Back to Journals » Vascular Health and Risk Management » Volume 16
Risk of Atrial Fibrillation, Ischemic Stroke and Cognitive Impairment: Study of a Population Cohort ≥65 Years of Age
Authors Clua-Espuny JL , Muria-Subirats E , Ballesta-Ors J , Lorman-Carbo B, Clua-Queralt J, Palà E, Lechuga-Duran I, Gentille-Lorente D, Bustamante A, Muñoz MA, Montaner J
Received 11 August 2020
Accepted for publication 2 October 2020
Published 28 October 2020 Volume 2020:16 Pages 445—454
DOI https://doi.org/10.2147/VHRM.S276477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Magnus Bäck
Jose-Luis Clua-Espuny,1 Eulalia Muria-Subirats,2 Juan Ballesta-Ors,3 Blanca Lorman-Carbo,4 Josep Clua-Queralt,5 Elena Palà,6 Iñigo Lechuga-Duran,7 Delicia Gentille-Lorente,7 Alejandro Bustamante,8 Miguel Ángel Muñoz,9 Joan Montaner6 On Behalf of the AFRICAT Research Group
1EAP Tortosa 1-Est, Institut Català Salut, Servei Atenció Primària, UUDD Terres De l’Ebre. Universidad Rovira I Virgili, Programa Doctorat, Tortosa, Spain; 2EAP Tortosa 1-Est, Institut Català Salut, SAP Terres De l’Ebre, España Universidad Rovira I Virgili. Programa Doctorat, Tortosa, Tarragona, Spain; 3EAP Tortosa 1-Est, Institut Català Salut, SAP Terres De l’Ebre, Universidad Rovira I Virgili. Programa Doctorat. Tortosa, Tarragona, Spain; 4EAP Tortosa 1-Est, Institut Català Salut, SAP Terres De l’Ebre, España Universidad Rovira I Virgili. Programa Doctorat. Tortosa, Tarragona, Spain; 5Universitat De Lleida, Lérida, Spain; 6Neurovascular Research Laboratory, Vall d’Hebron Institute of Research (VHIR)-Universitat Autónoma De Barcelona, Barcelona, Spain; 7Servicio Cardiología, Hospital Virgen De La Cinta, Institut Català Salut Tortosa, Tarragona, Spain; 8Department of Neurology, Hospital Universitari Vall d’Hebron, Barcelona, Spain; 9Institut d’Investigació En Atenció Primària IDIAP Jordi Gol, Unitat De Suport a La Recerca De Barcelona, Barcelona, Spain
Correspondence: Jose-Luis Clua-Espuny
EAP Tortosa 1-Est, Institut Català Salut, Servei Atenció Primària, UUDD Terres De l’Ebre. Universidad Rovira I Virgili, Programa Doctorat, CAP Temple, Carrilet Square s/Num. Tortosa, 43500, Spain
Tel +34 977 510018
Fax +34 977 44 57 28
Email [email protected]
Purpose: To evaluate a model for calculating the risk of AF and its relationship with the incidence of ischemic stroke and prevalence of cognitive decline.
Materials and Methods: It was a multicenter, observational, retrospective, community-based study of a cohort of general population ≥ 6ct 35 years, between 01/01/2016 and 31/12/2018. Setting: Primary Care. Participants: 46,706 people ≥ 65 years with an active medical history in any of the primary care teams of the territory, information accessible through shared history and without previous known AF. Interventions: The model to stratify the risk of AF (PI) has been previously published and included the variables sex, age, mean heart rate, mean weight and CHA2DS2VASc score. Main measurements: For each risk group, the incidence density/1000 person/years of AF and stroke, number of cases required to detect a new AF, the prevalence of cognitive decline, Kendall correlation, and ROC curve were calculated.
