Back to Journals » Cancer Management and Research » Volume 11
Risk factors of catheter-related thrombosis in early-stage breast cancer patients: a single-center retrospective study
Authors Tan L, Sun Y, Zhu L, Lei X, Liang D, Rao N, Su F, Chen K, Li S
Received 17 April 2019
Accepted for publication 19 August 2019
Published 13 September 2019 Volume 2019:11 Pages 8379—8389
DOI https://doi.org/10.2147/CMAR.S212375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Luyuan Tan,1,2,* Ya Sun,3,* Liling Zhu,1,2,* Xin Lei,4 Dongya Liang,5 Nanyan Rao,1,2 Fengxi Su,1,2 Kai Chen,1,2 Shunrong Li1,2
1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China; 2Department of Breast Surgery, Breast Tumor Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 3Department of Breast Oncology, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 4State Key Laboratory of Oncology in South China, Guangzhou, People’s Republic of China; 5Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shunrong Li; Kai Chen
Department of Breast Surgery, Breast Tumor Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, 107 Yanjiang West Road, Guangzhou 510120, People’s Republic of China
Tel +86 203 407 0091
Email [email protected]; [email protected]
Purpose: Totally implantable venous access devices (TIVADs) are widely used in cancer patients. The main purpose of our study is to observe the incidence and identified risk factors of catheter-related thrombosis (CRT) in breast cancer patients with TIVAD.
Patients and methods: We performed a retrospective cohort study of consecutive breast cancer patients who received the ultrasound-guided TIVAD implantation for the administration of chemotherapy from 2013 to 2016. The primary outcome was CRT (both symptomatic and asymptomatic detected by ultrasound). Univariable and multivariable logistic regression analyses were used to identify the risk factors for breast cancer TIVAD-related CRT.
Results: A total of 209 breast cancer patients with a newly implanted TIVAD for chemotherapy were included in this study. The average time of port duration was 7 months. Of the enrolled 209 patients, 33 patients (15.8%) had CRT, 2 of the 33 cases were symptomatic (1 pulmonary embolism, 1 deep-venous thrombosis [DVT]), the other 31 cases were asymptomatic detected by routine ultrasound examination of the catheter-associated vein before TIVAD removal with all cycles of chemotherapy completed. In total, 19 (57.6%) of CRT patients underwent directly TIVAD removal without any further treatments, 14 patients received anticoagulation treatments for 3–30 days followed by TIVAD removal. No DVT event was observed within at least 1.5 years of follow-up. In the multiple-variable analysis, tumor size >2 cm (OR 2.735, 95% CI 1.042–7.177; P=0.032), positive HbsAg (OR 2.803 95% CI 1.027–7.856; P=0.047) and low-density lipoprotein (LDL) >3.6 mmol/L (OR 2.360, 95% CI 1.059–5.351; P=0.040) were the significant independent risk factors of breast cancer TIVAD-related CRT.
Conclusion: CRT is a common complication in breast cancer patients with TIVAD for chemotherapy. Tumor size, HbsAg status and LDL level were independent predictors of breast cancer for TIVAD-related CRT. Removal of the port without anticoagulation treatments might be a feasible choice for asymptomatic TIVAD-related CRT.
Keywords: deep-venous thrombosis, hepatitis B virus, breast cancer, totally implantable venous access devices
Introduction
Patients with breast cancers often require long-term repeated administrations of chemotherapy. Most chemotherapeutic agents can induce venous toxicity and often lead to venous thromboembolism (VTE) or thrombophlebitis if peripheral veins are used. In contrast, applying central venous catheters (CVCs) in such patients not only reduces venous toxicity but also can be used continuously in all chemotherapy cycles.1,2 In 1982, totally implantable venous access devices (TIVADs), also known as portacaths or “ports,” were introduced.3 Later studies had demonstrated TIVAD to be an alternate choice of safe and reliable central venous access with improved convenience and cosmetic acceptability.4,5
VTE related to CVC or TIVAD was defined as catheter-related thrombosis (CRT).6 CRT is a frequent complication including deep-venous thrombosis (DVT) and pulmonary embolism (PE) in cancer patients with implanted port receiving chemotherapy.7 PE could be a fatal strike to a cancer patient and DVT sometimes leads to limbs swelling and TIVAD occlusion removal.6 Thus, the meaning of preventing CRT is significant.
