Back to Journals » Infection and Drug Resistance » Volume 14
Risk Factors of Drug Resistance and the Potential Risk of HIV-1 Transmission of Patients with ART Virological Failure: A Population-Based Study in Sichuan, China
Authors Zhou C, Kang R , Liang S, Fei T, Li Y , Su L, Li L, Ye L, Zhang Y, Yuan D
Received 2 September 2021
Accepted for publication 11 November 2021
Published 7 December 2021 Volume 2021:14 Pages 5219—5233
DOI https://doi.org/10.2147/IDR.S334598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Chang Zhou,1,* Rui Kang,2,* Shu Liang,1 Teng Fei,2 Yiping Li,1 Ling Su,1 Ling Li,1 Li Ye,1 Yan Zhang,1 Dan Yuan1
1Center for AIDS/STD Control and Prevention, Sichuan Center for Disease Control and Prevention, Chengdu, People’s Republic of China; 2School of Resource and Environmental Sciences, Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dan Yuan Email [email protected]
Background: Sichuan Province, in the interior of Southwest China, is the most severe HIV-affected area in China. Few data are currently available for people living with HIV/AIDS (PLWH) with virological failure of antiretroviral therapy (ART). Estimating the HIV-1 drug-resistant spread influencing factors and transmission patterns of the HIV-1 epidemic of PLWH with ART virological failure are critical in Sichuan.
Methods: We evaluated the drug-resistant transmission patterns on 5790 PLWH in 2018 with identified pol sequences of the five main HIV-1 subtypes (ie, subtype B, CRF08_BC, CRF85_BC, CRF07_BC, and CRF01_AE) in Sichuan Province, China. The multivariate logistic regression model was used to explore potential influencing factors of the spread of drug resistance (DR) clustering in the genetic transmission network. Spatial analyses were applied to demonstrate drug-resistant spatial clustering patterns of spatial connections of HIV-1 intercity transmission. Genetic transmission networks were performed by comparing sequences, calculating the pairwise distance, and visualizing the network.
Results: There were identified 452 transmission clusters containing 2159 of 5790 patients (37.29%) in the HIV-1 genetic transmission networks. Some clinical and demographic factors (eg, route of transmission, subtype) determined the DR clustering in the genetic transmission networks. The high drug-resistant clustering rates were mainly distributed in the Southern and Northeast of Sichuan Province (eg, Deyang, Neijiang), especially for CRF85_BC, which showed the highest clustering rate. Some cities had with strong intracity links (eg, Yibin, Neijiang), some cities had with strong transmission links with another city (eg, Ziyang and Guangyuan), 12 of 37 drug resistance mutation sites had a significant difference in the five subtypes (P < 0.001).
Conclusion: Our findings revealed the HIV-1 drug-resistant spread influencing factors and transmission patterns of PLWH with ART virological failure, which showed regions with high drug-resistant transmission of PLWH may not be a match for regions with severe epidemics in Sichuan, and it provided evidence-based to drug-resistant transmission targeting interventions.
Keywords: HIV-1, antiretroviral resistance, transmitted drug resistance, spatial analyses
Introduction
Since 1981, when the US reported the first case of AIDS, HIV has spread rapidly worldwide, it has been responsible for nearly 76 million infections globally,1 and approximately 0.85 million PLWH live in China. In view of its harm and prevalence, AIDS affects social security, and has become a major threat to human society, population structure and economic development.2
ART is the most effective method of AIDS treatment at present, which is highly effective in stopping reducing the risk of HIV transmissions.3,4 During antiviral treatment, strains carrying DR gene mutations are screened under drug pressure and accumulate over time, which will eventually reduce the efficacy of antiviral treatment, lead to the failure of antiviral treatment, and even the possibility of DR transmission.5,6
China had a massive floating population, studies have revealed that the floating population has become an important factor in the spread of HIV fast spreading bridge crowd and geographic differences may to some extent determine the patterns of HIV transmission.7,8 HIV genetic-transmission network analysis is an effective strategy for identifying high risk factors,9 which would be complementary found using traditional epidemiological approaches. Previous studies showed that young men, especially MSM, and some subtypes (eg, CRF55_01B, CRF01_AE) may have a higher transmission risk.10,11 In addition, reports showed that some demographic differences and HIV-related behaviors (eg, gender differences, ART adherence and ART regimens) lead to the risk of drug resistance with virological failure among PLWH, which were reported among PLWH in some countries, such as China,12–14 Uganda,15 Malawi,16 Cameroon.17 Yuan had reported that DR transmission in MSM with virological failure was concentrated in urban cities.18
Sichuan province (Figure 1) is in the interior of Southwest China, adjacent to Yunnan and Guizhou in the South and Tibet in the West. Sichuan has a population of approximately 85 million people, and is a developing area, Sichuan Province is the most severely HIV affected area in China.19 The universal coverage of ART led to a significant decline in morbidity and mortality among PLWH.20 However, the prevalence of virological failure on ART was high in Sichuan.14
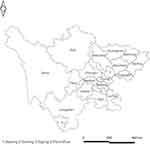 |
Figure 1 The distribution of people living with HIV/AIDS recruited in this study. |
Therefore, this study aimed to establish the HIV-1 drug-resistant spread influencing factors of PLWH with ART virological failure and evaluated the transmission patterns of the HIV-1 epidemic of PLWH with ART virological failure in Sichuan.
