Back to Journals » Journal of Pain Research » Volume 13
Risk of Postoperative Hyperalgesia in Adult Patients with Preoperative Poor Sleep Quality Undergoing Open-heart Valve Surgery
Authors Zhang Z, Wang H, Wang Y, Luo Q , Yuan S , Yan F
Received 18 July 2020
Accepted for publication 26 August 2020
Published 13 October 2020 Volume 2020:13 Pages 2553—2560
DOI https://doi.org/10.2147/JPR.S272667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Zhe Zhang, Hongbai Wang, Yuefu Wang, Qipeng Luo, Su Yuan, Fuxia Yan
Department of Anesthesiology, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Fuxia Yan; Su Yuan
Department of Anesthesiology, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 167 North Lishi Road, Xicheng District, Beijing 100037, People’s Republic of China
Tel +86 13641158173
; +86 13621019302
Fax +86 1088396628
Email [email protected]; [email protected]
Purpose: Studies have reported that preoperative poor sleep quality could decrease the pain threshold in patients undergoing noncardiac surgery. However, the risk of postoperative hyperalgesia (HA) in cardiac surgery patients with preoperative poor sleep quality remains unclear.
Patients and Methods: We retrospectively collected clinical data from patients undergoing open-heart valve surgery between May 1 and October 31, 2019, in Fuwai Hospital (Beijing). We assessed preoperative sleep quality and postoperative pain severity using the Pittsburgh sleep quality index (PSQI) and numerical pain rating scale (NPRS), respectively. A PSQI of six or greater was considered to indicate poor sleep quality, and a NPRS of four or greater was considered to indicate HA. Multivariable logistic regression analysis was used to study the risk of postoperative HA in patients with preoperative poor sleep quality.
Results: We divided 214 eligible patients into two groups based on postoperative HA; HA group: n=61 (28.5%) and nonHA group: n=153 (71.5%). Compared with nonHA patients, patients with postoperative HA showed a higher percentage of history of smoking, 17 (11.1%) vs 15 (24.6%) and alcohol abuse, 5 (3.3%) vs 6 (9.8%), higher intraoperative dose of sufentanil (median, 1.02 vs 1.12 μg/kg/h), and longer duration of ventilation with tracheal catheter (median, 760 vs 934 min). Preoperative poor sleep quality was associated independently with an increased risk of postoperative HA (adjusted odds ratio [AOR]: 2.66; 95%CI: 1.31– 5.39, P=0.007). Stratification by history of smoking revealed a stronger risk of postoperative HA in nonsmoking patients with preoperative poor sleep quality (AOR: 3.40; 95%CI: 1.51– 7.66, P=0.003). No risk was found in patients who had history of smoking (AOR: 0.83; 95%CI: 0.14– 4.75, P=0.832).
Conclusion: Preoperative poor sleep quality is an independent risk factor for postoperative HA in adult patients undergoing open-heart valve surgery who had no history of smoking.
Keywords: hyperalgesia, adult, poor sleep quality, cardiac surgery, smoking
Introduction
Postoperative enhancement of pain sensitivity, which is also termed as hyperalgesia (HA), is characterized by a strong response to nociceptive stimulation at the incision site and its surrounding tissues.1 Patients who have undergone cardiac surgery are susceptible to postoperative HA with an incidence of 37% in the first six months postsurgery.2 Previous studies have reported a significant association of postoperative HA with a high incidence of atelectasis, mental disorders, and delayed postsurgery recovery in patients undergoing cardiac surgery.3,4 Currently, analgesic management remains the most common method for alleviating postoperative HA.5 However, large analgesic doses could produce significant side effects.6–8 Therefore, there is a need to identify the risk factors for postoperative HA and address them to reduce the use of analgesics and allow rapid recovery from cardiac surgery.
