Back to Journals » Journal of Asthma and Allergy » Volume 15
Risk of Adverse Events of Live-Attenuated COVID-19 Vaccination Among Atopic Patients
Authors Chiewchalermsri C , Hengkrawit K, Srinithiwat P, Kiatsermkachorn W, Luecha O
Received 23 August 2022
Accepted for publication 28 October 2022
Published 10 November 2022 Volume 2022:15 Pages 1605—1621
DOI https://doi.org/10.2147/JAA.S386611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Chirawat Chiewchalermsri,1 Kitchawan Hengkrawit,2 Palakorn Srinithiwat,1 Wipawee Kiatsermkachorn,1 Orawin Luecha2
1Department of Medicine, Panyananthaphikkhu Chonprathan Medical Center, Srinakharinwirot University, Nonthaburi, Thailand; 2Department of Pediatrics, Panyananthaphikkhu Chonprathan Medical Center, Srinakharinwirot University, Nonthaburi, Thailand
Correspondence: Orawin Luecha, Department of Pediatrics, Panyananthaphikkhu Chonprathan Medical Center, Srinakharinwirot University, P.O.Box 222 Moo 1, Tiwanon Road, Pak Kret, Nonthaburi, 11120, Thailand, Tel +66 2 502 2345, Fax +662 502 2305, Email [email protected]
Purpose: Atopic patients have more risk of adverse drug reactions. COVID-19 vaccination is very important in the current situation. We still do not have data about risks of adverse effects from vaccine in atopic patients. The goal of our study was to evaluate atopic risks and adverse effects of live-attenuated Oxford/AstraZeneca COVID-19 vaccination.
Patients and methods: Data was collected using a prospective descriptive cohort study from participants 18 years old and above who came to the Outpatient Department, Panyananthaphikkhu Chonprathan Medical Center for live-attenuated COVID-19 vaccination between March and December 2021. The sample size was 3016 individuals. The information about adverse reactions at 6, 2, 72 hours and 7.30 and 60 days after each live-attenuated Oxford/AstraZeneca COVID-19 vaccination was collected by telephone. Participants with history of severe allergic reaction to vaccine components were excluded.
Results: There were 732 atopic patients and 2284 non-atopic patients. Atopic patients included 556 with allergic rhinitis, 83 with asthma, 23 with urticaria and 73 with food allergies. The underlying diseases of hypertension, hyperlipidemia and hyperthyroidism were more common in non-allergic patients, with p-value < 0.001, < 0.001 and 0.042, respectively. Atopic patients developed significantly more fever, nausea and vomiting, skin rash (urticaria), and local reaction than non-atopic patients, with p-values of < 0.001, 0.018, < 0.001 and < 0.001, respectively.
Conclusion: Atopic patients had more risk of adverse reactions to live-attenuated Oxford/AstraZeneca COVID-19 vaccination. No life-threatening adverse reaction was seen. Physicians should screen atopic risks in people who are getting vaccinated. Atopic patients should be knowledgeable about their risk and how to monitor clinical reactions by themselves.
Keywords: atopic, allergic rhinitis, asthma, urticaria, adverse effect of COVID-19 vaccination
Introduction
Since 2020, the COVID-19 pandemic has been causing catastrophic loss worldwide.1 Nowadays, there are multiple platforms of COVID-19 vaccine, most of which reduce morbidity and mortality of COVID-19 infection.2 In Thailand, the ministry of public health started a campaign for COVID-19 immunization on February 28, 2021. At the beginning of the program, the Thai FDA approved 2 platforms of vaccines, the viral vector ChAdOx1 nCoV-19/AZD1222 (University of Oxford, AstraZeneca, and the Serum Institute of India)3 and the inactivated vaccine CoronaVac (Sinovac Biotech, Beijing, China).4 Following vaccination for COVID-19, all vaccinated individuals should be monitored for immediate allergic reactions. A serious immediate reaction is anaphylaxis. Reported rates vary with the surveillance method, from 2.5 to 4.7 events per million for mRNA vaccine.5 Retrospective multicenter trial in Thailand collects anaphylaxis cases from CoronaVac (Sinovac Life Sciences, Beijing, China) COVID-19 vaccine. Anaphylaxis was classified by Ring and Messmer as grading 1 to 5. Anaphylaxis grading 1 to 3 accounted for 1, 9 and 2 cases, respectively. In total there were 12 cases. For time to anaphylaxis, minimum was 6 minutes and maximum was 180 minutes.6 In Korea, the Korea Disease Control and Prevention Agency reported 7.4 cases of anaphylaxis per 100,000 doses for the Oxford-AstraZeneca vaccine and 1.8 cases per 100,000 doses for the Pfizer-BioNTech vaccine.7 However, systematic review of multiple vaccine trials reported that most of the adverse effects were mild intensity, for example local reaction, fever, myalgia, and headache.8 Nevertheless, there were also reports of serious vaccine side effects following immunization, such as vaccine-induced immune thrombotic thrombocytopenia (VITT),9 myopericarditis,10 and other rare serious complications.