Methods
Study Subjects
Sichuan is located in the southwest of China, where the epidemic of HIV-1 is most serious in China, especially in central, eastern, and southern regions. Between January 2018 and December 2018, 7011 whole blood samples were collected from participants experiencing treatment failure in Sichuan Province. The study subjects were selected based on the following criteria: (1) receiving confirmatory HIV diagnosis, (2) receiving ART for at least 6 months; (3) being diagnosed as a virological failure in ART (namely HIV RNA level >1000 copies/mL), because gene sequencing was conducted in this population according to Chinese “National Guideline for Detection of HIV/AIDS (2020)”. The study subjects were excluded if they had used ART outside national guidelines or were missing treatment regimen information.
Among the 7011 PLWH, pol sequence information was successfully exported from 5926 (84.5%) samples. After eliminating duplicate samples and other HIV-1 subtype sequences, 5790 PLWH with pol sequences of the five main HIV-1 subtypes (ie, subtype B, CRF08_BC, CRF85_BC, CRF07_BC, and CRF01_AE) were finally included in this study.
Ethics Statement
The use of anonymous clinical/demographic and sequence data was reviewed, and the study protocol was approved by the Ethics Committee of the Sichuan Center for Disease Control and Prevention. The study was conducted following the Helsinki Declaration of 1964.
Laboratory Tests
About 5 mL of venous blood was extracted to test the CD4+ T cell. Flow cytometry was used to quantify the CD4+ T cells in the local CDCs. The plasma samples were isolated from each participant and preserved in a −80°C freezer.
Amplification, Sequencing, and HIV-1 Genetic Transmission Network Analysis
The viral nucleic acid was obtained from 200 mL plasma of PLWH by extraction machines (MagNA Pure LC 2.0 system, Roche, Branchburg, NJ). Similar to the previous reports, sequences were generated from the HIV-1 pol. Briefly, the Reverse Transcription-Polymerase Chain Reaction (RT-PCR) was used to amplify the full-length protease gene in the pol region and the first 300 codons of the reverse transcriptase gene. The PCR products were dealt with electrophoresis with 1% agarose gel, and the amplified positive products were purified and sequenced by Beijing Genomics Research Center Ltd. The detailed amplification and sequencing were as previously described.
The results of the HIV gene sequence were spliced, cleaned and edited by software sequencer 4.9, then the results of the splicing were corrected by bioedit software, and compared with the Los Alamos HIV database reference sequence. It should be explained that to avoid interference caused by ART, 62 DRMs were removed from all sample sequences by comparing reference subtype standard-strain sequences,21 considering our study focusing on the effect of DRMs on network characteristics, the length of the total sequence was 876 bp. Then, the phylogenetic tree was constructed by kimura 2 parameter model by neighbor joining method of mega 6.0 software, and 1000 times of evolution tree were constructed again. Bootstrap was used to construct the phylogenetic tree 1000 times, the results showed that the accuracy of genotyping was verified by the value of more than 70%, and the genotyping was determined.
The molecular transmission network was constructed by grouping the subtypes of the strains. The approximate maximum likelihood tree (ML) with different subtypes as the external group was constructed by fasttree 3.0. The nucleotide substitution model was GTR + G + I, and the node (branch point) value of the evolutionary tree was calculated using the SH test embedded in the software. The constructed mL evolutionary tree was introduced into cluster picker 1.2.5. The spread clusters were extracted according to bootstrap value >95% and gene distance < 3%, and the sequence in the cluster was included in the subsequent analysis. The gene distance between the clusters was calculated using the software hyphy 2.2.4 in TN93 model, and the relationship between the two sequences was determined by the minimum gene distance method. As the gene distance when the number of clusters is the most, it is the gene threshold of the molecular network. The visualization of communication network is realized using cytoscape.
We determined the characteristics of the network, including nodes (individuals in the network), edges (the link between two nodes, representing the potential transmission relationship between the two individuals), degrees (the number of edges linking one node to other nodes), network sizes (the number of individuals in a cluster), and clusters (groups of linked sequences). Drug-resistant individuals in the network are shown in Figure 2.
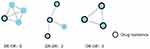 |
Figure 2 Resistance-drug transmission sketch map. |
 |
Figure 3 Continued. |
Data Collection
PLWH was screened and selected from the basic information system for AIDS Prevention and Control of the Sichuan Provincial Center for Disease Control and Prevention (CDC). Retrospective data were exported from their medical records in the system, including demographic characteristics (ie, ethnic, sex, age, and marital status), and HIV/AIDS-related characteristics (ie, route of HIV transmission, CD4+ T cell counts after diagnosis, disease stage, and sexually transmitted disease infection status).