Poor sleep quality is highly prevalent in patients with cardiovascular disease.9,10 And there is a mutually causal relationship between poor sleep quality and heart disease.11,12 A prospective observational study in our center discovered that the incidence of preoperative poor sleep quality in patients with cardiac valve disease was about 57%.13 Poor sleep quality can predispose individuals to pain conditions, and increasing pain severity further exacerbates their sleep quality.14,15 There have been reports of an increased risk of postoperative pain sensitivity in patients with preoperative poor sleep quality who have undergone noncardiac surgery.16–18 However, there have been few studies on preoperative poor sleep quality associated with postoperative HA in cardiac surgery patients. Therefore, this study aimed to assess the effect of preoperative poor sleep quality on postoperative HA in patients undergoing open-heart surgery.
Methods
We performed an unmatched case–control study. We retrospectively included adult patients who were hospitalized in No. 2 inpatient building of Fuwai Hospital and underwent elective open-heart valve surgery between May 1 and October 30, 2019. The inclusion criteria were being aged ≥18 years and having undergone assessment for preoperative sleep quality and HA (mainly at the incision site and its surrounding tissues) within the first five postoperative days. The exclusion criteria were as follows: (1) an age <18 years; (2) lacking data regarding preoperative sleep and/or postoperative pain evaluation; and (3) suffering from a coma or cognitive dysfunction (eg delirium) during the first five postoperative days. The included were divided into two groups based on whether postoperative HA occurred: cases were patients with HA, controls were patients without HA.
All eligible patients had undergone open-heart valve surgery on pump under general anesthesia. Intravenous anesthetics for induction of anesthesia included midazolam, etomidate, cisatracurium and sufentanil. Anesthetics for maintenance of anesthesia included midazolam, propofol, sufentanil, dexmedetomidine, and cisatracurium. A bispectral index (BIS) was used to monitor the anesthesia depth, which was maintained at an intraoperative value under 60. The normal body temperature was maintained except for light hypothermia (nasopharyngeal temperature: 30–34°C) during cardiopulmonary bypass (CPB). All the patients were equipped with a postoperative self-controlling analgesia infusion pump (total volume 100 mL: sufentanil 100 μg, dezocine 30 mg) for pain alleviation. The background infusion rate of the analgesic pump was 2 mL/h with a bolus volume of 2 mL. The locking time of the analgesic pump was 15 min.
Preoperative sleep quality was assessed on the day before surgery using the Pittsburgh sleep quality index (PSQI), which is comprised of 19 items in seven parts and has been shown as effective for evaluating sleep quality.19 The PSQI score ranges from 0–21 and is positively correlated with severity of poor sleep quality. A score of five is considered as the cutoff value for distinguishing between individuals with poor sleep quality and normal sleep quality.
The numerical pain rating scale (NPRS) has become a standard method for assessing acute pain, especially postoperative acute pain.20 The NPRS involves a line from 0 (no pain) to 10 (worst pain) where the patient describes the exact score indicating his/her pain severity. Within the first five postoperative days, the patient’s pain was assessed twice daily starting 24 h after the patient was fully awake. An HA diagnosis is identified if the highest NPRS score is ≥4 at rest. An additional analgesic dose (intravenous dezocine and/or oral amphenol hydroxycodone) was administered in case of postoperative HA.
Sample Size Calculation
The sample size was calculated using PASS 15.0 software (NCSS Corp., Kaysville, UT, USA) based on a preliminary retrospective study that included 30 patients undergoing open-heart valve surgery in our center. The preoperative poor sleep quality incidence in the nonHA patients was 52.9%; moreover, the OR for the risk of postoperative HA in patients with poor sleep quality was 2.963. The minimum sample size in the case and control groups were 55 and 72, respectively, with a power of 80% and a two-sided α of 0.05, calculated by PASS. Consequently, the minimum required cases and controls were 61 and 80, respectively, based on a 10% data missing rate.