Allergic diseases, eg, asthma, allergic rhinitis, atopic dermatitis, urticaria or food allergy, are not contra-indications for receiving COVID-19 vaccine. There is no evidence that allergic disease is a risk factor for COVID-19 vaccine allergy.11 As Greenhawt and colleagues reported, identification of potential risk factors associated with allergic reactions is still a knowledge gap.12 However, the CDC COVID-19 Vaccine Task Force stated that by January 18, 2021, after nearly 10 million doses of vaccination, there were 50 cases of anaphylaxis, most of them (80%) had history of allergy or allergic reaction to drug or food.13 This study aims to compare adverse effects of COVID-19 vaccine between atopic patients and non-atopic ones.
Method
Definition
Atopic patient: a patient who reported that they had at least one of the following conditions: asthma, allergic rhinitis, atopic dermatitis, food or drug allergy.
Vaccine adverse reaction: any symptom reported by a participant after receiving a vaccination, which was interpreted by an allergist as a significant adverse event.
Data Collection
This prospective descriptive cohort study collected data from participants 18 years old and above who came to the Outpatient Department, Panyananthaphikkhu Chonprathan Medical Center for COVID-19 vaccination during March–December 2021.
The sample size was initially 2688 individuals, calculated based on the number of citizens for whom our hospital has responsibility, which is roughly 90,000, and the first attempt of COVID vaccine campaign, which is 60% coverage. The risk of adverse effects of COVID vaccine from previous report was 0.021,14 with type I error rate = 1.96; an estimated difference in prevalence of adverse events between atopic group and non-atopic group of 1% would be significant. We also increased the number of participants to 12% for incomplete data, so final sample is 3016 individuals. Participants with history of severe allergic reaction to vaccine components were excluded as policy from Thai national guideline. Information on participants’ demographics, their underlying illnesses, and the number of their COVID vaccines, which in this trial use the viral vector ChAdOx1 nCoV-19/AZD1222, were collected after informed consent. The participants were observed for 30 minutes following vaccination to detect any immediate reaction. Afterwards, telephone follow up about adverse reaction was done at 6 hours, 24 hours, 3 days, 7 days, 30 days and 60 days from each dose of vaccine, for the total of 2 doses (primary series). The study protocols comply with the Declaration of Helsinki and were reviewed and approved by the Institutional Review Board of Panyananthaphikkhu Chonprathan Medical Center.
Data Analysis and Statistics
The number of participants with adverse events was reported as number and percentage of total number in each group. Population characteristics were reported as mean ± SE and median (min, max) for continuous data, and N (%) for numerical, categorical data. We used Pearson’s chi-square test, Fisher’s exact test, Chi-square test for independent inferential statistics and report as relative risk (RR) and 95% confidence interval (CI). Subgroup analysis was done on prevalent atopic diseases, allergic rhinitis and asthma. A p-value of <0.05 was considered significant.
Results
There were 732 atopic patients and 2284 non-atopic patients. Atopic patients included 556 with allergic rhinitis, 83 with asthma, 23 with urticaria and 73 with food allergies (Table 1). The underlying diseases of hypertension, hyperlipidemia and hyperthyroidism were more common in non-allergic patients, with p-values of <0.001, <0.001 and 0.042, respectively (Table 2).