Statistical Analysis
Univariate logistic regression analysis was used to identify risk factors associated with DR transmission in the genetic transmission network, and independent variables were drug-resistant mutation individuals in the study. Each DR subject was linked to two or more subjects, indicating the presence of the individuals with transmission risk in the network22 (1 = had more than 2 degrees in the genetic transmission network, 0 = ≤1 degree). Frequencies of demographic data (such as sociodemographic and AIDS-related characteristics) as the dependent variables were calculated. Multivariate logistic regression model was used to further test the significance of all micro-significant (P-value <0.1) variables in univariate logistic regression model (P-value <0.05). Statistical analysis was performed in SPSS 25.
HIV DR Transmission Network Among Cities
The network data were visualized using QGISv3.10 (QGIS.org (2020). QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.org). In the intensity matrix and flow graph, and the propagation network is visualized with different colors. Each PLWH is assigned to the city in which he/she lived and connected to other PLWH with infectious relationships through aside. The number of infectious diseases is represented by the color grading in the intensity matrix, and the darker color indicates that the two regions are more closely related to HIV infection. The graph shows the flowchart of the transmission network between cities, which is composed of edges representing HIV transmission links and edges with drug-resistant mutation links. The size of the circle represents the propagation intensity in the city, and the line width is measured by the spatial connection intensity consistent with the value of the spatial intensity matrix.
The Connection Degree of Drug-Resistant Mutation Sites in Subtypes
In each subtype virus cluster, the ratio of the number of links with common drug resistance mutation sites (DRMs) to the total number of total links in the network was analyzed. Significant differences in categorical variables were analyzed using chi-square tests. P values <0.05 indicated statistical significance.
Results
Characteristics of Transmission Networks
We used the HIV-1 transmission network to explore the risk associated with the potential transmission among PLWH with virological failure. Among the collected samples, 5926 were successfully sequenced, 5790 sequences were obtained after eliminating repeated sequences. HIV-1 transmission network was constructed and is shown in Figure 3. The most total number of clusters calculated threshold for each gene distance of the five main HIV-1 subtypes is 0.01, cluster picker identified 452 transmission clusters containing 2159 of 5790 patients (37.29%). Four hundred and fifty-two clusters were observed with the number of sequences per cluster ranging from 2 to 342. Of these clusters, 301 clusters had only two linked PLWH (one link), and 151 clusters had ≥2 potential transmission partners.
The network illustrates the risk-specific distribution of PLWH with ART virological failure. To explore the geographic dimension of HIV-1 transmission, among all 21 cities in Liangshan (16.67%), Yibin (15.28%), Neijiang (11.25%), Luzhou (10.33%) showed the largest number of PLWH, followed by Chengdu (7.87%), Deyang (7.69%). CRF07_BC accounted for 1107 (51.27%) nodes, CRF01_AE accounted for 566 (26.22%) nodes, CRF08_BC accounted for 290 (13.43%) nodes, CRF85_BC accounted for 171 (7.92%) nodes, and B subtype accounted for only 25 (1.16%) of the clustering nodes. Of the 452 networks, of which among the infection routes, 80.9% of cases occurred among heterosexual transmission, 6.4% among men who have sex with men (MSM), 9.4% among injection drug users (IDUs), 1.4% among sexual transmission and IDUs, and 1.6% among the mother to child transmission. In the network, 761 (35.25%) DR sequences were observed in the transmission network, among these, 647 subjects (13.36%) were linked to other DR sequences (DR-DR) (Figure 3D).
In the clusters within the molecular network, the largest four clusters accounting for 127 (5.88%), 156 (7.23%), 206 (9.54%) and 342 (15.84%) of nodes, respectively, the transmission route with the highest clustering rate was the heterosexual transmission, IDUs and MSM, moreover we found that drug injection and heterosexual contact were often cross-linked Figure 3C). Furthermore, there was one large cluster in the network (n = 127 persons), which mainly belonged to Yibin city (CRF85_BC) sequences. CRF07_BC has two large clusters that mainly belonged to Liangshan and Chengdu. Moreover, the largest CRF01_AE clusters reported the PLWH in the cities of Deyang, Neijiang, Chengdu, Dazhou were cross-linked. Additionally, the clusters were more linked within the city (Figure 3A).