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Continuous variables were presented as median, IQR. Categorical variables were expressed as number (percentage). Continuous variables were analyzed using Mann–Whitney U-test while categorical variables were analyzed using Pearson’s chi-squared test. Multivariate logistic regression analysis was used to study the risk of postoperative HA in patients with preoperative poor sleep quality. Patients with a normal PSQI score (≤5) were the reference category. In multivariate regression analysis, preoperative PSQI score was defined as an independent variable, while postoperative NPRS score was defined as the dependent variable. The multivariate logistic regression model was adjusted with the following variables: sex, age (as a continuous variable), history of smoking, history of alcohol abuse, dose of sufentanil (as a continuous variable) and duration of ventilation with tracheal catheter (as a continuous variable). On the basis of univariate analysis and a previous meta-analysis,21 we performed subgroup analyses in which we studied the influence of smoking on the association between preoperative poor sleep quality and postoperative HA. An area under the curve (AUC) was generated from the receiver operating characteristic (ROC) curve to indicate the discrimination of the multivariate logistic regression model. Hosmer–Lemeshow goodness of fit test was used to evaluate the calibration of the multivariate logistic regression model. Statistical significance was set at P<0.05.
Results
Between May 1 and October 30, 2019, 238 adult patients underwent open-heart valve surgery on pump; among them, 24 cases were excluded based on the exclusion criteria. The remaining 214 eligible patients (preoperative poor sleep quality: 121 cases; no preoperative poor sleep quality: 93 cases) were divided into the HA group, n= 61 (28.5%) and the nonHA group, n=153 (71.5%) (Figure 1).
 |
Figure 1 Flow chart of patient screening and grouping. |
Table 1 shows the univariate analyses of the baseline values and perioperative characteristics of the eligible patients. Compared with patients without postoperative HA, patients with postoperative HA showed a higher percentage of history of smoking, 17 (11.1%) vs 15 (24.6%) and alcohol abuse, 5 (3.3%) vs 6 (9.8%), higher intraoperative dose of sufentanil (median, 1.02 vs 1.12 μg/kg/h), and longer duration of ventilation with tracheal catheter (median, 760 vs 934 min). Preoperative poor sleep quality was associated independently with an increased risk of postoperative HA (adjusted OR: 2.66; 95%CI: 1.31–5.39; P=0.007) (Table 2). Stratification by history of smoking revealed a stronger risk of postoperative HA in nonsmoking patients with preoperative poor sleep quality (AOR: 3.40; 95%CI: 1.51–7.66; P = 0.003) (Table 3). No risk was found in patients who had a history of smoking (AOR: 0.83; 95%CI: 0.14–4.75; P=0.832) (Table 3).
 |
Table 1 Baseline Characteristics and Comparison Between HA and NonHA Patients |
 |
Table 2 Association Between Preoperative Poor Sleep Quality and Postoperative HA |
 |
Table 3 Stratification by Smoking |
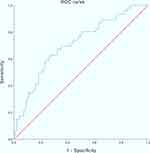
ROC curve analysis showed that the multivariate logistic regression model had a reasonable predictive ability for postoperative HA in nonsmoking patients (AUC: 0.708; 95%CI: 0.622–0.794; P<0.001) (Figure 2). The accuracy of the estimated probabilities in nonsmoking patients was ensured by the Hosmer–Lemeshow goodness of fit test of the multivariate logistic regression model (χ2: 6.138; P=0.632).
Discussion
Our study indicates that preoperative poor sleep quality (PSQI >5) is associated with an increased risk of postoperative HA (NPRS ≥4) in patients undergoing cardiac valve surgery, which is consistent with previous studies of noncardiac surgery.16–18 This association appears to be mainly attributable to a stronger increased risk of postoperative HA in nonsmoking patients. Among these nonsmokers, preoperative poor sleep quality was associated with a 3.4-fold increased risk of postoperative HA compared with patients with normal sleep quality.