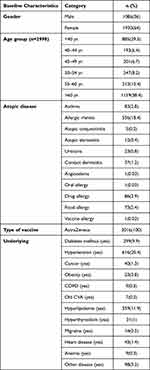 |
Table 1 Baseline Characteristics (n=3016) |
 |
Table 2 Characteristic Data (n=3016) |
Within the first 6 hours after first dose vaccination, atopic patients developed significantly more fever, skin rash (urticaria), and local reaction than non-atopic patients, with p-values of <0.002 and 0.002, respectively. Within the first 6 hours after second dose vaccination, atopic group had significantly more fever than non-atopic group, with p-value of <0.025 (Figures 1–4).
 |
Figure 1 Percentages of fever adverse effect in atopic and non-atopic patients and their atopic subgroups. |
 |
Figure 2 Percentages of skin rash (urticaria) adverse effect in atopic and non-atopic patients and their atopic subgroups. |
 |
Figure 3 Percentages of local reactions adverse effect in atopic and non-atopic patients and their atopic subgroups. |
 |
Figure 4 Percentages of nausea and vomiting adverse effect in atopic and non-atopic patients and their atopic subgroups. |
Within the first 24 hours of first dose vaccination, atopic patients developed significantly more fever, nausea and vomiting, skin rash (urticaria), and local reaction than non-atopic patients, with p value <0.001, 0.018, < 0.001 and < 0.001, respectively. In first 24 hours of second dose vaccination, Atopic patients developed significantly more fever, nausea and vomiting, skin rash (urticaria), and local reaction than non-atopic patients, with p-values of <0.001, 0.02, <0.001 and <0.002, respectively.
At 3 days after first dose vaccination, atopic group developed significantly more fever, nausea and vomiting, skin rash (urticaria), and local reaction than non-atopic group, with p-values of <0.001, 0.018, <0.001 and <0.001, respectively. At 3 days after second dose vaccination, atopic group developed significantly more fever, skin rash (urticaria), and local reaction than non-atopic group, with p-values of <0.001, <0.001 and <0.001, respectively.
Seven days after first dose vaccination, atopic group developed significantly more fever, nausea and vomiting, skin rash (urticaria), and local reaction than non-atopic group, with p-values of <0.001, 0.034, <0.001 and <0.001, respectively. Seven days after second dose vaccination, atopic group developed significantly more fever, skin rash (urticaria), and local reaction than non-atopic group, with p-values of <0.001, <0.001 and <0.001, respectively (Figures 1–4).
By subgroup analysis (Figures 1–4), we separated atopic group into asthmatic and non-asthmatic group, allergic rhinitis and non-allergic rhinitis group, urticaria and non-urticaria group, food allergy and non-food allergy group. Non-asthmatic group had other atopic conditions, such as allergic rhinitis, urticaria and food allergy. Non-allergic rhinitis group, non-urticaria group and non-food allergy group had other atopic conditions.
Within 6 hours of first and second dose vaccinations, the adverse effects in asthmatic group did not differ from non-asthmatic group.
In first 24 hours after first dose vaccination, asthma group developed significantly more fever than non-asthmatic patients, with p-value of 0.049. In first 24 hours after second dose vaccination, asthmatic group developed significantly more skin rash (urticaria) than non-asthmatic patients, with p-value of 0.002.
At 3 days after first dose vaccination, asthma group were developing significantly more fever than non-asthma group, with p-value of 0.018. Seven days after first and second dose vaccinations, the adverse effects in asthmatic group did not differ from non-asthmatic group.
By subgroup analysis (Figures 1–4), within 6 hours after first dose vaccinations, the adverse effects in urticaria group did not differ from non-urticaria group. Within 6 hours fter second dose vaccinations, urticaria group developed significantly more skin rash (urticaria) than non-urticaria group, with p-value of 0.001.
In first 24 hours after first dose vaccination, urticaria group developed significantly more skin rash (urticaria) and local reaction than non-urticaria patients, with p-value of 0.008 and 0.022. In first 24 hours after second dose vaccination, urticaria group developed significantly more fever and skin rash (urticaria) than non-urticaria patients, with p-values of <0.001 and 0.048, respectively.
Three days after second dose vaccination, urticaria group were developing significantly more skin rash (urticaria) than non-urticaria group, with p-value of 0.027. Seven days after first and second dose vaccinations, the adverse effects in urticaria group did not differ from non-urticaria group.
By subgroup analysis (Figures 1–4), within 6 hours after first dose vaccinations, the adverse effects in allergic rhinitis group did not differ from non-allergic rhinitis group. Within 6 hours of second dose vaccinations, allergic rhinitis group developed significantly more skin rash (urticaria) than non-allergic rhinitis group, with p-value of 0.001.