The network clustering rate of drug-resistant PLWH in 21 cities in Sichuan Province is shown in Table 1. The total clustering rate in Sichuan Province was 37.29% (2159/5790), and the total drug resistance clustering rate was 13.14% (761/5790). The clustering rate of Deyang (69.17%, 166/240) and Neijiang (61.99%, 243/392) was much higher than the average clustering rate, while the clustering rate of Liangshan (20.88%, 360/1724) was the lowest in the whole province. Nanchong (24.84%, 38/153) and Neijiang (24.74%, 97/392) had the highest drug resistance clustering rate in the province, while the drug resistance clustering rate in five cities (ie, Chengdu, etc.) was only 7%.
 |
Table 1 Urban Distribution of the Individuals Clustering of DR |
Risk Factors of Drug-Resistant Clustering
Links (degrees) between each (node) for the subjects (nodes) ranged from 1 to 178. A total of 2443 PLWH were DR-associated individuals, 761 DR individuals (35.25%) in the transmission network, 373 of 2433 drug-resistant PLWH showed DR-associated transmission links≥2. Table 2 shows the sociodemographic and AIDS-related characteristics of the DR participants with ART virological failure.
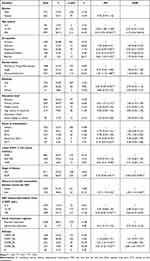 |
Table 2 Risk Factors of Drug Resistant Nodes with Drug Resistance-Associated Transmission Links ≥2 |
To explore factors associated with DR-associated transmission links≥2, we investigated the influence of 13 groups of dependent variables on DR transmission by using a logistic regression model (Table 2). For the univariate analysis, age, occupation, marital status, ethnicity, education level, route of transmission, latest CD4+ T cell counts, stage of disease, history of sexually transmitted diseases, time of ART, subtype were significantly associated with DR-associated transmission links≥2 (P < 0.05). However, no statistically significant difference was found in terms of the gender and initial treatment regimen (P > 0.05).
Multivariate logistic regression analyses showed that individuals with ≥2 links were more likely to be a history of sexually transmitted diseases (OR: 1.69, 95% CI: 1.15–2.48), IDUs (OR: 1.70, 95% CI: 1.05–2.76) and CRF85_BC subtype (OR: 2.86, 95% CI: 1.81–4.51). Additionally, employed (OR: 0.70, 95% CI: 0.51–0.97), AIDS-sufferer being involved in clinical stage (OR: 0.69, 95% CI: 0.53–0.89), ART time >36 years (OR: 0.66, 95% CI: 0.47–0.92) subjects were associated with lower likelihood of DR-associated transmission links ≥2.
The Geographic Dimension of HIV-1 DR Transmission Links
We have constructed spatial explicit transmission routes among PLWH with virological failure at the city scale (Figure 4). The strength of transmission links between cities was diverse. Specifically, the intensity matrix shows that the pattern of DR-DR links in the network is different from general transmission links.
In terms of general transmission links, among all 21 cities in Sichuan, Yibin had the strongest intracity links, followed by Neijiang, Liangshan and Deyang, while major cities which had cross-regional HIV-1 transmission were Chengdu Neijiang, Liangshan, Guang’an and Mianyang. The results showed that most cities had strong intracity links (eg, Neijiang and Yibin), and some cities also showed strong transmission links with other cities (eg, Mianyang and Chengdu) (Figure 4A).
In terms of DR-DR links, except for a few cities that have only intracity links such as Dazhou and Bazhong, Leshan (16/17), Yibin (126/134), and Neijiang (138/148) ranked the top three in terms of the proportion of intracity transmission links in all DR-DR links. As for the absolute quantity, Neijiang showed the largest number of local transmissions. For the cross-regional DR-DR link strength ratio, Ziyang (22/23) ranked first, followed by Guangyuan (12/13) and Mianyang (38/49). Totally, DR-DR links were mainly intracity transmission, but cross-regional transmission should not be ignored (Figure 4B).
Analysis Links of HIV DRMs with Subtype in Transmission Network
A total of 2159 nodes were observed in the transmission network, 761 of 2159 DR nodes (35.25%) at least linked one other node, among them 647 nodes (13.36%) were linked to common DRMs (Figure 3D). Table 3 lists all DR-DR links DRMs with five different subtypes among PLWH with virological failure, 12 of 37 DRMs had a significant difference in the subtypes (P < 0.001). In our analysis, the DRMs frequency to non-nucleoside reverse transcriptase inhibitors (NNRTIs, 93.20%, 603/647) was much higher than that to nucleoside reverse transcriptase inhibitors (NRTIs, 46.37%, 300/647) and protease inhibitors (PIs, 2.01%, 13/647), and the DRMs of PI had no significant difference in subtypes (P > 0.001). Analysis Links of HIV DRMs with subtype in transmission network, the highest rate of drug resistance was observed for subtype B (26.67%, 4/15), followed by CRF08_BC (18.22%, 92/505), CRF07_BC (15.49%, 306/1975), CRF85_BC (12.05%, 108/896) and CRF01_AE (9.45%, 137/1450). But, these DRMs largely resided in CRF07_BC (47.30%, 306/647), CRF01_AE (21.17%, 137/647), CRF85_BC (16.69%, 108/647) and CRF08_BC (14.22%, 92/647), there were few individuals of subtype B (0.62%, 4/647).