Progress has been made in the research of mechanism underlying the association of sleep deprivation with HA.22 Increased adenosine activity plays an important role in sleep-pain regulation and has been shown to enhance pain sensitivity.23 Moreover, systemic and central nervous system inflammatory responses promoted by sleep disturbances increase pain sensitivity through multiple signaling pathways.24–27 In addition, long-term poor sleep quality can continuously activate the hypothalamus-pituitary-adrenal axis, which produces a large amount of corticotropin and cortisol.28 Study on healthy volunteers has reported that high corticosteroid levels in the body could enhance pain sensitivity.29
In our study, preoperative sleep quality was assessed by the PSQI, which has a sensitivity of 89.6% and specificity of 86.5% in distinguishing normal and poor sleep quality.19 The PSQI can comprehensively assess sleep quality over the preceding month, including sleep latency, sleep duration, sleep efficiency, sleep habits, and obstructive sleep apnea.30 Sleep quality is affected by a variety of cultural, social, psychological, behavioral, pathophysiological and environmental influences.31 Although a lot of studies have shown that poor sleep quality has a negative effect on pain-related outcomes in clinical populations and in the general population.16–18,32 And there are many ways to improve perioperative sleep quality.33,34 There is, however, insufficient clinical evidence to suggest a clear positive effect of sleep improvement on pain.35 Therefore, whether the improvement of sleep quality before cardiac surgery could be an effective method for attenuating postoperative pain severity still needs further research.
After determining preoperative poor sleep quality as an independent risk factor of postoperative HA, we evaluated the clinical application value of multivariate regression model in nonsmoking patients. ROC curve analysis showed that AUC >0.7, indicating that the model has a reasonable predictive ability for nonsmoking cardiac patients in prediction of postoperative HA.36 Therefore, we consider that clinicians should understand and inform patients about the clinical significance of preoperative sleep quality and its impact on postoperative pain. In addition, we suggest that perioperative sleep quality assessment could be routinely carried out in the clinical practice for patients with cardiac disease.
According to the results of our study, we should pay more attention to postoperative pain assessment in nonsmokers with poor sleep quality before cardiac surgery, as these patients are more likely to develop postoperative HA. In a separate analysis, we included postoperative NPRS score and preoperative PSQI score as continuous variables for linear regression analysis. The regression model was not statistically significant (adjusted R2: 0.015; F=3.682, P=0.057), and preoperative sleep quality could not explain the severity of postoperative pain. We consider that the above results are related to the use of postoperative analgesics. Based on previous literature and our own clinical experience, more analgesic treatment is needed if a patient develops postoperative HA.5
Based on previous studies,1,37 smoking is considered to be associated with postoperative HA, which is also confirmed in our study. Both the univariate (OR: 2.61; 95%CI: 1.21–5.64, P=0.013) and multivariate analyses (AOR: 2.32; 95%CI: 0.94–5.72, P=0.069) in our study show that smoking is a risk factor for postoperative HA. In patients who had history of smoking, we found no association between preoperative poor sleep quality and postoperative HA. Because of the relatively small number of smoking patients in our study (n=32), we cannot exclude that the absent risk of postoperative HA in smokers with preoperative poor sleep quality is the result of this limited sample size. In addition, smoking is not only a risk factor for elevation of pain severity, but also a risk factor for sleep disturbances.38–40 Therefore, the relationship between preoperative poor sleep quality and postoperative pain is complex in smokers. Consequently, the relationship between sleep and pain in smokers cannot be concluded in our study. Further studies are needed to focus on the complex relationship between sleep and pain in smoking patients undergoing cardiac surgery.