In Saber’s meta-analysis8 including 5636 subjects from 5 RCTs and 7 prospective studies, a history of DVT, subclavian venipuncture site, improper catheter tip location and PICC versus port were identified as the risk factors of CRT for cancer patients. Recently, the incidence of CRT had been decreased since the modifications in catheter composition, coating and the improvement in inserting operation technique.9 In 2015, Leung et al10 summarized 25 articles to find out the consistent risk factors of CRT reported by different studies. However, there were no consistent significant associations for CRT across the 25 studies. The inconsistent conclusion raised our attention to the heterogeneity of cancer sites and study populations.
Endocrine therapy drugs for breast cancer such as tamoxifen affect the coagulation function and lipid metabolism of the patients,11 which might probably influence the incidence of CRT. The incidence and risk factors of developing CRT in breast cancer patients using TIVAD are so far unclear. Therefore, the main purpose of our study is to observe the incidence and identified risk factors in breast cancer patients with TIVAD. Meanwhile, the management and follow-up outcomes of these patients will also be discussed.
Methods
Data source
We used data from the Redcap database created in 2014 of Sun Yat-sen Memorial Hospital Breast Tumor Center, a tertiary hospital located in Guangzhou, China. Patients were mostly from the southern area of China. Patients’ information was prospectively collected by the specialized research assistants after obtaining the informed consent from the patients. The cutoff date of this study is December 31, 2018. The study protocol was censored and approved by the Institutional Ethics Committee of Sun Yat-sen Memorial Hospital (approval number: SYSEC-KY-KS-024). Patient consent was achieved by informed consent forms. The study was conducted in accordance with the Declaration of Helsinki.
Study population and materials
All breast cancer cases who received a TIVAD for chemotherapy were retrieved. Eligibility criteria included pathologically diagnosed breast cancer between 2013 and 2016, age between 18 and 80 years, no prior history of DVT, no previous history of breast cancer or chemotherapy and no prior history of TIVAD implantation.
For each enrolled case, age, body mass index (BMI), TNM stage, pathology type, estragon receptor (ER) status, progestogen receptor (PR) status, human epidermal growth factor receptor-2 (HER-2) status, surgery type, chemotherapy type, anticoagulation treatment, TIVAD brands, interval time of implantation and removal, baseline blood test such as white blood cell (WBC) count, platelet count (PLT), hemoglobin (Hb), alanine transaminase (ALT), aspartate transaminase (AST), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), triglycerides (TGs), prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer, hepatitis B surface antigen (HbsAg) status, comorbidities and information of the CRT were retrieved. BMI, WBC, Hb and PLT were classified based on the Khorana score system.12 All the variables reported in our study were completely measured in all participants.
The procedure of TIVAD implantations and maintenance
According to the standard procedure of our center, the internal jugular vein (IJV) was considered a priority without abnormal conditions (internal jugular vein thrombosis, tumor infiltration or infection, puncture failure). Three brands of ports, Bard (Bard Limited, Salt Lake City, USA), Braun (Braun Melsungen AG, Chasseneuil, France) and Medicom (A. R. Medicom, Shanghai, China), were chosen according to the surgeon’s preference. Patients were placed in a supine position on the operation table. The pectoral region of the planned implantation side, as well as the collar region, was scrubbed with betadine and a maximum sterile barrier precaution was provided. In order to prevent clot formation and catheter blockage, TIVAD was flushed with 20 mL saline before implantation. Local anesthesia was administrated subcutaneously, restricted to the port implantation and the venous puncture area. The IJV was punctured on average 2–3 cm above the clavicle with an 18-gauge needle and under continuous ultrasound guidance. Seldinger technique was applied to insert the catheter of the TIVAD. The catheter was tunneled to connect to the port placed and sutured in an infraclavicular subcutaneous pocket (2–3 cm caudal to the clavicle). 5 mL of a solution containing 50 U/mL heparin was injected into the TIVAD to prevent clot formation. After the implantation, the correct positioning of the tip of the catheter was confirmed by X-ray to be localized in the vena cava superior.