Results: The prognostic index was obtained in 37,731 cases (80.8%) from lowest (Q1) to highest risk (Q4). A total of 1244 new AFs and 234 stroke episodes were diagnosed. Q3-4 included 53.8% of all AF and 69.5% of strokes in men; 84.2% of all AF and 85.4% of strokes in women; and 77.4% of cases of cognitive impairment. There was a significant linear correlation between the risk-AF score and the Rankin score (p < 0.001), the Pfeiffer score (p < 0.001), but not NIHSS score (p 0.150). The overall NNS was 1/19.
Conclusion: Risk stratification allows identifying high-risk individuals in whom to intervene on modifiable risk factors, prioritizing the diagnosis of AF and investigating cognitive status.
Keywords: vascular risk score, atrial arrhythmia, cerebrovascular disease, silent stroke, cognitive decline
Introduction
The Action Plan in Europe (2018–2030)1 prioritizes the availability of detection and treatment programs to address the risk factors for stroke in all European countries. Due to demographic aging, it is estimated that the prevalence of AF will increase from 1.9% (2008) to 3.5% (2050), and the number of AF-related ischemic strokes in people >80 years will triple (2010–2060).2 If we consider that >60% of strokes occur in people >80 years of age,3,4 the proposed objective is extremely important.
Approximately one-third of ischemic strokes are related to the presence of unknown atrial fibrillation (AF).5–7 In this case, strokes are more severe, more disabling, associated with cognitive decline, and with a higher risk of institutionalization than strokes from other causes.8 Consequently, it is clear that the costs related to AF will increase, constituting a major public health problem.
While the strategy for detecting AF after a stroke episode accumulates evidence9,10 at the hospital level, the same does not occur in the primary care setting before the stroke episode. Although different screening strategies have been recommended9 by international organizations,10 they have not demonstrated greater effectiveness in the general population than their non-implementation,11 and there is little evidence of their impact on the incidence of comorbidities, especially the stroke.12 For all these reasons, the new priorities point towards the identification of individuals at high risk of having AF in whom to carry out screening and evaluate its effectiveness13,14 both in its detection and in the incidence of strokes.15
The main objective of this study is to apply a five-year model for calculating the risk of AF16 in the general population ≥65 years and to describe its possible relationship with the incidence of ischemic stroke and the prevalence of cognitive decline in the primary care setting.
Materials and Methods
Type of Study
Multicenter, observational, retrospective, community-based study of a cohort of general population ≥65 years between 01/01/2016 - 12/31/2018 with no diagnosis of atrial fibrillation in the Primary Care setting. The research protocol of the study was approved by the Ethical Committee of the Institut Universitari d’Investigació en Atenció Primària IDIAP Jordi Gol with the code number (P18/118).
Territorial Scope
The SAP Terres de l’Ebre territory has 11 primary care teams, all managed by the Catalan Institute of Health (ICS), in which 98.2% of the census population with an active medical history is assigned, and a reference general teaching public hospital for the entire territory and three regionally managed hospitals. All centres in the territory share clinical information (HC3).
The data were obtained from the databases of the electronic clinical records (e-cap, E-SAP) by the Information Systems department for the exploitation of the record known as the “Minimum Basic Data Set” (CMBD) and delivered to the main researcher in an anonymised format, supervised and analyzed according to the general regulation on Spanish and European data protection (02/01/2017). There was no contact with the cases included. The specific codes of the International Classification of Diseases (10th version; ICD-10) were used for atrial fibrillation (I48), cerebrovascular disease (I60-I69), and cognitive impairment (G31).