Obesity has become an increasing problem worldwide during the past few decades. The association of obstructive sleep apnea (OSA) with obesity is quite common.41,42 There is a high prevalence of OSA in the surgical population, however, a significant proportion of patients are undiagnosed.43 Therefore, preoperative OSA screening is crucial in the obese patient.44 Postoperative opioid-based pain management of cardiac patients suffering from OSA may present challenges because of concerns over severe ventilatory compromise. However, life-threatening opioid-related respiratory events are rare under intensive postoperative surveillance.45 In our study, no severe OSA-related complications occurred when patients received patient-controlled analgesia and additional medication for postoperative HA. To avoid serious OSA-related complications, postoperative analgesia should be given to patients on the recommendations of guidelines and literatures, as well as the clinical experience of local medical institutions.46,47
Our study has several limitations. Firstly, this was a retrospective study and there could be inevitable data loss. This study collected incomplete NPRS scores in 15 patients due to coma or delirium occurrence. These patients were excluded before analysis. Secondly, in the retrieved medical records, no patient showed a history of chronic pain or took painkillers before surgery. However, we are not sure whether a reliable assessment of chronic pain has been performed before surgery, which could be a potential confounder. Thirdly, we did not obtain data regarding the postoperative sleep quality of patients, which may weaken the outcome reliability.48 Finally, we cannot exclude the possibility that recall bias and other inherent limitations of self-report measurement influenced the results.
Conclusion
To our knowledge, this is the first case–control study that investigated the risk of postoperative HA in adult patients with preoperative poor sleep quality undergoing open-heart valve surgery. Our results suggest that preoperative poor sleep quality is associated with a considerably increased risk of postoperative HA in nonsmoking patients. This increased risk should be taken into consideration when assessing perioperative sleep quality and providing postoperative analgesia for patients undergoing cardiac surgery.
Abbreviation
HA, hyperalgesia; BIS, bispectral index; PSQI, Pittsburgh sleep quality index; NPRS, numerical pain rating scale; ROC, receiver operating characteristic; AUC, area under curve; OSA, obstructive sleep apnea.
Ethics
This study was approved by the Ethics Committee of Fuwai Hospital and conducted in accordance with the Declaration of Helsinki. The Ethics Committee waived the need for patient consent owing to retrospective data collection. The patient records and information were anonymized and de-identified before analysis.
Acknowledgments
We would like to thank all residents who retrieved the data of included patients.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Supported by “the Fundamental Research Funds for the Central Universities” (3332018065) and “Sanming Project of Medicine in Shenzhen” (SZSM202011013).
Disclosure
All authors declared that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest and report no conflicts of interest in this work.
References
1. Malik OS, Kaye AD, Urman RD. Perioperative hyperalgesia and associated clinical factors. Curr Pain Headache Rep. 2017;21(1):4. doi:10.1007/s11916-017-0602-3
2. Guimarães-Pereira L, Reis P, Abelha F, et al. Persistent postoperative pain after cardiac surgery: a systematic review with meta-analysis regarding incidence and pain intensity. Pain. 2017;158(10):1869–1885. doi:10.1097/j.pain.0000000000000997
3. Tenenbein PK, Debrouwere R, Maguire D, et al. Thoracic epidural analgesia improves pulmonary function in patients undergoing cardiac surgery. Can J Anaesth. 2008;55(6):344–350. doi:10.1007/BF03021489
4. Kumar AK, Jayant A, Arya VK, et al. Delirium after cardiac surgery: a pilot study from a single tertiary referral center. Ann Card Anaesth. 2017;20(1):76–82. doi:10.4103/0971-9784.197841
5. Weinbroum AA. Postoperative hyperalgesia-a clinically applicable narrative review. Pharmacol Res. 2017;120:188–205. doi:10.1016/j.phrs.2017.02.012
6. Nagappa M, Weingarten TN, Montandon G, et al. Opioids, respiratory depression, and sleep-disordered breathing. Best Pract Res Clin Anaesthesiol. 2017;31(4):469–485. doi:10.1016/j.bpa.2017.05.004