Experienced nurses flushed the TIVAD every 4 weeks with 10 mL of a heparin-sodium solution 100 IE/mL or at the end of each cycle of chemotherapy infusions to maintain patency. No prophylaxis anticoagulant was given during chemotherapy. No attempt was made to detect occult thrombosis during chemotherapy. Symptomatic thrombosis was treated with TIVAD removal and appropriate anticoagulant when appeared. Within 1 month after completing chemotherapy, we used ultrasound to examine the catheter-associated vein (IJV in the study population) before removing the TIVADs to detect CRT existence. Treatment of the asymptomatic CRT discovered before the TIVAD removal was based on the clinical practice of each physician.
Statistics analysis
The logistic regression analysis was adopted to identify the risk factors of CRT and a stepwise method was used to develop the multivariate predictive model. Chi-square test or Fisher’s exact test was applied to identify the appearance of the CRT in different risk levels of both our model and Khorana model.12 A two-sided level of significance of 0.05 was applied. All of the statistical analysis was performed using STATA (Version 13.0).
Results
Patient’s characteristics
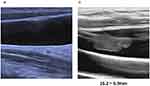
From 2013 to 2016, a total of 209 consecutive patients in our center with pathologically diagnosed breast cancer receiving TIVAD implantation were enrolled. All patients’ clinicopathological characteristics are summarized in Table 1. The median (range) age of the population was 48.2 (46.7–49.6) years. The TIVADs of our study patients were all implanted in the IJV method. The ports and catheters were removed after completing all cycles of chemotherapy with an average port duration time of 7 months. A total of 33 (15.8%) patients had CRT in the period of chemotherapy, among which one patient presented as PE and ultrasound detected IJV mural thrombosis, one was discovered to develop IJV thrombosis due to TIVAD device malfunctioning, others were asymptomatic and identified by the routine ultrasound examination before TIVAD removal when chemotherapy was completed. All the CRT was attached to the catheter surface and part of the IJV vessel wall (Figure 1).
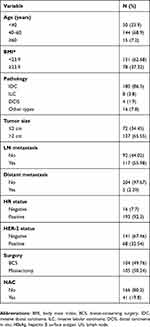 |
Table 1 Patients’ characteristics |
Treatment of patients with CRT
The case with PE was transferred to the angiology surgery department immediately for intensive care and anticoagulation treatment with the TIVAD removal. The patients with TIVAD malfunctioning accepted one-week rivaroxaban ahead of the port removal. Among the other 31 patients with CRT detected before TIVAD removal, 19 (61.29%) removed TIVAD directly, while 12 (38.71%) received 3–30 days of anticoagulation (rivaroxaban, enoxaparin or aspirin) followed by TIVAD removal (Table 2). Until the study cutoff date (December 31, 2018), none of the 33 patients progressed to any symptomatic DVT within at least 1.5 years of follow-up.
 |
Table 2 Treatment to patients with catheter-related thrombosis |
Risk factors of CRT
In the univariate analysis, tumor size >2 cm (OR 2.700, 95% CI 1.059–6.883; P=0.037), positive HbsAg (OR 3.200, 95% CI 1.177–8.702; P=0.023) and LDL >3.6 mmol/L (OR 2.283, 95% CI 1.042–4.999; P=0.039) was associated with breast cancer CRT. Age, BMI, breast cancer characteristics (LN metastasis status, distant metastasis status, HR status, HER-2 status and pathology type), treatments (surgery type, neoadjuvant chemotherapy or not), TIVAD-related factors (brand, inserting duration) and baseline laboratory results (Hb, WBC, PLT, AST, ALT, LDL, HDL, TC, TG, PT, APTT and D-dimer) were not associated with CRT (Table 3). BMI, WBC, Hb and PLT were all reported as risk factors for VTE in cancer patients according to the Khorana score system.12 Then, we select BMI, WBC, Hb and PLT into the stepwise multivariate analysis with the three significant univariate risk factors.