 |
Table 3 Links of HIV DRMs with Subtype in Transmission Network |
In 647 DR-DR links, K103N/S/E/Q was the most frequent DRMs, accounting for 45.60% (295/647), followed by M184V/I (27.20%, 176/647), Y181C (17.62%, 114/647), V179D/E (16.38%, 106/647), and K70R/E/T/S/Q/H/G (14.06%, 91/647). In DRMs of CRF07_BC, 164 links (8.30%) shared a common DRMs of K103N/S/E/Q in the NNRTI coding region and 81 links (4.10%) shared the common site of M184V/I in the NRTI coding region, furthermore, Q58E (0.51%, 10/1975) was the highest mutation probability in the PI coding region, and only detected in CRF07_BC. The most frequent DRMs in CRF01_AE was K103N/S/E/Q (3.17%, 46/1450); moreover, M184V/I (2.41%, 35/1450) was quite more. The most common DRMs in CRF08_BC was V108I (11.09%, 56/505), followed by Y181C (10.30%, 52/505), M184V/I (10.30%, 52/505) and K70R/E/T/S/Q/H/G (10.10%, 51/505), in addition, the mutation frequency of D67N (7.13%, 36/505), T215F/Y/S (8.91%, 45/505), V108I were significantly higher than that of other subtypes. Although there were few kinds of mutation sites of CRF85_BC, they were mostly concentrated in K103N/S/E/Q (5.58%, 50/896), V179D/E (3.46%, 31/896). It was suggested that the linked sequences with DR tended to have common DRMs and they were interlinked, indicating potential transmitted DR.
Discussion
We used HIV transmission network analysis to characterize DR transmission links of those infected with ART virological failure. We found the characteristic determining DR phylogenetic clustering and multiple links in the genetic transmission networks. Furthermore, we found that the spatial distribution of DR phylogenetic clustering was mainly clustered in Southern of Sichuan, except for Liangshan. The links within and between the cities, and the strength of the transmission connection are fully expounded. CRF07_BC was the most widespread subtype, while CRF85_BC (66.3%) showed the highest clustering rate. DR-DR links among the transmission network showed that there was a significant correlation between subtypes and common DRMs.
The HIV-1 transmission network is concentrated, four networks contained 831 (38.49%) individuals, with the largest network containing 342 individuals. Among this network, 761 DR subjects were observed, further analysis showed that 373 (49.01%) of individuals had more than 2 DR-DR links, indicating the presence of the individuals with transmission risk in the network,22 187 (24.57%) subjects had more than 4 links and were considered high-risk spreaders and more likely to spread HIV-1, one individual had 178 links and was considered a super spreader, which differs from those in other provinces of China.11 This super spreader is fit with previous studies that individuals with more linkages may have a higher transmission risk because of their high viral load and high risk behavior (ie, inject drug),22–25 therefore, these individuals may function as super-spreaders. Most of the individuals linked with the super-spreader were from Liangshan, but they had intercity connections with 14 regions. This finding reveals the existence of a wide spread transmission network of PLWH with virologically failed drug resistance.
Multivariate regression analysis shows among that those who had a history of sexually transmitted diseases, IDUs and CRF85_BC subtype are more likely to be potential drug-resistant spreaders. PLWH had a history of sexually transmitted diseases, and clustering may be prone to be formed, because of the high-risk behavior features of this population, including multiple sex partners, low rates of condom use.19,26 Those who inject drug use have more active high-risk behavior, and who reported previous or current injecting drug use were statistically significantly associated with higher rates of antiretroviral resistance,24 therefore, they have a higher risk of drug resistance transmission. The PLWH of CRF85_BC due to the short time of formation and transmission in Sichuan, and most of the infected people were concentrated in one area,27 so, clustering was more likely to occur in the transmission network. This result was similar to that of previous study that different HIV-1 subtypes can affect the presence of drug resistance.28 In Sichuan, subtype CRF07_BC and CRF01_AE subtypes were the main epidemic strains, and studies had shown that the possibility of resistance was higher in CRF01_AE subtypes,19 but CRF85_BC was the subtype with the highest DR phylogenetic clustering rate in our study. This finding reflects the characteristics of HIV-1 DR transmission, which differ from those in other provinces of China,22,29 and reveals why CRF85_BC has been widely disseminated in Yibin region recently. Since subtype CRF85_BC is mainly found in elderly people infected by non-married heterosexual transmission as a previous report,27 which might be attributed to the hidden high-risk populations (eg, sex workers). Some cities (eg, Luzhou) showed the spread trend except for Yibin; hence, additional molecular epidemiology surveillance is required for CRF85_BC.
Besides, we find that employed, AIDS-sufferer being involved in clinical stage, ART time >36 years were protective factor for the spread of drug resistance. Compared with farmers, employed patients have higher opportunities for health education than farmers, so the risk of transmission was reduced and further drug resistance was reduced. For AIDS stage patients, the transmission intensity of patients was limited. The patients in this stage may have various complications, which affected their activities, so the transmission rate of drug resistance is reduced. And that time of ART suggests that ART plays an important role in inhibiting drug-resistant transmission, emphasizing the need for early diagnosis and timely antiretroviral treatment.
