Back to Journals » Cancer Management and Research » Volume 12
Risk Factors for Urethral Recurrence in Men After Radical Cystectomy with Orthotopic Urinary Diversion for Urothelial Carcinoma: A Retrospective Cohort Study
Received 2 May 2020
Accepted for publication 16 July 2020
Published 3 August 2020 Volume 2020:12 Pages 6739—6746
DOI https://doi.org/10.2147/CMAR.S260979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Dong Hyeon Lee,1 Wan Song2
1Department of Urology, Ewha Womans University Medical Center, Ewha Womans University School of Medicine, Seoul, Korea; 2Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
Correspondence: Wan Song
Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea
Tel +82-2-3410-3558
Fax +82-2-3410-3027
Email [email protected]
Purpose: To evaluate the risk factors affecting urethral recurrence (UR) in men after radical cystectomy (RC) with ileal orthotopic neobladder (IONB).
Materials and Methods: We retrospectively reviewed 348 men who underwent RC with IONB for bladder cancer between January 2010 and December 2017. Clinicopathologic characteristics, including tumor location (trigone and/or bladder neck), prostatic urethral and/or stromal involvement, presence of carcinoma in situ (CIS), pathologic T and N stage, and urethral resection margin status, were identified. Kaplan–Meier survival analysis was used to illustrate urethral recurrence-free survival (URFS), and Cox proportional hazard models were applied to identify factors predicting UR.
Results: Of the 348 patients, UR was identified in 7 (2.0%) patients during the mean follow-up of 33.3 months. The 2-, 3-, and 5-year URFS rates were 97.6%, 96.3%, and 93.8%, respectively. On multivariable analysis, prostatic urethral involvement (P = 0.033, hazard ratio: 6.25, 95% confidence interval: 1.06– 36.96) was an independent predictor of UR. When patients were divided according to prostatic urethral involvement (negative vs positive), the 2- and 3-year URFS rates were significantly different (93.8% and 96.8%, respectively, vs 92.0% and 92.0%, respectively; P = 0.020). All 7 patients with UR underwent transurethral surgery and maintained their IONB.
Conclusion: In this series, UR occurred in approximately 2% of men after RC with IONB. Prostatic urethral involvement was the only significant prognostic factor for UR. Follow-up strategies considering UR risk should be adopted to facilitate early detection in those at high risk of UR.
Keywords: bladder cancer, neobladder, radical cystectomy, risk factor, urethral recurrence
Introduction
Radical cystectomy (RC) with pelvic lymph node dissection (PLND) is the standard treatment for muscle invasive or recurrent, high-risk, non-muscle invasive urothelial carcinoma (UC) of the bladder.1,2 In addition, ileal orthotopic neobladder (IONB) is currently the preferred method for continent urinary diversion due to the resulting improved quality of life, with a particular benefit to sexual function.3,4 However, as UC is a disease that can affect the remnant urothelium after RC,5,6 the chance of urethral recurrence (UR) has been described as a deterring factor against performing IONB. Previous reports have suggested that the rate of UR after RC with IONB ranged from 1.4% to 5.0%.5–9 Therefore, urologists are reluctant to perform IONB in patients at high risk for UR.
To date, several studies have examined the risk factors for UR after RC with IONB.6,9-12 Previously reported risk factors include tumor location, multifocality, presence of carcinoma in situ (CIS), prostatic involvement, and a positive urethral margin.13–17 However, when these risk factors were applied as selection criteria, the unaffected majority of patients would be eliminated from receiving an orthotopic urinary substitution. In addition, in previous studies, conflicting results have been reported on the association of pathologic features with UR; some studies reported that prostatic involvement was significantly associated with a higher risk of UR.14,17,18 On the other hand, others reported that tumors located at the bladder neck, multifocal tumors, and positive urethral margin were associated with a higher risk of UR, and therefore IONB should not be aborted based on prostatic involvement.6,19
For its rarity, UR after RC with IONB raises a diagnostic and therapeutic challenge. A better understanding of the risk factors for UR after RC with IONB is necessary both for patient counselling and for an individualized, evidence-based strategy for the surveillance of patients at risk for UR. Therefore, in this study, we evaluated the risk factors affecting UR in men who underwent RC with IONB.