 |  |  |
Table 3 Univariate and multivariate logistic regression of risk factors for catheter-related thrombosis |
Tumor size >2 cm (OR 2.735, 95% CI 1.042–7.177; P=0.032), positive HbsAg (OR 2.803, 95% CI 1.027–7.856; P=0.047) and LDL >3.6 mmol/L (OR 2.360, 95% CI 1.059–5.351; P=0.040) were confirmed to be significant risk factors for breast cancer CRT (Table 3). Among the 21 HBsAg-positive patients, CRT was observed in 5 out of the 13 (38.5%) patients receiving anti-HBV treatment and 2 out of the 8 (20%) without anti-HBV treatment (Fisher's exact test P=0.223).
Predictive models for CRT in breast cancer patients
We assigned points for each risk factor based on the regression coefficients attained from the final model constructed with tumor size, HbsAg status and LDL level. Study individuals were divided into 3 risk categories based on the score from our breast cancer CRT risk model (BC-RCT model): low (score 0), intermediate (score 1) and high risk (score 2–3; Table 4).
 |
Table 4 Breast cancer catheter-related thrombosis risk score |
Rates of CRT the cohort classified by BC-RCT model were 6.25% in low-risk, 12.17% in intermediate-risk and 34.78% in high-risk category. The Fisher's exact test showed a significant P-value of 0.001 (Table 5). We also classified risk of CRT of the study population based on the classic cancer VTE risk predicted model Khorana score12 including factors such as cancer site (breast cancer was not considered as high-risk site; point 0 for all patients), BMI >35 kg/m2 (none in our cohort; point 0 for all patients), PLT >350×109/L, Hb<100 g/L and WBC >11×109/L (1 point each). The Fisher's exact test showed a significant P-value of 0.503 for the Khorana model (Table S1).
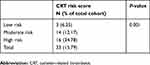 |
Table 5 Catheter-related thrombosis stratified by CRT risk score and Khorana model |
Discussion
Incidence of CRT
The incidence of CRT varied considerably between 12% and 74% in different types of cancer. Tumor subtypes, stages of diseases, chemotherapy regimens and catheter-related factors were the major determinants of the occurrence of CRT.13 In studies examining the TIVAD-associated complications in cancer patients, the rate of symptomatic CRT varied from 1.06% to 12.8%.5,14–17 The incidence of undefined CRT was 4–7% in breast cancer patients.18,19 With prophylactic anticoagulant treatment, the incidence dropped to 0.6–2%.19,20 In our study, the incidence of CRT in breast cancer patients was 15.79% (33/209) without any prophylactic anticoagulant treatments, while 0.9% (2/209) of patients with CRT were symptomatic. A possible explanation for the higher rate of CRT in our study was that ultrasound was routinely used to screen for thrombosis before TIVAD removal when chemotherapy was completed, whereas other studies did not.18,19 Also, the high prevalence of HBV in the Chinese population may account for the increased incidence of CRT. However, the incidence of symptomatic CRT remains, even without prophylactic anticoagulant treatments in our study.
CRT risk factor
To the best of our knowledge, this is the first study to explore the risk factors of breast cancer patients with TIVAD developing CRT, and those who developed CRT had significantly larger tumor size (OR 2.735), positive HbsAg (OR 2.803) and high serum-level of LDL (OR 2.360).
