc calculation of this cohort, we had 80% power to detect a hazard ratio of 1.42 or greater. Nevertheless, though a risk of more modest magnitude may be present, it would pale in comparison to the established risk factors for severe Covid-19 outcomes such as older age, hypertension, and diabetes.25 The scope of our study precluded an evaluation of whether Covid-19 was associated with gastrointestinal symptoms in CeD patients; a variety of gastrointestinal manifestations have been reported in patients with Covid-19.33 Strengths of this study include its population-based setting and use of multiple control groups to test the robustness of our findings. The fact that older patients and males in this cohort had a higher incidence of our primary and secondary outcomes lends internal validity to this study.
In conclusion, we found that patients with CeD were not at increased risk of hospitalization for Covid-19 in this population-based study; nor were they at increased risk for Covid-19-related severe outcomes including intensive care unit admission or mortality; nor was overall mortality elevated in people with CeD during the first six-months of the Covid-19 pandemic in Sweden. Based on these data, having a diagnosis of CeD does not appear to place an individual at increased risk for severe Covid-19.
Data Sharing Statement
Other researchers can apply for our data through the different Swedish pathology departments, and through the Swedish National Board of Health and Welfare.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
BL: The Louis and Gloria Flanzer Philanthropic Trust. JFL: Karolinska Institute.
Disclosure
Dr Benjamin Lebwohl reports grants from The Louis and Gloria Flanzer Philanthropic Trust, during the conduct of the study; grants from Celiac Disease Foundation, personal fees from Takeda, personal fees from Anokion, outside the submitted work.
Dr. Joseph Murray has received study grants from Nexpep/ImmusanT, National Institutes of Health, Immunogenix, Takeda Pharmaceutical, Allakos, Oberkotter, and Cour; consultancy fees from Bionix, Lilly Research Laboratory, Johnson & Johnson, Dr. Schar USA, UCB Biopharma, Celimmune, Intrexon Corporation, Chugai Pharma, Kanyos, and Boehringer Ingelheim; holds patents licensed to Evelo Biosciences; and receives royalties from Torax Medical., during the conduct of the study.
Professor Jonas F Ludvigsson reports research support from Janssen, outside the submitted work. The authors report no other conflicts of interest related to this work.
References
1. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391(10115):70–81. doi:10.1016/S0140-6736(17)31796-8
2. Thomas HJ, Wotton CJ, Yeates D, Ahmad T, Jewell DP, Goldacre MJ. Pneumococcal infection in patients with coeliac disease. Eur J Gastroenterol Hepatol. 2008;20(7):624–628. doi:10.1097/MEG.0b013e3282f45764
3. Simons M, Scott-Sheldon LAJ, Risech-Neyman Y, Moss SF, Ludvigsson JF, Green PHR. Celiac disease and increased risk of pneumococcal infection: a systematic review and meta-analysis. Am J Med. 2018;131(1):83–89. doi:10.1016/j.amjmed.2017.07.021
4. Röckert Tjernberg A, Bonnedahl J, Inghammar M, et al. Coeliac disease and invasive pneumococcal disease: a population-based cohort study. Epidemiol Infect. 2017;145(6):1203–1209. doi:10.1017/S0950268816003204
5. Mårild K, Fredlund H, Ludvigsson JF. Increased risk of hospital admission for influenza in patients with celiac disease: a nationwide cohort study in Sweden. Am J Gastroenterol. 2010;105(11):2465–2473. doi:10.1038/ajg.2010.352
6. Ludvigsson JF, Choung RS, Marietta EV, Murray JA, Emilsson L. Increased risk of herpes zoster in patients with coeliac disease - nationwide cohort study. Scand J Public Health. 2018;46(8):859–866. doi:10.1177/1403494817714713
7. Ludvigsson JF, Olén O, Bell M, Ekbom A, Montgomery SM. Coeliac disease and risk of sepsis. Gut. 2008;57(8):1074–1080. doi:10.1136/gut.2007.133868
8. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. doi:10.1053/j.gastro.2020.05.032
9. Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE. Risk factors for severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Clin Infect Dis. 2020;71(16):2199–2206. doi:10.1093/cid/ciaa576