Materials and Methods
Study Population
This retrospective study was approved by the Institutional Review Board of our institution (IRB No. 2018-10-040), and the need for informed consent was waived due to the study design. We retrospectively reviewed a prospectively maintained database of 395 men who underwent RC for bladder cancer between January 2010 and December 2017 by a single urologic oncology surgeon. In the entire cohort, patients who underwent ileal conduit urinary diversion (ICUD) (n = 40) or cystectomy for non-urothelial carcinoma or benign etiologies (n = 7) were excluded from analysis. Ultimately, 348 men who underwent Studer IONB following RC were analyzed in this study. All patients were staged cM0 preoperatively.
The oncologic and functional exclusion criteria for IONB were: tumor staged cT4b on computed tomography (CT) and/or magnetic resonance imaging (MRI) of the abdomen and pelvis, bulky lymph node (LN) involvement (cN3, not cN1-2), estimated glomerular filtration rate (eGFR) ≤ 60 mL/min, severe hepatic insufficiency, urethral sphincter dysfunction, or severe urethral stricture preoperatively.
Data Collection
Clinical and pathologic characteristics of patients, including age at surgery, primary tumor location (bladder neck and/or trigone involvement), pathologic tumor stage and LN tumor involvement, underlying histology, presence of CIS, lymphovascular and/or perineural invasion, prostatic involvement (prostatic urethra and/or stroma), resection margin status in the urethra and adjuvant chemotherapy were obtained from the medical records at the time of surgery. Positive urethral resection margin was defined as either CIS or high-grade non-invasive or invasive UC at the distal urethral margin on permanent sectioning.20 Prostatic urethral involvement was defined as involving the prostatic urethra and/or ducts without stromal invasion.17
Histologic Assessment
The surgical technique of RC with IONB in our institution has been described previously.21 All RCs were performed as open procedures and included removal of the prostate and seminal vesicle. Standard bilateral pelvic lymphadenectomy was performed. RC specimens, as well as those after transurethral resection of the bladder (TURB), were processed on formalin-fixed, paraffin-embedded sections and reviewed by the same, experienced genitourinary pathologist. Pathologic staging and tumor grading were determined according to the 2010 TNM classification from the American Joint Committee on Cancer (AJCC) and the 2004 World Health Organization (WHO)/International Society of Urologic Pathology consensus classification.
Follow-Up
Each patient was followed up according to recommendations and institutional protocols. In general, after RC with IONB, patients were scheduled for a follow-up at 1 month postoperatively and then every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. During the follow-up, a physical examination with laboratory tests, urine analysis with cytology, chest radiography, and radiologic evaluation including CT or MRI for the chest, abdomen, and pelvis were carried out at every visit to identify local recurrence and/or distant metastasis. Cystoscopy was performed when there was an abnormal finding in urine cytology or when symptoms (hematuria or irritative voiding) were present. Bone scintigraphy scan was performed when clinically indicated.
Disease recurrence included local recurrence at the surgical bed or regional LNs and/or distant metastasis. Recurrence-free survival (RFS) was measured from the date of RC to the date of first documented recurrence or the date of last follow-up when the patient had not yet experienced disease recurrence. Urethral recurrence (UR) was defined as a tumor in the urethra pathologically confirmed via endoscopy, and urethral-RFS (URFS) was measured from the date of RC to the date of UR or last follow-up.
Statistical Analysis
Quantitative variables are presented as median (range) or mean (standard deviation, SD), and qualitative variables are presented as absolute value (percentage). Descriptive statistics were obtained for demographic variables. Kaplan–Meier survival analysis was used to estimate overall RFS and URFS, and differences were assessed with a Log-rank test. Multivariable Cox proportional hazards models were used to identify potential risk factors associated with UR. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp. Armonk, NY, USA). Two-tailed P values <0.05 were considered statistically significant.
Results
The baseline characteristics of 348 men who underwent RC with IONB for bladder cancer are summarized in Table 1. In the entire cohort, the median (range) age at RC was 65.0 (27.0–84.0) years. Pure UC was presented in 79.3% (276/348) of patients, and a total of 124 (35.6%) showed locally advanced tumor stage (≥ pT3) at RC. Pathologic analysis demonstrated LN involvement in 83 (23.9%) patients, and concomitant CIS was found in 194 (55.7%) patients. When tumor location was examined, bladder tumors were located at the trigone in 202 (58.0%) patients and at the bladder neck in 141 (40.5%) patients. Prostatic urethral and stromal involvement was identified in 39 (11.2%) and 29 (8.3%) patients, respectively. On the final pathology findings, 12 (3.4%) patients had a positive urethral resection margin, and one of them developed subsequent UR (1/12, 8.3%). UR rates by risk factor are shown in Table 2.