The model to stratify the risk of suffering AF at five years has been previously published16 and it is intended to be applied to the general population ≥65 years. It includes the variables: sex, age, average heart rate, and average weight and CHA2DS2VASc value. The mathematical formula of the model was applied to the population older than 65 years without a diagnosis of AF and the quartiles of the distribution from lowest to highest risk were defined (Q1-Q4). For each risk group, the incidence density/1000 people/year (ID) was calculated; number of screening (NNS) necessary to detect a new AF; the incidence of strokes and the registered prevalence of cognitive decline. The NNS was calculated on the number of new AFs/number of cases with record of screening in each quartile. In the clinical electronic records (E-cap) of people aged ≥60 years, opportunistic screening10 of the rhythm is registered as a variable “A” (arrhythmic) vs “R” (rhythmic) in the e-cap (Basic Activities of Prevention and Promotion of Health in Primary Care) accompanied by an alert (appears in a different colour if pending). The patient’s record indicated whether case finding was performed. Case finding or screening for AF was defined as pulse palpation during routine general practitioner consultations at least once a year, together with a 12-lead ECG confirmation of an irregular or regular pulse—for instance, during an annual cardiac disease review, with the result recorded as either “rhythmic” or “arrhythmic” (AR). The AF diagnosis requires at least 30 sec of absolutely irregular RR intervals and no discernible, distinct P waves on the electrocardiogram (ECG). Its registration as made occurs when the professional performing the activity enters the option (rhythmic/arrhythmic).
Target Population
Subjects ≥65 years who met the inclusion criteria: active medical history in any of the health centres in the territory with information accessible through the shared history (HC3), without prior AF, residence in the territory and assignment to any of the Teams Primary Care (EAP) of the same. The non-availability or loss of accessibility to the information necessary for the study was considered as a reason for exclusion. The follow-up was carried out until the end of the study, the death of the patient or the clinical loss of records (new residence outside the scope of the study) during follow-up.
Variables
The main variable was the incidence rate of new diagnosis of AF in the follow-up period and, secondarily, the incidence rate of ischemic stroke, simultaneous or subsequent to the diagnosis of AF. All cases of new AF were confirmed by referring Cardiology and clinical records. In each quartile, the cognitive impairment and dementia diagnoses were obtained from the different datasets got from the former available records of all patients in the timeframe of the study. The Pfeiffer score represents the average of the included individuals in each quartile. Two researchers independently reviewed the record of stroke and cognitive impairment for confirmation and its severity according to the NIHSS and Rankin scales, considering them as the simultaneous incidence of AF and stroke when the difference in the diagnostic time of both was not greater than 30 days. The Rankin and NIHSS scores are the only assessed in individuals with stroke. Sociodemographic, clinical, comorbidities, Charlson and Pfeiffer scores, registration as a Chronic Complex Patient37 (PCC), if institutionalized in long-term stay (≥3 months), were included as independent variables. The mortality rate is total from any cause and evaluated by the Kaplan-Meier method.
Statistic Analysis
The statistical analysis was descriptive to define the characteristics of the population with basic statistics of centralization and dispersion; and for the identification of differences in registration and functional and risk assessment among population subgroups. Other subgroups were considered based on the emerging results. In the bivariate analysis of normal distributions, the T test was used for independent samples in the case of quantitative variables and the Chi2 test or Fisher’s exact test in the case of categorical variables. The incidence density/1000 people/year, NNS to diagnose a new case AF, incidence rate, and the ROC curve and the area under the curve were obtained to evaluate the discriminative capacity of the model. The Kendall correlation for AF risk, Pfeiffer, NIHSS, and Rankin scores because of a smaller gross error sensitivity and a smaller asymptotic variance. P ≤0.05 was considered a significant statistical difference, and 95% confidence intervals (95% CI) were calculated. For statistical analysis, the IBM SPSS Statistics version 19.0 program was used.
Results
Baseline Characteristics
Forty-six thousand seven hundred and six people without known AF were included. Table 1 describes the baseline characteristics according to the presence or absence of AF. Screening was performed in 53.2% of the population without AF and in 79.6% of the population diagnosed with a new AF (p <0.001). The AF population presented a significantly higher value in all parameters. The CHA2DS2VASc, age and mortality are significantly higher in women. Mortality was significantly higher in both those with AF and those with strokes.