High drug-resistant clustering rates of PLWH were distributed in Southern and Northeast Sichuan Province, which reflected the spread of DR in these areas. Previous studies had shown that regions for relatively severe HIV-1 transmission dynamics might be due to human mobility, relatively developed traffic, and strong economic activity,30,31 notably, regions with high drug-resistant clustering rates of PLWH who virological failure for ART may not be matched for regions with severe epidemics in this study, it may be related to PWLH in southern Sichuan was mainly of age ≥50, as ART virological failure usually occurs the elderly people.14,19 As the worst-hit HIV epidemic region in China,14 Liangshan had a low drug resistance phylogenetic clustering rate (7.54%), which may be attributed to poor traffic and economic activity. Besides, China’s 13th five-year-plan has achieved huge effect for PLWH in Liangshan,32 to a certain extent, inhibited the spread of HIV, thus affecting the DR phylogenetic clustering rate. In contrast, several of the cities showed higher DR phylogenetic clustering rates with less severe HIV epidemics, such as Neijiang. The result reflects that regions, where the HIV epidemic is not severe in the past, may not be particularly concerned for possible high DR phylogenetic clustering rates, which should arouse attention. Furthermore, previous research proved that spatial clustering can be used to show the geographic transmission of HIV.33 The high drug resistance phylogenetic clustering rate regions in our study provide a new focus for our geographic intervention.
Exploring the geographic dimension of HIV-1 DR transmission among PLWH with virological failure, we found Liangshan exhibited the most linkage with the other cities. Interestingly, the major cities of cross-regional HIV-1 transmission with Liangshan are Mianyang and Ziyang, and the geographical distance between Liangshan from the two places is relatively far apart, it may be related to the fact that Ziyang compulsory detoxification center of Sichuan Province guards many IDUs from Liangshan. However, the intensity of transmission of these neighboring cities to Liangshan may not be that strong enough, which indicated a noteworthy long-distance strong interaction. Besides, Yibin, Neijiang, Luzhou showed obvious characteristics of intracity DR-DR links intensity ratio, among these cities with more than 40 DR-DR links, suggesting that there was more likely regional transmission in these areas. Furthermore, among the transmission network, most of DR (85.02%) subjects were linked to other DR sequences, indicating that PLWH with drug resistance were more likely to link with those with drug resistance. Of course, there may be deeper social factors that determine the spatial flow of the virus. As a previous study has indicated, the increase of human exposure and the increase in population mobility lead to the high risk of disease transmission among numerous urban population, cities become incubators that meet all the conditions of the outbreak, and urbanization may lead to the strengthening of disease transmission links between cities in the future.34
We analyzed the links of DRMs in the transmission network to further confirm the transmission of DR in PLWH with virological failure. Six hundred and forty-seven of 761 links (85.02%) were linked to common DRMs, suggesting that HIV-1 resistant strains would spread out. The most common linked DRMs found in our study were directed against at NNRTIs, which is consistent with this category drug widely used as standard first-line China’s National ART Guidelines,35,36 the more widespread use of NRTIs and NNRTIs may be the major cause of the higher prevalence of resistance to NNRTI than to PI in Sichuan. In our study, it was found that the drug resistance mutation of DR-DR links in the network significantly differ among the five main subtypes, 12 of 37 drug resistance mutation sites had significant difference (P < 0.001), which was different from the results of previous study in other region.35 The results showed that different subtypes of HIV-1 acquired different drug-resistant mutation sites could occur when exposing them to ART for long term, and these mutation sites may be stably passaged; thus, it provides some clues of adaptability of the virus and renewal of the ART regime.
Limitations
The study has some limitations. First, to compare the drug-resistant links with different subtypes, we selected the threshold value of the highest cumulative cluster number of five subtypes, which may have some deviation from CRF85_BC, for the subtype is prevalent in Sichuan for a short time. Second, we only used the sequences of Sichuan province, which may not fully reflect the actual situation of drug-resistant transmission, thus, may strengthen regional cooperation especially in Chongqing, which is the province with the most cross-regional HIV-1 transmission of Sichuan; therefore, the result provides direction, which may strengthen regional cooperation. Third, a potential sampling bias, we could analyze only the individuals with ART virological failure, but those that ART naive newly diagnosed individuals could not be included in the analysis, there may be the possibility of the same drug resistance mutations after ART, therefore, to avoid the interference caused by ART drug-resistant mutation, 62 drug-resistant mutation sites were removed from all sample sequences by comparison reference subtype standard-strain sequences.