Within first 24 hours after first dose vaccination, allergic rhinitis group developed significantly more fever, nausea and vomiting, skin rash (urticaria) and local reaction than non-allergic rhinitis patients, with p-values of <0.001, 0.004, <0.001 and <0.001, respectively. In first 24 hours after second dose vaccination, allergic rhinitis group developed significantly more fever, nausea and vomiting, skin rash (urticaria) and local reaction than non-allergic rhinitis patients, with p-values of <0.001, 0.018, <0.001 and 0.008, respectively.
Three days after first dose vaccination, allergic rhinitis group developed significantly more fever, nausea and vomiting, skin rash (urticaria) and local reaction than non-allergic rhinitis patients, with p-values of <0.001, 0.004, <0.001 and 0.002, respectively. Three days after second dose vaccination, allergic rhinitis group developed significantly more fever, nausea and vomiting, skin rash (urticaria) and local reaction than non-allergic rhinitis patients, with p-values of <0.001, 0.018, 0.001 and <0.001, respectively.
Seven days after first dose vaccination, allergic rhinitis group developed significantly more fever, nausea and vomiting, skin rash (urticaria) and local reaction than non-allergic rhinitis patients, with p-values of <0.001, 0.007, <0.001 and 0.001, respectively. Seven days after second dose vaccination, allergic rhinitis group developed significantly more fever, nausea and vomiting, skin rash (urticaria) and local reaction than non-allergic rhinitis patients, with p-values <0.001, 0.018, <0.001 and <0.001, respectively.
By subgroup analysis (Figures 1–4), within 6 hours of first and second dose vaccinations, the adverse effects in food allergy group did not different compared to non-food allergy group.
Within first 24 hours after first dose vaccination, food allergy group developed significantly more fever, nausea and vomiting, skin rash (urticaria) and local reaction than non-food allergy group, with p-values of 0.035, 0.001, 0.043 and 0.002, respectively. Within first 24 hours after second dose vaccination, food allergy group developed significantly more fever and local reaction than non-food allergy group, with p-values <0.001 and 0.007, respectively.
Three days after first dose vaccination, food allergy group developed significantly more fever and local reaction than non-food allergy group, with p-values of 0.001 and 0.002, respectively. Three days after second dose vaccination, food allergy group developed significantly more fever and local reaction than non-food allergy group, with p-values of <0.001 and 0.013, respectively.
Seven days after first dose vaccination, food allergy group developed significantly more local reaction than non-food allergy group, with p-value of 0.01. Seven days after second dose vaccination, food allergy group developed significantly more fever and local reaction than non-food allergy group, with p-values of <0.001 and 0.015, respectively.
By subgroup analysis, within 6 hours and within 24 hours of first and second dose vaccinations, the adverse effects in hypertension group did not differ from non-hypertension group. Non-immediate phase reaction in both first and second dose vaccination (within 3 days and after 7 days) there were significant differences in local reaction and a non-significant difference in skin rash (Table 3).
 |
Table 3 Differences in Adverse Reactions of Live-Attenuated Oxford/AstraZeneca COVID-19 Vaccination Between Hypertension (n=616) and Non-Hypertension (n=2400) Groups |
By subgroup analysis, within 24 hours of first dose vaccination, hyperthyroid group developed significantly more fever than non-hyperthyroid group, with p-value of 0.04. Twenty-four hours after first and second dose vaccinations, the adverse effects in hyperthyroid group did not differ from non-hyperthyroid group (Table 4).
 |
Table 4 Differences in Adverse Reactions of Live-Attenuated Oxford/AstraZeneca COVID-19 Vaccination Between Hyperthyroid (n=31) and Non-Hyperthyroid (n=2985) Groups |
By subgroup analysis, within 24 hours of first and second dose vaccinations, the adverse effects in hyperlipidemia group developed more fever than non-hyperlipidemia group, with p-values of 0.019 and 0.001, respectively. Non-immediate phase reaction in first dose vaccination (within 3 days and after 7 days) there were significant differences in skin rash (urticaria) and in second dose vaccination (within 3 days and after 7 days), there were significant differences in nausea and vomiting (Table 5).
 |
Table 5 Differences in Adverse Reactions of Live-Attenuated Oxford/AstraZeneca COVID-19 Vaccination Between Hyperlipidemia (n=359) and Non-Hyperlipidemia (n=2657) Groups |
Fever, local reaction, urticaria, nausea/vomiting after first dose vaccination were common at 24 hours after vaccination; fewer participants experienced these reactions after 30 minutes in both groups. Fever, local reaction, urticaria, nausea/vomiting after first dose vaccination could be start at 3–7 days after vaccination in both groups (Figures 1–4).