 |
Table 1 Baseline Characteristics of Cases with AF vs without AF |
AF-Risk Stratification results
The prognostic index was obtained in 37,731 cases (80.8%) distributed in risk levels (Table 2). One thousand two hundred and forty-four new AFs (ID 10.5/1000/year 95% CI 9.9–11.2) were identified, appreciating an increasing case gradient as risk increases (p <0.001). The incidence rate for the Q4/mean group was 1.61 [95% CI 1.45–1.79 p <0.001]. Although the highest ID of AF occurs in Q4 (17.0 95% CI 15.5–18.6), Q3-4 include 53.8% of all AF in men and 84.2% of all AF in women, with a global NNS of 1/19. There was a significant difference between the risk-AF groups in the AF incidence (p <0.001), stroke incidence (p <0.001), and cognitive impairment prevalence (p <0.001). The area under the curve was 61.9 (95% CI 60.3–63.4).
 |
Table 2 Distribution of Cases According to Calculated AF Risk (N 37,731) |
Stroke Incidence Density
Two hundred and thirty-four stroke episodes were confirmed (Table 3), which presented higher comorbidity, higher mean number of visits and higher mortality than those without strokes. Stroke incidence increased progressively with risk levels as well as the mean value (p <0.001) on the Rankin scale. The Q4/mean incidence ratio was 1.98 [95% CI 1.57–2.50 p <0.001]. In Q3-Q4 levels, 85.4% of episodes were concentrated in women, while in men they represented 69.5% of the total. The percentage of AF screening in those with strokes was significantly higher (72.6% vs 53.8%).
 |
Table 3 Baseline Characteristics of Stroke vs Non-Stroke Cases |
Regarding the temporal relation between strokes/AF, most of the strokes (78.5%) occurred in people without AF, concentrated in Q3-Q4 risk levels, especially among women (88.17%). The cumulative percentage of strokes associated with the simultaneous diagnosis of AF was 9.8% of the total [ID 5.8 cases (95% CI 3.4–8.1)/1000] new AFs/year. In addition, the 57.1% of simultaneous diagnosis of stroke and AF happened in the group Q4, presented greater NIHSS (7.25 ± 8.62 vs 4.55±5.74, p 0.002).
Cognitive Impairment Prevalence
Eventually there was a progressive increase in the prevalence of cognitive deterioration and the mean value on the Pfeiffer scale with the increase of the risk of AF. 77.4% of the cases with cognitive impairment were concentrated in the Q3-Q4 risk levels. There was a significant linear correlation between the risk-AF score and the Rankin score (p < 0.001), the Pfeiffer score (p < 0.001), but not NIHSS score (p 0.150).
Discussion
The study uses data from the general population ≥65 years without previous atrial fibrillation to compare frequencies of incident strokes and prevalence of cognitive deterioration in the different risk groups obtained during the same period of time, but excludes causal relationships. The clinical stratification model used16 can discriminate those individuals with a higher risk (17.0/1000/year) of suffering an AF at five years, who are associated with a higher prevalence of strokes and cognitive deterioration, as well as differences by sex. Furthermore, the NNS (n 19) for the diagnosis of a new case of AF was much lower than that reported (n 147) by previous studies in the general population.21,22
The risk stratification in Primary Care would identify those individuals with the highest risk: men [Q3-4 and/or CHA2DS2VASc ≥3], women [Q4 and/or CHA2DS2VASc ≥4] in which to intervene on modifiable risk factors, additional explorations, and to investigate which methodology could be more effective13,14,17 for the diagnosis of AF before its debut associated with strokes and whether anticoagulation should be started. The results of the CHA2DS2VASc are similar to the previous evidence18 and although the combination of the CHA2DS2VASc with an AF load >5–6 min is required to produce an increased risk of stroke or the presence of ECG abnormalities,19,20 there is uncertainty about the efficacy and safety of starting anticoagulation. Opportunistic screening in 79.6% of cases of new AF would support its performance over usual care,21,22 but its impact on the incidence of stroke is unknown. Furthermore, the total NNC <20 is much lower than that published23 in the general population; and, at least, it would allow interventions on modifiable factors.24 There is little evidence about the added value of using echocardiographic criteria and biomarkers25–28 in this higher-risk population.