Conclusions
This study shows that transmission network links analysis based on molecular methods can be used to explore the HIV-1 drug-resistant spread influencing factors and transmission patterns of PLWH with ART virological failure. Additionally, we found regions with high drug-resistant transmission of PLWH may not be matched for regions with severe epidemics. Using the network links to infer the patterns of HIV transmission and identify the high-risk populations of drug resistance, the spatial distribution and clustering patterns of the clustering and spatial links of HIV-1 between cities showed the geographic variations in HIV-1 drug-resistant transmission and high-risk areas. Our findings will be helpful in strengthening the evidence-based to drug-resistant transmission targeting interventions.
Ethics Approval
This study was approved by the local Ethics Committee at Sichuan Center for Disease Control and Prevention, according to the Helsinki II Declaration. All participants in this study provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, CZ and DY designed the study; RK and TF participated in the spatial clustering analysis process; YL, YZ, LL and LY participated in the process of sequences editing and phylogenetic analyses; CZ and DY performed statistical analysis; SL, DY and LS collected sample and the demographic data; CZ, RK and DY participated in the writing process. All authors have agreed on the journal to which the article has been submitted.
Funding
This study was supported by grants from the Scientific research project of Sichuan Health and Health Commission (20PJ121) and Sichuan Science and Technology program (2020YJ0449).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Union: AIDS. Available from: https://www.un.org/zh/sections/issues-depth/aids/index.html.
2. Piot P, Bartos M, Ghys PD, et al. The global impact of HIV/AIDS. Nature. 2001;410(6831):968–973. doi:10.1038/35073639
3. Cohen MS, Ying QC, Mccauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):1934–1935. doi:10.1056/NEJMoa1105243
4. Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4). doi:10.1001/jama.2012.7961
5. Phillips AN, Cambiano V, Miners A, et al. Effectiveness and cost-effectiveness of potential responses to future high levels of transmitted HIV drug resistance in antiretroviral drug-naive populations beginning treatment: modelling study and economic analysis. BMC Infect Dis. 2014;1(2):e85–e93. doi:10.1016/S2352-3018(14)70021-9
6. Liao L, Xing H, Su B, et al. Impact of HIV drug resistance on virologic and immunologic failure and mortality in a cohort of patients on antiretroviral therapy in China. Aids. 2013;27(11):1815–1824. doi:10.1097/QAD.0b013e3283611931
7. Li X, Fang XD, Mao R, et al. HIV/STD risk behaviors and perceptions among rural-to-urban migrants in China. AIDS Educ Prev. 2004;16(6):538–556. doi:10.1016/j.physletb.2004.01.084
8. Xiao C, Liu D, Jia P, et al. The changing modes of human immunodeficiency virus transmission and spatial variations among women in a minority prefecture in southwest China: an exploratory study. Medicine. 2020;99(6):e18776. doi:10.1097/MD.0000000000018776
9. Hassan AS, Pybus OG, Sanders EJ, et al. Defining HIV-1 transmission clusters based on sequence data. AIDS. 2017;31(9):1211–1222. doi:10.1097/QAD.0000000000001470
10. Jean LF, Domercant JW, Ignacio C, et al. High prevalence of HIV-1 drug resistance and dynamics of transmission among high-risk populations in Port-Au-Prince, Haiti. J Acquir Immune Defic Syndr. 2020;85(4):416–422. doi:10.1097/QAI.0000000000002475
11. Zheng M, Yu M, Cheng S, et al. Characteristics of HIV-1 molecular transmission networks and drug resistance among men who have sex with men in Tianjin, China (2014–2018). Virol J. 2020;17(1). doi:10.1186/s12985-020-01441-8
12. Jing W, Cui H, Hsi JH, et al. Virological outcomes and drug resistance in Chinese patients after 12 months of 3TC-based first-line antiretroviral treatment, 2011–2012. PLoS One. 2014;9(2):e88305. doi:10.1371/journal.pone.0088305
13. Kan W, Teng T, Liang S, et al. Predictors of HIV virological failure and drug resistance in Chinese patients after 48 months of antiretroviral treatment, 2008–2012: a prospective cohort study. BMJ Open. 2017;7(9):e16012. doi:10.1136/bmjopen-2017-016012
14. Yuan D, Liu M, Jia P, et al. Prevalence and determinants of virological failure, genetic diversity and drug resistance among people living with HIV in a minority area in China: a population-based study. BMC Infect Dis. 2020;20(1). doi:10.1186/s12879-020-05124-1
15. Namale G, Kamacooko O, Bagiire D, et al. Sustained virological response and drug resistance among female sex workers living with HIV on antiretroviral therapy in Kampala, Uganda: a cross-sectional study. Sex Transm Infect. 2019:2018–53854. doi:10.1136/sextrans-2018-053854