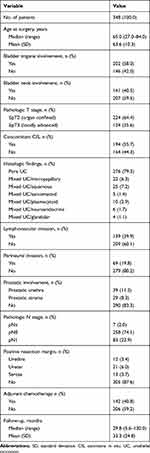 |
Table 1 Clinicopathologic Characteristics of 348 Men After Radical Cystectomy with Ileal Orthotopic Neobladder |
 |
Table 2 Urethral Recurrence Rates by Risk Factor |
During the mean (SD) follow-up of 33.3 (24.8) months, overall recurrence was noted in 75 (21.6%) patients. Figure 1 shows the overall RFS rates using the Kaplan–Meier estimation method. The 3-year and 5-year overall RFS rates were 67.8% and 55.1%, respectively. In the entire cohort, UR was identified in 7 (2.0%) patients. The 2-, 3-, and 5-year URFS rates were 97.6%, 96.3%, and 93.8%, respectively (Figure 2). However, when patients were stratified according to prostatic urethral involvement, there was a significant difference in URFS (log-rank test, P = 0.020) (Figure 3): the 2-year and 3-year URFS rates were 98.3% and 96.8%, respectively, for patients with negative prostatic urethral involvement, compared to 92.0% and 92.0%, respectively, for patients with positive prostatic urethral involvement.
The outcomes of Cox proportional hazards regression analysis to assess the prognostic factors for UR after RC with IONB are presented in Table 3. In multivariable analysis, prostatic urethral involvement was the only significant prognostic factor for UR after RC with IONB (HR = 6.25, 95% CI: 1.06–36.96, P = 0.033). Bladder tumor location, pathologic T and N stage, CIS, and resection margin status in the urethra had no significant effect on UR. All 7 patients with UR underwent transurethral surgery and maintained their IONB. The pathologic results of the 7 patients with UR after endoscopic surgery are summarized in Table 4.
 |
Table 3 Cox Proportional Hazards Regression Analyses to Predict Urethral Recurrence After Radical Cystectomy with Ileal Orthotopic Neobladder |
 |
Table 4 Condition of Risk Factors and Pathologic Results of Patients (n = 7) with Urethral Recurrence After Transurethral Surgery |
Discussion
In the present study, among the 348 men who underwent RC with IONB for bladder cancer, UR was identified in 7 (2.0%) patients, and the 2-year URFS rate was 97.6% for all patients. However, when patients were divided according to prostatic urethral involvement, a significant difference in UR was identified: 2-year URFS was 98.3% with negative prostatic urethral involvement and 92.0% with positive prostatic urethral involvement. We found that only prostatic urethral involvement was a significant prognostic factor that predicted UR after RC with IONB. Our results can be used to help counsel patients who underwent RC with IONB regarding the possibility of UR. To the best of our knowledge, this is one of the largest studies to evaluate the risk factors for UR after RC with IONB.
When considering RC with IONB, it is important to identify patients at highest risk of UR. In our study, when examined the UR rates by risk factor, the highest UR rate was identified in patients with positive urethral resection margin (1/12, 8.3%). However, these results have to be interpreted carefully owing to the relatively low number of included cases.
To date, the prostatic involvement of UC is the most important risk factor associated with UR.14,17,18 Our results showed that prostatic involvement of UC was confirmed in 58 (16.7%) patients who underwent RC, among which UR occurred in 3 (5.2%) patients. This shows a statistically significant difference compared to the UR rate (4/290, 1.4%) in the remaining patients (P = 0.02). On the other hand, UR did not occur in 55 of 58 (94.8%) patients with prostatic involvement of UC. One possible explanation is that negative urethral resection margin was identified in approximately 90% of the patients who underwent RC, even if they had prostatic involvement. Taking all these results into account, over 90% of patients with prostatic involvement would be candidates for IONB.