HBV infection is a major worldwide public health problem with nearly 350 million infected cases, while the HBV-infected patients in China account for one-third of the cases in the world.21 All of the patients in our study had normal liver and coagulation functions. None of them had a history of liver cirrhosis or liver failure. The incidences of thrombosis were not significantly different between the HBsAg-positive patients with and without anti-HBV drugs, probably due to the small sample size. Some randomized trials also demonstrated that these anti-HBV drugs did not increase the risk of thrombosis formation.22–24 The underlying mechanism of how HBV infection leads to a higher rate of CRT was still unclear. Results from several studies reveal the plausible hypothesis. Xiang et al25 showed in rat models that early thrombus formation may transform to a catheter sleeve, which consisted of smooth muscle cells and collagen covered by endothelial cells to provide an ideal place for HBV to colonize. An evidence of HBV colonized in the vessel was a study reported by Mason et al.26 They investigated the extrahepatic tissues of 4 patients who died of chronic HBV infection and two uninfected controls using in situ hybridization and immunohistochemical staining, observing the existence of HBV in the vascular endothelium. In addition, a previous study27 showed that patients with inactive chronic hepatitis B disease tend to have an increased rate of PLT activation accounting for higher risk of thrombosis. PLT activation was defined as the increased mean PLT volume other than the increased number of PLT, which explained the irrelevant relationship of PLT count and thrombosis in our study.
Another interesting risk factor discovered by our study was tumor size, and patients with breast cancer >2 cm were at higher risk of developing CRT. Cancer increased the incidence of VTE (4–20%) and arterial thrombosis (2–5%)12,28 by several pathways. Increased levels of leukocytes,29 platelets,30 secretion of thrombosis-associated cytokines such as P-selectin31 and tissue factor–positive (TF+) microvesicles32 are all potential factors that alone or in combination could increase cancer-associated thrombosis. Larger tumors allow more active cancer cells to involve in the intricate procoagulation procedure. Besides, the mechanical compression from a larger tumor tend to slow the local blood speed which may lead to a higher risk of thrombosis.28
In previous studies, the association between venous thrombosis and dyslipidemia had been demonstrated.33–35 Our study found that high serum level of LDL was associated with CRT, and consistent results were also reported in studies on VTE risk factors.34,35 But the relation with CRT was not observed in HDL, TC and TG, which was inconsistent with other studies that showed that low level of HDL,33,34 hypercholesterolemia36 and high level of TG33 were strong predictors for VTE. However, the relationship between high levels of TGs, TC, LDL cholesterol and VTE is controversial, due to different study populations (with regard to disease, age and sex) and study designs. Our study mainly focused on the population of breast cancer patients with TIVAD, so the risk factors identified were specific for predicting the risk of CRT in this population.
The Khorana score was the most accepted risk model developed to improve stratification of VTE risk based on 5 risk factors in cancer patients planning on receiving chemotherapy.12 We also tried to validate the ability of Khorana score to predict CRT in breast cancer patients with TIVAD. In our patient cohort, there was no statistically significant difference in overall CTR rates between Khorana high-risk, intermediate-risk and low-risk patients. First of all, Khorana score was developed for all cancer patients for cancer-related VTE. Although CRT is an important component of cancer-related VTE, the related risk factors of these two could be different. Site of cancer is an important predictor of Khorana score. However, the risk of breast cancer to develop VTE was comparably low according to the Khorana score (score 0). Also, none of the patients in our cohort had a BMI >35 (mean 22.7). Besides, the appearance of leukemoid reaction and thrombocytosis were more often observed in gastrointestinal and lung cancer than breast cancer, and most of the cohort patients were in the early stage which leads to a low rate of anemia. All the above weakened the predictive performance of Khorana score in our study cohort.
CRT treatment
In the present study, 33 patients in total developed CRT, 32 of 33 were DVT in the catheter-related vein (IJV) and 1 one patient had PE. Among all the DVT cases, only 1 had symptom as catheter blockage while others were asymptomatic and detected before in-plan TIVAD removal by routine ultrasound examination. The 9th edition of American College of Chest Physicians Evidence-Based Clinical Practice Guidelines on the Antithrombotic Therapy of Thrombosis37 recommends a 3-month anticoagulation therapy over shorter periods for DVT. The PE and symptomatic DVT patients in our study did receive standard anticoagulation treatment; among the 31 asymptomatic DVT patients, 61.29% removed TIVAD without any treatment. No thrombosis event was observed during follow-up, which implied the insufficient necessity of anticoagulation treatment in asymptomatic CRT developed in breast cancer patients with TIVAD.