7. Dewan KC, Dewan KS, Idrees JJ, et al. Trends and outcomes of cardiovascular surgery in patients with opioid use disorders. JAMA Surg. 2019;154(3):232–240. doi:10.1001/jamasurg.2018.4608
8. Gupta K, Nagappa M, Prasad A, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open. 2018;8(12):e024086. doi:10.1136/bmjopen-2018-024086
9. Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. doi:10.1016/j.chest.2017.01.026
10. Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi:10.1016/j.jacc.2016.11.069
11. Martino TA, Young ME. Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. J Biol Rhythms. 2015;30(3):183–205. doi:10.1177/0748730415575246
12. Madsen MT, Huang C, Zangger G, et al. Sleep disturbances in patients with coronary heart disease: a systematic review. J Clin Sleep Med. 2019;15(3):489–504. doi:10.5664/jcsm.7684
13. Wang H, Zhang L, Luo Q. et al. Effect of sleep disorder on delirium in post-cardiac surgery patients. Can J Neurol Sci. 2020;47(5):627-633. doi:10.1017/cjn.2020.62
14. Andersen ML, Araujo P, Frange C, et al. Sleep disturbance and pain: a tale of two common problems. Chest. 2018;154(5):1249–1259. doi:10.1016/j.chest.2018.07.019
15. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi:10.1016/j.jpain.2013.08.007
16. Wang JP, Lu SF, Guo LN, et al. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery: a prospective cohort study. Medicine (Baltimore). 2019;98(44):e17708. doi:10.1097/MD.0000000000017708
17. Orbach-Zinger S, Fireman S, Ben-Haroush A, et al. Preoperative sleep quality predicts postoperative pain after planned caesarean delivery. Eur J Pain. 2017;21(5):787–794. doi:10.1002/ejp.980
18. Luo ZY, Li LL, Wang D, et al. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthop Surg Res. 2019;14(1):378. doi:10.1186/s13018-019-1446-9
19. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
20. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10(4):390–392. doi:10.1197/aemj.10.4.390
21. Yang MMH, Hartley RL, Leung AA, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019;9(4):e025091. doi:10.1136/bmjopen-2018-025091
22. Xue J, Li H, Xu Z, et al. Paradoxical sleep deprivation aggravates and prolongs incision-induced pain hypersensitivity via bdnf signaling-mediated descending facilitation in rats. Neurochem Res. 2018;43(12):2353–2361. doi:10.1007/s11064-018-2660-2
23. Hambrecht-Wiedbusch VS, Gabel M, Liu LJ, et al. Preemptive caffeine administration blocks the increase in postoperative pain caused by previous sleep loss in the rat: a potential role for preoptic adenosine a2a receptors in sleep-pain interactions. Sleep. 2017;40(9). doi:10.1093/sleep/30.6.729
24. Mills PJ, von Känel R, Norman D, et al. Inflammation and sleep in healthy individuals. Sleep. 2007;30(6):729–735. doi:10.1093/sleep/30.6.729
25. Díaz AF, Polo S, Gallardo N, et al. Analgesic and antidepressant effects of oltipraz on neuropathic pain in mice by modulating microglial activation. J Clin Med. 2019;8(6):890. doi:10.3390/jcm8060890
26. Su Y, Xiong S, Lan H, et al. Molecular mechanism underlying anti-inflammatory activities of lirioresinol B dimethyl ether through suppression of NF-κB and MAPK signaling in in vitro and in vivo models. Int Immunopharmacol. 2019;73:321–332. doi:10.1016/j.intimp.2019.05.020
27. Weinstock LB, Myers TL, Walters AS, et al. Identification and treatment of new inflammatory triggers for complex regional pain syndrome: small intestinal bacterial overgrowth and obstructive sleep apnea. Case Rep. 2016;6(9):272–276. doi:10.1213/XAA.0000000000000292
28. Mohammadi H, Rezaei M, Amiri SM, et al. Sleep architecture and hypothalamic-pituitary-adrenal activity in paradoxical and psychophysiological insomnia. Basic Clin Neurosci. 2018;9(6):397–407. doi:10.32598/bcn.9.6.397
29. Benson S, Siebert C, Koenen LR, et al. Cortisol affects pain sensitivity and pain-related emotional learning in experimental visceral but not somatic pain: a randomized controlled study in healthy men and women. Pain. 2019;160(8):1719–1728. doi:10.1097/j.pain.0000000000001579
30. Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi:10.1016/j.smrv.2015.01.009
31. Cappuccio FP, Miller MA. Sleep and cardio-metabolic disease. Curr Cardiol Rep. 2017;19(11):110. doi:10.1007/s11886-017-0916-0
32. Afolalu EF, Ramlee F, Tang NKY. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. 2018;39:82–97. doi:10.1016/j.smrv.2017.08.001