Although the number of strokes is relatively low, there is a higher incidence of AF and stroke in Q4, especially among women, reflecting different possible pathways of cardiovascular disease26,29 as well as differences in the CHA2DS2-VASc value.15,26 The risk stratification of AF could also be a criterion for deciding the type of monitoring after having suffered a stroke, and the sensitivity of the existing scales could be improved. Although the result in the ROC curve may be a relative limitation, it will not modify the clinical decision to optimize the approach to modifiable risk factors; and the significantly incremental densities and incidence ratios of both AF and stroke at the different risk levels should be sufficient to test clinical stratification to improve AF screening results. The inclusion of echocardiographic criteria (19), biomarkers (27,28), and the use of different technologies (13,14,17) will define different post-test probabilities (Figure 1).
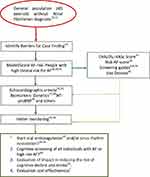 |
Figure 1 Atrial fibrillation Primary Care Pathway (proposal). |
Given the AF meets the characteristics for population screening and opportunistic, pulse taking is recommended in primary care,30 we have no evidence of which diagnostic protocol could be with better results or its impact on the prevention of strokes and deterioration cognitive. Although we have technology that expands diagnostic opportunities in different contexts (population campaigns, clinical consultation and home) and we have data on high-risk population subgroups, it would be necessary to develop research proposals in Primary Care that would allow not only to improve diagnostic effectiveness of AF but also to measure its burden and to evaluate the cost-effectiveness on the prevention of stroke episodes and cognitive deterioration.8,31 The prevalence of cognitive disorder is higher in each quartile in relation to that described in the general population.32 How studies included individuals with the baseline dementia or prevalent MCI may affect the prevalence figures. Finally, one must consider that estimates for MCI from general population studies include all cases, regardless of their likelihood of being detected in the health-care system or the underlying disease etiology. In addition to the epidemiological estimate associated with demographic aging, the greater cardiovascular comorbidity, frequency, average drug consumption, mortality and severity of stroke would confirm the estimate of the increase in costs associated with the treatment of AF associated with a stroke episode. The presence of a progressive increase in the prevalence and severity of cognitive impairment with the risk of AF would support a possible etiopathogenic interrelation between both processes in the general population,33 as well as the need to protocolize its detection.34 A model of comprehensive care for AF has shown a 45% reduction in mortality from any cause,35 but its analysis is subsequent to the diagnosis of AF.
As potential limitations of the study, the authors consider the under-registration of diagnoses; the cross-sectional format does not allow defining causal relationships between AF and cognitive impairment even in the absence of stroke; and the results are limited to a generic AF and cognitive decline without stratification according to the age, type of AF and cognitive dysfunction. Among its strengths, are remarkable the considerable number of cases, had with long follow-ups, and were conducted in general population. The target population to screen has yet to be established particularly with regards to the impact of oral anticoagulation on cognitive outcomes. At present, ideal strategies to screen for AF remains to be defined. This article aims to provide content that allows new research including the interrelation of different form of AF, cognitive impairment vs dementia and age with the risk-AF model to be developed.
Future research should focus on the cost-effectiveness analysis of a protocol that includes the systematic identification of patients at high risk of suffering from AF13,16 according to age-specific ranges, type and cognitive impairment; the modification of risk factors; the use of echocardiographic criteria and biomarkers;25–28 and the prevalence of cognitive disorder and dementia separately along the AF risk scale (Q1 to Q4) may resolve uncertainties related to the most effective type of monitoring,17 whether to start anticoagulant treatment36 or not, and favour the definition of the potential mechanisms leading to cognitive dysfunction37–39 and therapeutic strategies to prevent AF-related cognitive decline.
Conclusions
The clinical stratification model used16 can discriminate those individuals with a higher risk (17.0/1000/year) of suffering an AF at five years, who are associated with a higher prevalence of strokes and cognitive deterioration, as well as differences by sex in whom to carry out screening and evaluate its effectiveness.