When considering the depth of prostatic involvement, in our study, only prostatic urethral involvement was a significant prognostic factor to predict UR, and not prostatic stromal invasion. In general, a higher UR rate is reported when prostatic stromal invasion is confirmed,17 but our study showed contradictory results. A possible explanation for this discrepancy is as follows. Of 29 patients with prostatic stromal invasion, histologic evidence of intraurethral spread was confirmed in 19 patients, while extravesical spread was seen in 10 patients. Eventually, prostatic urethral involvement was identified in 39 of 58 (67.2%) patients, which increased the risk of UR. However, these results have to be interpreted carefully owing to the relatively low number of included cases.
In our study, UR was detected in 4 of 7 patients by positive urinary cytology and in the remaining 3 patients by microscopic hematuria. All patients with UR underwent endoscopic surgery, and pathologic results of all patients were identified as non-invasive disease (1 CIS, 4 pTa, and 2 pT1). To date, various regimens for surveillance after RC have been proposed with different imaging modalities and different intensities of follow-up schedules.22,23 Although data is scarce on the optimal follow-up after IONB, in our institution, urine analysis with cytology was performed at every follow-up, and urethroscopy was performed when abnormal findings and/or hematuria were identified. Urine cytology is a non-invasive and useful diagnostic tool, allowing for early detection and treatment of UR at a less advanced stage.11,24 A recent systemic review reported that detection of asymptomatic recurrence was associated with an approximately 30% reduction in mortality (HR = 0.69, 95% CI: 0.59–0.79).25 Therefore, it is important that UR is identified at the earliest possible stage since detection at an early stage is associated with improved survival.1,10
The general consensus in the recent American Urological Association (AUA) and European Association of Urology (EAU) guidelines is to assure a negative margin in the urethra prior to RC with IONB, and the optimal predictive parameter is intraoperative frozen section analysis (FSA) at the time of surgery.1 However, this procedure was not routinely performed at our institution. In our study, the positive urethral margin rate was 3.4%, which is slightly higher than other studies where the rate ranged from 1.1% to 2.4%,26,27 but the results might be affected by selection bias because previous studies excluded patients with positive intraoperative FSA. However, despite the increase in positive urethral margin rate, our UR rate is comparable to those in other series.5–9
In addition, a positive urethral margin in intraoperative FSA is the main reason for conversion to incontinent urinary diversion during RC.28 However, in our study, there was no statistically significant difference in the UR rate based on urethral margin status (P = 0.22). Furthermore, a clinical dilemma is that there is a discrepancy between intraoperative FSA and the final pathology in the urethra. In a study by Kates et al,27 a total of 298 patients with RC were retrospectively reviewed, and the accuracy of intraoperative FSA was analyzed. They reported that all negative FSA was confirmed to be negative on final pathology with 100% negative predictive value, but 53% of patients with positive FSA were ultimately determined to be negative during re-review or final pathology.
In our study, pure UC was presented in 79.3% of patients, while mixed histology was found in 20.7%. However, all URs were observed in patients with pure UC. These results suggest that the risk of UR emanates mainly from UC histology.29
Despite the potential clinical implications of our study, there are several limitations. First, this study was a retrospective design and was conducted at a single, tertiary referral center, thus raising concerns for selection bias. Nonetheless, this study used a prospectively maintained database and reflects real-world clinical practice. Second, as the mean follow-up period was 33.3 months, which is relatively short, we identified only one end-point (URFS) and did not analyze other clinically significant oncologic outcomes such as cancer-specific survival and overall survival. However, since most URs are reported within 3 years,18,30 this study was sufficiently powered to confirm UR after RC. Finally, this study represents one of the largest series with regard to UR after RC with IONB. However, due to the low incidence of UR, the number of cases is limited. Therefore, confirmation via a large, multi-institutional study is warranted to verify the implications of our results.