16. Gupta-Wright A, Fielding K, van Oosterhout JJ, et al. Virological failure, HIV-1 drug resistance, and early mortality in adults admitted to hospital in Malawi: an observational cohort study. Lancet HIV. 2020;7(9):e620–e628. doi:10.1016/S2352-3018(20)30172-7
17. Meriki HD, Tufon KA, Afegenwi MH, et al. Immuno-haematologic and virologic responses and predictors of virologic failure in HIV-1 infected adults on first-line antiretroviral therapy in Cameroon. Infect Dis Poverty. 2014;3(1):5. doi:10.1186/2049-9957-3-5
18. Yuan D, Du Z, Zhou J, et al. HIV-1 subtype diversity, drug resistance, and genetic transmission networks in men who have sex with men with virologic failure in antiretroviral therapy in Sichuan, China, 2011 to 2017. Medicine. 2019;98(43):e17585. doi:10.1097/MD.0000000000017585
19. Dong K, Ye L, Leng Y, et al. Prevalence of HIV-1 drug resistance among patients with antiretroviral therapy failure in Sichuan, China, 2010–2016. Tohoku J Exp Med. 2019;247(1):1–12. doi:10.1620/tjem.247.1
20. Zhang F, Pan J, Yu L, et al. Current progress of China’s free ART program. Cell Res. 2005;15(11):877–882. doi:10.1038/sj.cr.7290362
21. Wertheim JO, Kosakovsky Pond SL, Forgione LA, et al. Social and genetic networks of HIV-1 transmission in New York City. PLoS Pathog. 2017;13(1):e1006000. doi:10.1371/journal.ppat.1006000
22. Pang X, Wei H, Huang J, et al. Patterns and risk of HIV-1 transmission network among men who have sex with men in Guangxi, China. Sci Rep. 2021;11(1). doi:10.1038/s41598-020-79951-2
23. Arruda GD, Barbieri AL, Rodriguez PM, et al. Role of centrality for the identification of influential spreaders in complex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2014;90(3):32812. doi:10.1103/PhysRevE.90.032812
24. Werb D, Mills EJ, Montaner J, et al. Risk of resistance to highly active antiretroviral therapy among HIV-positive injecting drug users: a meta-analysis. Lancet Infect Dis. 2010;10(7):464–469. doi:10.1016/S1473-3099(10)70097-9
25. Xi C, Mo P, Jinghua L, et al. Factors associated with drug use among HIV-infected men who have sex with men in China. AIDS Behav. 2020;24(6):1612–1620. doi:10.1007/s10461-019-02660-z
26. Chen L, His JH, Wu X, et al. Disparities in HIV and syphilis prevalence and risk factors between older male clients with and without steady sex partners in southwestern rural China. BMC Infect Dis. 2017;17(1):269. doi:10.1186/s12879-017-2367-z
27. Su L, Feng Y, Liang S, et al. The origin and spread of CRF85_BC, driven by heterosexual transmission among older people in Sichuan, China. BMC Infect Dis. 2020;20(1). doi:10.1186/s12879-020-05488-4
28. Zhang F, Li L, Sun M, et al. An analysis of drug resistance among people living with HIV/AIDS in Shanghai, China. PLoS One. 2017;12(2):e165110. doi:10.1371/journal.pone.0165110
29. Huang L, Wu H, Yan H, et al. Syphilis testing as a proxy marker for a subgroup of men who have sex with men with a central role in HIV-1 transmission in Guangzhou, China. Front Med. 2021;8. doi:10.3389/fmed.2021.662689
30. Bulstra CA, Hontelez J, Giardina F, et al. Mapping and characterising areas with high levels of HIV transmission in sub-Saharan Africa: a geospatial analysis of national survey data. PLoS Med. 2020;17:e1003042. doi:10.1371/journal.pmed.1003042
31. Eshraghian EA, Ferdos SN, Mehta SR. The impact of human mobility on regional and global efforts to control HIV transmission. Viruses. 2020;12(1):67. doi:10.3390/v12010067
32. Han M. Forty years of fighting AIDS, we are on the road to ending the AIDS epidemic. Chinese J Exp Clin Virol. 2021;35(2):3. doi:10.3760/cma.j.cn112866-20210312-00046
33. Ratmann O, Kagaayi J, Hall M, et al. Quantifying HIV transmission flow between high-prevalence hotspots and surrounding communities: a population-based study in Rakai, Uganda. Lancet HIV. 2020;7(3):e173–e183. doi:10.1016/S2352-3018(19)30378-9
34. Alirol EP, Getaz LM, Stoll BM, et al. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis. 2011;11(2):131–141. doi:10.1016/S1473-3099(10)70223-1
35. Lu X, Zhao H, Zhang Y, et al. HIV-1 drug-resistant mutations and related risk factors among HIV-1-positive individuals experiencing treatment failure in Hebei Province, China. AIDS Res Ther. 2017;14(1). doi:10.1186/s12981-017-0133-3
36. Yuan D, Liu M, Li Y, et al. Genetic transmission networks of HIV-1 CRF07_BC strain among HIV-1 infections with virologic failure of ART in a minority area of China: a population-based study. BMC Infect Dis. 2020;20(1). doi:10.1186/s12879-020-05347-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