Abbreviations
AF, atrial fibrillation; CCP, complex chronic patient; CI, confidence interval; e-SAP, electronic clinical records in primary care; CMBD, minimum basic data set – catalonian acronym for Conjunt Mínim Bàsic de Dades (CMBD); ICD-10, International Classification of Diseases; ID, incidence rate, 1000/person-years; NNS, number needed to screen to detect one case of AF; SIRE, Integrated Electronic Prescription System – catalonian acronym for Sistema Integrat de Recepta Electrònica (SIRE).
Data Sharing Statement
Detailed information is available at the AFRICAT study protocol (NCT03188484). The datasets generated, used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The research protocol of the study was approved by the Ethical Committee of the Institut Universitari d’Investigació en Atenció Primària IDIAP Jordi Gol with the code number (P18/118) and complies with the Helsinki Declaration and the local ethics committee requirements for clinical research. Registry information was collected from the government-run healthcare provider responsible for all inpatient care in the county without contact with participants in order to gather data from the study. For this type of study formal consent is not required and the requirement for the informed consent of patients was waived prior to the inclusion of their medical data in this study.
Acknowledgments
The authors thank to Jesus Carot and Sistemes d’Informació i Comunicació of the Territorial Management of l’ICS in Terres de l’Ebre for their invaluable material and technical collaboration in the management of the bases of original data, as well as all the primary care professionals who participated directly or indirectly in the elaboration of the Results.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The study received a research grant by Fundació Marató de TV3 in the research call “La Marató 2014: Malalties del Cor”. Grant number: 201528-30-31-3 and received funding from the Spanish Society of Family and Community Medicine -semFYC- by being the winner of a grant for the completion of doctoral theses Isabel Fernández 2020.
Disclosure
The authors declare that they have no conflict of interest and have not received commercial sponsorship for the research and publication of the results. There are also no direct or indirect financial interests. This research received no specific grant from funding agencies in the commercial or for-profit sectors.
References
1. Ictus: Plan de Actuación en Europa (2018-2030). Ed Stroke Alliance for Europe (SAFE). 2018. www.safestroke.eu.
2. Gabriel SC, Yiin DPJ, Nicola LM. Rothwell, on behalf of the Oxford Vascular Study. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: a population-based study. Circulation. 2014;130(15):1236–1244. doi:10.1161/CIRCULATIONAHA.114.010942
3. Clua-Espuny JL, Piñol-Moreso JL, Panisello-Tafalla A, et al. Estudio Ebrictus. Resultados funcionales, supervivencia y años potenciales de vida perdidos después del primer episodio de ictus. Aten Primaria. 2012;44(4):223–231. doi:10.1016/j.aprim.2011.04.004
4. Proietti M, Laroche C, Opolski G, et al. Investigators. Real-word’ atrial fibrillation management in Europe: observations from the 2-year-follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace. 2017;19(5):722–733. doi:10.1093/europace/euw112