33. Ramar K, Olson EJ. Management of common sleep disorders. Am Fam Physician. 2013;88(4):231–238.
34. Hu RF, Jiang XY, Chen J, et al. Non-pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. 2015;2015(10):CD008808.
35. Bjurström MF, Irwin MR. Perioperative pharmacological sleep-promotion and pain control: a systematic review. Pain Pract. 2019;19(5):552–569. doi:10.1111/papr.12776
36. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi:10.1148/radiology.143.1.7063747
37. Oh TK, Kim K, Jheon S, et al. Relationship between pain outcomes and smoking history following video-assisted thoracic surgery for lobectomy: a retrospective study. J Pain Res. 2018;11:667–673. doi:10.2147/JPR.S157957
38. Stipelman BA, Augustson E, McNeel T. The relationship among smoking, sleep, and chronic rheumatic conditions commonly associated with pain in the National Health Interview Survey. J Behav Med. 2013;36(5):539–548. doi:10.1007/s10865-012-9447-8
39. Cox F. Cigarette smoking increases persistent pain intensity and interference, impairs function and sleep. Evid Based Nurs. 2020;23(4):112. doi:10.1136/ebnurs-2019-103174
40. Zhou B, Ma Y, Wei F, et al. Association of active/passive smoking and urinary 1-hydroxypyrene with poor sleep quality: a cross-sectional survey among Chinese male enterprise workers. Tob Induc Dis. 2018;16:23. doi:10.18332/tid/90004
41. De Jong A, Verzilli D, Chanques G, et al. Preoperative risk and perioperative management of obese patients. Rev Mal Respir. 2019;36(8):985–1001. doi:10.1016/j.rmr.2019.01.009
42. Carron M, Safaee Fakhr B, Ieppariello G, et al. Perioperative care of the obese patient. Br J Surg. 2020;107(2):e39–e55. doi:10.1002/bjs.11447
43. Madhusudan P, Wong J, Prasad A, et al. An update on preoperative assessment and preparation of surgical patients with obstructive sleep apnea. Curr Opin Anaesthesiol. 2018;31(1):89–95. doi:10.1097/ACO.0000000000000539
44. Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of anesthesia and sleep medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg. 2016;123(2):452–473. doi:10.1213/ANE.0000000000001416
45. Lam KK, Kunder S, Wong J, et al. Obstructive sleep apnea, pain, and opioids: is the riddle solved? Curr Opin Anaesthesiol. 2016;29(1):134–140. doi:10.1097/ACO.0000000000000265
46. Wolfe RM, Pomerantz J, Miller DE, et al. Obstructive sleep apnea: preoperative screening and postoperative care. J Am Board Fam Med. 2016;29(2):263–275. doi:10.3122/jabfm.2016.02.150085
47. Memtsoudis SG, Cozowicz C, Nagappa M, et al. Society of anesthesia and sleep medicine guideline on intraoperative management of adult patients with obstructive sleep apnea. Anesth Analg. 2018;127(4):967–987. doi:10.1213/ANE.0000000000003434
48. Rampes S, Ma K, Divecha YA, et al. Postoperative sleep disorders and their potential impacts on surgical outcomes. J Biomed Res. 2019;34(4):271–280. doi:10.7555/JBR.33.20190054
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.