10. Siniscalchi M, Zingone F, Savarino EV, D’Odorico A, Ciacci C. COVID-19 pandemic perception in adults with celiac disease: an impulse to implement the use of telemedicine. Dig Liver Dis. 2020;52:1071–1075. doi:10.1016/j.dld.2020.05.014
11. Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–114. doi:10.2147/CLEP.S191914
12. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi:10.1016/j.ijid.2020.03.017
13. Abedi V, Olulana O, Avula V, et al. Racial, Economic, and Health Inequality and COVID-19 Infection in the United States. J Racial Ethn Health Disparities. 2020. doi:10.1007/s40615-020-00833-4
14. Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. doi:10.2147/CLEP.S90589
15. Lebwohl B, Green PHR, Söderling J, Roelstraete B, Ludvigsson JF. Association between celiac disease and mortality risk in a swedish population. JAMA. 2020;323(13):1277–1285. doi:10.1001/jama.2020.1943
16. Canova C, Ludvigsson J, Baldo V, Barbiellini Amidei C, Zanier L, Zingone F. Risk of bacterial pneumonia and pneumococcal infection in youths with celiac disease - a population-based study. Dig Liver Dis. 2019;51(8):1101–1105. doi:10.1016/j.dld.2019.02.010
17. Ludvigsson JF, Sanders DS, Maeurer M, Jonsson J, Grunewald J, Wahlström J. Risk of tuberculosis in a large sample of patients with coeliac disease–a nationwide cohort study. Aliment Pharmacol Ther. 2011;33(6):689–696. doi:10.1111/j.1365-2036.2010.04572.x
18. Bouziat R, Hinterleitner R, Brown JJ, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356(6333):44–50. doi:10.1126/science.aah5298
19. Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101(10):2333–2340. doi:10.1111/j.1572-0241.2006.00741.x
20. Kahrs CR, Chuda K, Tapia G, et al. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort. BMJ. 2019;364:l231. doi:10.1136/bmj.l231
21. Zingone F, D’Odorico A, Lorenzon G, Marsilio I, Farinati F, Savarino EV. Risk of COVID-19 in celiac disease patients. Autoimmun Rev. 2020;19:102639. doi:10.1016/j.autrev.2020.102639
22. Zhen J, Stefanolo JP, Temprano M, et al. The risk of contracting COVID-19 is not increased in patients with celiac disease. Clin Gastroenterol Hepatol. 2020;19:391–393. doi:10.1016/j.cgh.2020.10.009
23. Emilsson L, Lebwohl B, Sundström J, Ludvigsson JF. Cardiovascular disease in patients with coeliac disease: a systematic review and meta-analysis. Dig Liver Dis. 2015;47(10):847–852. doi:10.1016/j.dld.2015.06.004
24. Elfström P, Sundström J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40(10):1123–1132. doi:10.1111/apt.12973
25. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, china. JAMA Intern Med. 2020;180:934. doi:10.1001/jamainternmed.2020.0994
26. Hormati A, Ghadir MR, Zamani F, et al. Are there any association between COVID-19 severity and immunosuppressive therapy? Immunol Lett. 2020;224:12–13. doi:10.1016/j.imlet.2020.05.002
27. Faye AS, Lee KE, Laszkowska M, et al. Risk of adverse outcomes in hospitalized patients with autoimmune disease and COVID-19: a matched cohort study from New York City. J Rheumatol. 2020. doi:10.3899/jrheum.200989
28. Kamerlin SCL, Kasson PM. Managing COVID-19 spread with voluntary public-health measures: Sweden as a case study for pandemic control. Clin Infect Dis. 2020;71:3174–3181. doi:10.1093/cid/ciaa864
29. Baker MG, Peckham TK, Seixas NS, Nelson CC. Estimating the burden of United States workers exposed to infection or disease: A key factor in containing risk of COVID-19 infection. PLoS One. 2020;15(4):e0232452. doi:10.1371/journal.pone.0232452
30. Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020; 383:1522–1534.
31. Choung RS, Unalp-Arida A, Ruhl CE, Brantner TL, Everhart JE, Murray JA. Less hidden celiac disease but increased gluten avoidance without a diagnosis in the United States: findings from the national health and nutrition examination surveys from 2009 to 2014. Mayo Clin Proc. 2016. doi:10.1016/j.mayocp.2016.10.012
32. Salisbury H. Helen Salisbury: when will we be well again? BMJ. 2020;369:m2490. doi:10.1136/bmj.m2490
33. Hormati A, Shahhamzeh A, Afifian M, Khodadust F, Ahmadpour S. Can COVID-19 present unusual GI symptoms? J Microbiol Immunol Infect. 2020;53(3):384–385. doi:10.1016/j.jmii.2020.03.020
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