5. Clua-Espuny JL, Lechuga-Duran I, Bosch-Príncep R, et al. Prevalence of undiagnosed atrial fibrillation and of that not being treated with anticoagulant drugs: the AFABE study. Rev Esp Cardiol. 2013;66:545–552. doi:10.1016/j.rec.2013.03.003
6. Clua-Espuny JL, Piñol-Moreso JL, Panisello-Tafalla A, et al. Resultados de prevención cardiovascular primaria y secundaria en pacientes con ictus. Riesgo de recurrencia y supervivencia asociada. Estudio Ebrictus. Rev Neurol. 2012;54:81–92. doi:10.33588/rn.5402.2011464
7. Ratajczak-Tretel B, Lambert AT, Johansen H, et al. (2019. Atrial fibrillation in cryptogenic stroke and transient ischaemic attack. The Nordic Atrial Fibrillation and Stroke (NOR-FIB) Study: rationale and design. Eur Stroke J. 2019;4(2):172–180. doi:10.1177/2396987319837089
8. Hachinskia V, Einhauplb K, Gantenc D, et al. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimer’s & Dementia. 2019;15:961–984. doi:10.1016/j.jalz.2019.06.001
9. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J. Searching for Atrial Fibrillation Poststroke: A White Paper of the AF-SCREEN International Collaboration. Circulation. 2019;140(22):1834–1850. doi:10.1161/CIRCULATIONAHA.119.040267
10. Kirchhhof P, et al. ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with EACTS. Rev Esp Cardiol (Engl Ed), 2017. 70 (1):50.documento de consenso de múltiples sociedades (EHRA, HRS, APHRS y SOLAECE). Europace. 2016;2017(19):1589–1623. doi:10.1093/ejcts/ezw313
11. Moran PS, Teljeur C, Ryan M, Smith SM. Systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev. 2016;6:. doi:10.1002/14651858.CD009586.pub3
12. Vermond RA, Geelhoed B, Verweij N, et al. Incidence of Atrial Fibrillation and Relationship With Cardiovascular Events, Heart Failure, and Mortality. A Community-Based Study From the Netherlands. J Am Coll Cardiol. 2015;66(9):1000–1007. doi:10.1016/j.jacc.2015.06.1314
13. Clua-Espuny JL, Muñoz Perez MA, Bustamante-Rangel A, Montaner Villalonga J, Pedrote Martínez AA on behalf members of AFRICAT Group. Stepwise High Risk Individuals Screening for Atrial Fibrillation Using Sequential Clinical-electro-biological Register: the AFRICAT Study (Atrial Fibrillation Research In CATalonia). ClinicalTrials. 2020.
14. Uittenbogaart SB, Gurp NV-V, Petra MG, et al. Stoffers. Detecting and Diagnosing Atrial Fibrillation (D2AF): study protocol for a cluster randomised controlled trial. Trials. 2015;16:478. doi:10.1186/s13063-015-1006-5
15. Thijs V. Atrial Fibrillation Detection. Fishing for An Irregular Heartbeat Before and After Stroke. Stroke. 2017;48(10):2671–2677. doi:10.1161/STROKEAHA.117.017083
16. Muria-Subirats E, Clua-Espuny JL, Ballesta-Ors J, Lorman-Carbó B, Lechuga-Durán I, Fernández-Sáez J. Pla-Farnós R. Incidence and Risk Assessment for Atrial Fibrillation at 5 Years: hypertensive Diabetic Retrospective Cohort. Int J Environ Res. 2020;17(10):3491. doi:10.3390/ijerph17103491
17. Blay C, Limón E (Coord.) Bases para un modelo catalán de atención a las personas con necesidades complejas: conceptualización e introducción a los elementos operativos. [Internet] Barcelona: departament de Salut; 2016. Available from: http://salutweb.gencat.cat/web/.content/_ambits-actuacio/Linies-dactuacio/Estrategies-de-salut/Cronicitat/Documentacio-cronicitat/arxius/bases_modelo_personas_complejidad_v_6.pdf.