Fever, local reaction, urticaria after first dose vaccination were common at 24 hours after vaccination; fewer participants experienced these reactions after 30 minutes in both groups. Fever, local reaction, urticaria, nausea/vomiting after first dose vaccination could start at 3 days after vaccination in both groups. All side effects were more common in first dose vaccination than second dose vaccination (Figures 1–4).
Discussion
From our study, we found that atopic patients had significantly higher risk of adverse effects from live-attenuated Oxford/AstraZeneca COVID-19 vaccination, such as fever, nausea and vomiting, skin rash (urticaria), and local reaction than non-atopic patients. Also, in subgroup analysis of atopic group, those with asthma, allergic rhinitis, urticaria and food allergy had significantly higher risk of immediate and non-immediate adverse reactions. Fever, nausea and vomiting, skin rash (urticaria), and local reaction were significantly higher in allergic rhinitis patients than non-allergic patients and in patients with history of food allergy compared to patients without history of food allergy. Urticaria patients got more skin rash (urticaria) adverse effects than non-urticaria patients. Fever and skin rash (urticaria) were significantly higher in asthmatic patients than non-asthmatic patients.
A previous cohort study by Emel Atayik and Gokhan Aytekin showed patients with allergic diseases and adverse effects of CoronaVac and Pfizer-BioNTech COVID-19 vaccines. The 648 allergic disease patients included 35.2% with allergic rhinitis, 22.8% with asthma and 26.5% with chronic urticaria. Two-hundred and ninety-three patients (45.2%) reported side effects after their first dose of COVID-19 vaccine. Most local adverse effects developed within 4 hours (26.9%). One hundred and fifty-six patients (24.1%) reported systemic side effects after their first dose of COVID-19 vaccine. A systemic effect was fatigue (14.5%) within 24–72 hours (13.9%) after COVID-19 vaccine. Anaphylaxis developed in 2 patients (0.3%); 104 patients (19.1%) developed local adverse effects; 67 patients (12.3%) reported systemic side effects after their second dose of COVID-19 vaccine. Anaphylaxis was not reported.15 There has been one small cohort study of 68 atopic patients on subcutaneous immunotherapy who received mRNA COVID vaccine without reporting any allergic reaction. Although limited by a small sample size, Dages and team reported that atopy may not be a considerable risk factor for an immediate allergic reaction to the mRNA COVID-19 vaccines.13
Side effects of the live-attenuated Oxford/AstraZeneca COVID-19 vaccine in an Egyptian population was studied by Marwa et al. Among a total of 168 participants, 80 received BBIBP-CorV vaccine, 63 received ChAdOx1 and 25 received the BNT162 vaccine. The most common side effects of ChAdOx1 vaccine were local reaction (90.5%); muscle pain (71.5%); fatigue and lethargy (57%); joint pain (52%); fever and headache (38%); dizziness, abdominal pain, convulsions and tremors (14%); inflammation of the nervous system, including numbness, tingling, and loss of sensation (13.5%); decreased appetite, nausea, and vomiting (9.5%); cough, allergies, rashes, and runny nose (5%); and sore throat (4.5%). Only 5% of the participants who received the ChAdOx1 vaccine did not feel any side effects.16 Comparison of adverse effects of live-attenuated ChAdOx1 COVID-19 vaccine in atopic and non-atopic patients has never been studied before. From latest review, cutaneous adverse effects of live-attenuated Oxford/AstraZeneca COVID-19 vaccine were injection site reactions, exanthemas, urticaria, leukocytoclastic vasculitis, purpura/petechiae, acute generalized exanthematous pustulosis (AGEP) and Stevens–Johnson syndrome (SJS).17
Our study of adverse effects from ChAdOx1 COVID-19 showed increased urticaria, injection site reaction and fever in atopic rather than non-atopic patients in both the immediate and non-immediate phases. Nausea or vomiting also increased in atopic rather than non-atopic patients in the immediate phase. All reactions were consistent with previous studies but our study did not see any severe reactions such as anaphylaxis, AGEP and SJS. In subgroup analysis, immediate-phase reaction in patients with non-atopic diseases, such as hypertension, hyperthyroid or hyperlipidemia, resulted in no skin reactions. This is the first study demonstrating that those with atopic underlying diseases are at risk of adverse effects from live-attenuated Oxford/AstraZeneca COVID-19 vaccine.