In our institution, we regularly recommend patients with a CRT size >1 cm and elevated D-dimer to receive 1–2 weeks of anticoagulation treatment until the D-dimer returns to normal. For patients with a thrombosis size <1 cm and a normal D-dimer, immediate TIVAD removal was observed to be safe and feasible.
Limitations
Several limitations should be noted for this study. First, the study was limited by its retrospective design and relatively small sample size which required a future external validation in a larger population. Second, details such as the operator of the port insertion were not recorded in the database, and there might be operator bias in the incidence of CRT.38 Finally, ultrasound examination was not routinely performed after removing the TIVAD. Thus, the asymptomatic DVT could not be observed during follow-up.
Conclusion
CRT is a common complication in breast cancer patients with TIVAD for chemotherapy; most of them are asymptomatic. Tumor size >2 cm, HBsAg positivity and LDL serum-level >3.6 mmol/L are correlated risk factors. Removal of the port without anticoagulation treatments might be a feasible choice for asymptomatic TIVAD-related CRT.
Acknowledgment
This study was funded by the Yat-Sen Scholarship of Young Scientist program of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (recipient: Kai Chen) and by the grants from the Sun Yat-sen Clinical Research Cultivating Program of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (recipient: Kai Chen), and the grants from the China Anti-aging Promoting Association (recipient: Shunrong Li).
Author contributions
Luyuan Tan: literature research, data analysis and interpretation, manuscript editing; Ya Sun: literature research, data analysis and interpretation, drafting the article; Liling Zhu: literature research, data analysis and interpretation; Xin Lei and Dongya Liang: literature research, data acquisition; Nanyan Rao and Fengxi Su: study design; Shunrong Li: study concepts; Kai Chen: manuscript revision, guarantor of integrity of entire study. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Broviac JW, Cole JJ, Scribner BH. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet. 1973;136(4):602–606.
2. Hickman RO, Buckner CD, Clift RA, Sanders JE, Stewart P, Thomas ED. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet. 1979;148(6):871–875.
3. Gyves J, Ensminger W, Niederhuber J, et al. Totally implanted system for intravenous chemotherapy in patients with cancer. Am J Med. 1982;73(6):841–845. doi:10.1016/0002-9343(82)90774-4
4. Kreis H, Loehberg CR, Lux MP, et al. Patients’ attitudes to totally implantable venous access port systems for gynecological or breast malignancies. Eur J Surg Oncol. 2007;33(1):39–43.
5. Ignatov A, Hoffman O, Smith B, et al. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfaction. Eur J Surg Oncol. 2009;35(3):241–246.
6. Geerts W. Central venous catheter-related thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014(1):306–311.
7. Lee AY, Levine MN, Butler G, et al. Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol. 2006;24(9):1404–1408.
8. Saber W, Moua T, Williams EC, et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
9. Plumhans C, Mahnken AH, Ocklenburg C, et al. Jugular versus subclavian totally implantable access ports: catheter position, complications and intrainterventional pain perception. Eur J Radiol. 2011;79(3):338–342.
10. Leung A, Heal C, Perera M, Pretorius C. A systematic review of patient-related risk factors for catheter-related thrombosis. J Thromb Thrombolysis. 2015;40(3):363–373. doi:10.1007/s11239-015-1175-9
11. Oberhoff C, Szymeczek J, Hoffmann O, Winkler UH, Kaiser S, Schindler AE. Adjuvant antiestrogen treatment with tamoxifen in postmenopausal women with breast cancer: a longitudinal study of blood coagulation and fibrinolysis. Breast Cancer Res Treat. 1998;50(1):73–81.
12. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi:10.1182/blood-2007-10-116327
13. Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665–3675. doi:10.1200/JCO.2003.08.008
14. Biffi R, Orsi F, Pozzi S, et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. 2009;20(5):935–940. doi:10.1093/annonc/mdn701
15. Heibl C, Trommet V, Burgstaller S, et al. Complications associated with the use of port-a-caths in patients with malignant or haematological disease: a single-centre prospective analysis. Eur J Cancer Care (Engl). 2010;19(5):676–681. doi:10.1111/j.1365-2354.2009.01115.x
16. Decousus H, Bourmaud A, Fournel P, et al. Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood. 2018;132(7):707–716. doi:10.1182/blood-2018-03-837153
17. Biffi R, Pozzi S, Agazzi A, et al. Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol. 2004;15(2):296–300. doi:10.1093/annonc/mdh049
18. Orlando L, Colleoni M, Nole F, et al. Incidence of venous thromboembolism in breast cancer patients during chemotherapy with vinorelbine, cisplatin, 5-fluorouracil as continuous infusion (ViFuP regimen): is prophylaxis required? Ann Oncol. 2000;11(1):117–118. doi:10.1023/a:1008364801718
19. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet (London, England). 1994;343(8902):886–889. doi:10.1016/s0140-6736(94)90008-6
20. Curigliano G, Balduzzi A, Cardillo A, et al. Low-dose aspirin for the prevention of venous thromboembolism in breast cancer patients treated with infusional chemotherapy after insertion of central vein catheter. Support Care Cancer. 2007;15(10):1213–1217. doi:10.1007/s00520-007-0277-0
21. Janahi EM. Prevalence and risk factors of hepatitis B virus infection in Bahrain, 2000 through 2010. PLoS One. 2014;9(2):e87599. doi:10.1371/journal.pone.0087599
22. Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–1010. doi:10.1056/NEJMoa051285
23. Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354(10):1011–1020. doi:10.1056/NEJMoa051287
24. Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357(25):2576–2588. doi:10.1056/NEJMoa066422
25. Xiang DZ, Verbeken EK, Van Lommel AT, Stas M, De Wever I. Composition and formation of the sleeve enveloping a central venous catheter. J Vasc Surg. 1998;28(2):260–271. doi:10.1016/s0741-5214(98)70162-4
26. Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology (Baltimore, Md). 1993;18(4):781–789.
27. Turhan O, Coban E, Inan D, Yalcin AN. Increased mean platelet volume in chronic hepatitis B patients with inactive disease. Med Sci Monit. 2010;16(4):Cr202–Cr205.
28. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. doi:10.1182/blood-2013-04-460121
29. Pabinger I, Posch F. Flamethrowers: blood cells and cancer thrombosis risk. Hematology Am Soc Hematol Educ Program. 2014;2014(1):410–417. doi:10.1182/asheducation-2014.1.410
30. Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014;41(3):302–310. doi:10.1053/j.seminoncol.2014.04.009
31. von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–835. doi:10.1084/jem.20112322
32. Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122(11):1873–1880. doi:10.1182/blood-2013-04-460139
33. Cheng Y, Cui T, Fu P, Liu F, Zhou L. Dyslipidemia is associated with tunneled-cuffed catheter-related central venous thrombosis in hemodialysis patients: a retrospective, multicenter study. Artif Organs. 2013;37(8):E155–E161. doi:10.1111/aor.12086
34. Garcia Raso A, Ene G, Miranda C, Vidal R, Mata R, Llamas Sillero MP. [Association between venous thrombosis and dyslipidemia]. Med Clin (Barc). 2014;143(1):1–5. doi:10.1016/j.medcli.2013.07.024
35. Hsu SH, Jang MH, Torng PL, Su TC. Positive association between small dense low-density lipoprotein cholesterol concentration and biomarkers of inflammation, thrombosis, and prediabetes in non-diabetic adults. J Atheroscler Thromb. 2019 Jul 1;26(7):624-635.
36. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102. doi:10.1161/CIRCULATIONAHA.107.709204
37. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–e496S. doi:10.1378/chest.11-2301
38. Grove JR, Pevec WC. Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11(7):837–840.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.