18. Özcan EE. Bülent Görenek1. Lessons from the current European Heart Rhythm Association consensus document on screening for atrial fibrillation. Anatol J Cardiol. 2018;19:222–224. doi:10.14744/AnatolJCardiol.2018.37043
19. Ballesta-Ors J, Clua-Espuny JL, Gentille-Lorente DI, et al. Results, barriers and enablers in atrial fibrillation case finding: barriers in opportunistic atrial fibrillation case finding-a cross-sectional study. Fam Pract. 2020;27:
20. Zungsontiporn N, Link MS. Newer technologies for detection of atrial fibrillation. BMJ. 2018;363:k3946. doi:10.1136/bmj.k3946
21. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke Risk as a Function of Atrial Fibrillation Duration and CHA 2 DS 2-VASc Score. Circulation. 2019;140(20):1639–1646. doi:10.1161/CIRCULATIONAHA.119.041303
22. Bayés-de-Luna A, Martínez-Sellés M, Elosua R, et al. Relation of Advanced Interatrial Block to Risk of Atrial Fibrillation and Stroke. Am J Cardiol. 2020;16:
23. Haeusler KG, Tütüncü S, Schnabel RB. Detection of Atrial Fibrillation in Cryptogenic Stroke. Curr Neurol Neurosci Rep. 2018;18(10):66. doi:10.1007/s11910-018-0871-
24. Lowres N, Olivier J, Chao TF, et al. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single times screening: a multi country patient-level meta-analysis of 141,220 screened individuals. PLoS Med. 2019;16(9):e1002903. doi:10.1371/journal.pmed.1002903
25. Efremov L, Lacruz ME, Tiller D, et al. Metabolically Healthy, but Obese Individuals and Associations with Echocardiographic Parameters and Inflammatory Biomarkers: results from the CARLA Study. Diabetes Metabolic Syndrome Obesity. 2020;13:2653–2665. doi:10.2147/DMSO.S263727
26. Middeldorp ME, Ariyaratnam J, Lau D, Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart. 2020;106(5):325–332. doi:10.1136/heartjnl-2019-315327
27. Lau ES, Paniagua SM, SawallaGuseh J, et al. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J Am Coll Cardiol. 2019;74:12. doi:10.1016/j.jacc.2019.06.077
28. García-Berrocoso T, Palà E, Consegal M, et al. Cardioembolic Ischemic Stroke Gene Expression Fingerprint in Blood: a Systematic Review and Verification Analysis. Transl Stroke Res. 2019. doi:10.1007/s12975-019-00730-x
29. Palà E, Bustamante A, Clúa-Espuny JL, et al. N-Terminal Pro B-Type Natriuretic Peptide’s Usefulness for Paroxysmal Atrial Fibrillation Detection Among Populations Carrying Cardiovascular Risk Factors. Front Neurol. 2019;10:1226. doi:10.3389/fneur.2019.01226
30. Wu VC, Wu M, Aboyans V, et al. Female sex as a risk factor for ischaemic stroke varies with age in patients with atrial fibrillation. Heart. 2019;26:
31. Cuixart CB. Recomendaciones preventivas cardiovasculares. Actualización PAPPS. 2018. doi:10.1016/S0212-6567(18)30360-3
32. Gillis C, Mirzaei F, Potashman M, Ikram MA, Maserejian N. The incidence of mild cognitive impairment: A systematic review and data synthesis. Alzheimers Dement. 2019;11:248–256. doi:10.1016/j.dadm.2019.01.004
33. Kim D, Yang PS, Yu HT, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J. 2019;40(28):2313–2323. doi:10.1093/eurheartj/ehz386
34. Dagres N, Chao TF, Fenelon G, et al. Zhang S and Chung MK. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: what is the best practice? Heart Rhythm. 2018;15:e37–e60.
35. Lei C, Deng Q, Haijiang L, Zhong L. Association Between Silent Brain Infarcts and Cognitive Function: A Systematic Review and Meta-Analysis. J Stroke Cerebrovascular Dis. 2019;28:2376–2387. doi:10.1016/j.jstrokecerebrovasdis.2019.03.036
36. Carline J. Integrated management of atrial fibrillation in primary care: results of the ALL-IN cluster randomized trial. Eur Heart J. 2020;ehaa055. doi:10.1093/eurheartj/ehaa055
37. Friberg L, Andersson T, Rosenqvist M. Less dementia and stroke in low-risk patients with atrial fibrillation taking oral anticoagulation. Eur Heart J. 2019;40(28):2327–2335. doi:10.1093/eurheartj/ehz304
38. Gallinoro E, D’Elia S, Prozzo D, et al. Cognitive Function and Atrial Fibrillation: from the Strength of Relationship to the Dark Side of Prevention. Is There a Contribution from Sinus Rhythm Restoration and Maintenance? Medicina. 2019;55(9):587. doi:10.3390/medicina55090587
39. Rivard L, Mechanisms KP, Significance C. Prevention of Cognitive Impairment in Patients with Atrial Fibrillation. Can J Cardiol. 2017;33(12):1556–1564. doi:10.1016/j.cjca.2017.09.024
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