Limitation
Adverse effects were evaluated by telephone, so if the adverse reaction was a skin reaction, it was hard to differentiate the type of rash, such as urticaria or maculopapular rash. This might limit our study’s ability to define the type of rash. Further study should be evaluated by video call or follow up at a clinic to correct this problem.
Conclusion
Atopic patients had more risk of adverse reactions to live-attenuated Oxford/AstraZeneca COVID-19 vaccination. No life-threatening adverse reaction was seen. Physicians should screen atopic risks in people who are getting vaccinated. Atopic patients should be knowledgeable about their risk and how to monitor clinical reactions by themselves.
Acknowledgment
Vaccination unit, Panyananthaphikkhu Chonprathan Medical Center, Srinakharinwirot University, Nonthaburi, Thailand.
Funding
Academic affairs and Research, Panyananthaphikkhu Chonprathan Medical Center, Srinakharinwirot University, Nonthaburi, Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Júnior Borges Do Nascimento I, Cacic N, Mohamed Abdulazeem H, et al. Novel coronavirus infection (covid-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9(4):941.
2. Higdon MM, Wahl B, Jones CB, et al. Systematic review of coronavirus disease 2019 vaccine efficacy and effectiveness against severe acute respiratory syndrome coronavirus 2 infection and disease. Open Forum Infect Dis. 2022;9(6):1–19. doi:10.1093/ofid/ofac138
3. Knoll MD, Wonodi C. Oxford-AstraZeneca covid-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi:10.1016/S0140-6736(20)32623-4
4. Fadlyana E, Rusmil K, Tarigan R, et al. A Phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of sars-cov-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520. doi:10.1016/j.vaccine.2021.09.052
5. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA covid-19 vaccines in the us-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102. doi:10.1001/jama.2021.1967
6. Laisuan W, Wongsa C, Chiewchalermsri C, et al. Coronavac covid-19 vaccine-induced anaphylaxis: clinical characteristics and revaccination outcomes. J Asthma Allergy. 2021;14:1209–1215. doi:10.2147/JAA.S333098
7. Kim MA, Lee YW, Kim SR, et al. Covid-19 vaccine-associated anaphylaxis and allergic reactions: consensus statements of the kaaaci urticaria/angioedema/anaphylaxis working group. Allergy Asthma Immunol Res. 2021;13(4):526–544. doi:10.4168/aair.2021.13.4.526
8. Kaur RJ, Dutta S, Bhardwaj P, et al. Adverse events reported from covid-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021;36(4):427–439. doi:10.1007/s12291-021-00968-z
9. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680. doi:10.1056/NEJMoa2109908
10. Fazlollahi A, Zahmatyar M, Noori M, et al. Cardiac complications following mRNA covid-19 vaccines: a systematic review of case reports and case series. Rev Med Virol. 2022;32(4):e2318. doi:10.1002/rmv.2318
11. Nilsson L, Brockow K, Alm J, et al. Vaccination and allergy: eaaci position paper, practical aspects. Pediatr Allergy Immunol. 2017;28(7):628–640. doi:10.1111/pai.12762
12. Greenhawt M, Abrams EM, Shaker M, et al. The risk of allergic reaction to sars-cov-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, grade assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;3567:3546.
13. Dages KN, Pitlick MM, Joshi AY, Park MA. Risk of allergic reaction in patients with atopic disease and recent coronavirus disease 2019 vaccination. Ann Allergy Asthma Immunol. 2021;127(2):257–258. doi:10.1016/j.anai.2021.04.024
14. Blumenthal KG, Robinson LB, Camargo CA
15. Atayik E, Aytekіn G. The course of covid-19 in allergic rhinitis patients receiving allergen specific immunotherapy. medRxiv. 2022;2022. doi:10.1101/2022.01.29.22270072
16. Marwa OE, Ahmed OE-G, Sarah M, Tarek Yehia M, Mohamed EAA, Ahmed MS. Side effects and efficacy of covid-19 vaccines among the Egyptian population. Vaccines. 2022;10(109):109. doi:10.3390/vaccines10010109
17. Francesco B, Martina M, Paolo G, Giampiero G. Cutaneous adverse reactions associated with sars-cov-2 vaccines. J Clin Med. 2021;10(5344):5344. doi:10.3390/jcm10225344
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