In conclusion, this study provides some insight regarding the incidence and risk factors of recurrence in retained urethra after RC with IONB. UR occurs only in a small proportion of patients following RC with IONB, and prostatic urethral involvement is the only significant risk factor for UR. Therefore, follow-up strategies to ameliorate UR risk should be adopted to facilitate early detection in those at high risk of UR. These data provide further evidence for continued evaluation of the urethra after RC with IONB.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45–57. doi:10.1016/j.eururo.2012.08.009
2. Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol. 2001;19(3):666–675. doi:10.1200/JCO.2001.19.3.666
3. Ahmadi H, Skinner EC, Simma-Chiang V, et al. Urinary functional outcome following radical cystoprostatectomy and ileal neobladder reconstruction in male patients. J Urol. 2013;189(5):1782–1788. doi:10.1016/j.juro.2012.11.078
4. Gakis G, Stenzl A. Considerations for orthotopic diversions in women. Curr Opin Urol. 2015;25(6):550–554. doi:10.1097/MOU.0000000000000224
5. Perlis N, Turker P, Bostrom PJ, et al. Upper urinary tract and urethral recurrences following radical cystectomy: review of risk factors and outcomes between centres with different follow-up protocols. World J Urol. 2013;31(1):161–167. doi:10.1007/s00345-012-0905-2
6. Fahmy O, Khairul-Asri MG, Schubert T, et al. Urethral recurrence after radical cystectomy for urothelial carcinoma: a systematic review and meta-analysis. Urol Oncol. 2018;36(2):54–59. doi:10.1016/j.urolonc.2017.11.007
7. Giannarini G, Kessler TM, Thoeny HC, Nguyen DP, Meissner C, Studer UE. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58(4):486–494. doi:10.1016/j.eururo.2010.05.041
8. Hassan JM, Cookson MS, Smith JA
9. Chan Y, Fisher P, Tilki D, Evans CP. Urethral recurrence after cystectomy: current preventative measures, diagnosis and management. BJU Int. 2016;117(4):563–569. doi:10.1111/bju.13370
10. Boorjian SA, Kim SP, Weight CJ, Cheville JC, Thapa P, Frank I. Risk factors and outcomes of urethral recurrence following radical cystectomy. Eur Urol. 2011;60(6):1266–1272. doi:10.1016/j.eururo.2011.08.030
11. Gakis G, Black PC, Bochner BH, et al. Systematic review on the fate of the remnant urothelium after radical cystectomy. Eur Urol. 2017;71(4):545–557. doi:10.1016/j.eururo.2016.09.035
12. Huguet J, Palou J, Serrallach M, Sole Balcells FJ, Salvador J, Villavicencio H. Management of urethral recurrence in patients with Studer ileal neobladder. Eur Urol. 2003;43(5):495–498. doi:10.1016/S0302-2838(03)00096-4
13. Mitra AP, Quinn DI, Dorff TB, et al. Factors influencing post-recurrence survival in bladder cancer following radical cystectomy. BJU Int. 2012;109(6):846–854. doi:10.1111/j.1464-410X.2011.10455.x
14. Sherwood JB, Sagalowsky AI. The diagnosis and treatment of urethral recurrence after radical cystectomy. Urol Oncol. 2006;24(4):356–361. doi:10.1016/j.urolonc.2005.11.027
15. Balci U, Dogantekin E, Ozer K, Gorgel SN, Girgin C, Dincel C. Patterns, risks and outcomes of urethral recurrence after radical cystectomy for urothelial cancer; over 20 year single center experience. Int J Surg. 2015;13:148–151. doi:10.1016/j.ijsu.2014.12.006
16. Varol C, Thalmann GN, Burkhard FC, Studer UE. Treatment of urethral recurrence following radical cystectomy and ileal bladder substitution. J Urol. 2004;172(3):937–942. doi:10.1097/01.ju.0000135626.91587.c8
17. Stein JP, Clark P, Miranda G, Cai J, Groshen S, Skinner DG. Urethral tumor recurrence following cystectomy and urinary diversion: clinical and pathological characteristics in 768 male patients. J Urol. 2005;173(4):1163–1168. doi:10.1097/01.ju.0000149679.56884.0f
18. Huguet J, Monllau V, Sabate S, et al. Diagnosis, risk factors, and outcome of urethral recurrences following radical cystectomy for bladder cancer in 729 male patients. Eur Urol. 2008;53(4):
19. Bulbul MA, Wazzan W, Nasr R, Hemady K. The value of cystoscopy, prostate biopsy and frozen-section urethral biopsy prior to orthotopic neobladder substitution. Can J Urol. 2001;8(3):1290–1292.
20. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22(12):1435–1448. doi:10.1097/00000478-199812000-00001
21. Song W, Yoon HS, Kim KH, et al. Role of bowel suspension technique to prevent early intestinal obstruction after radical cystectomy with ileal orthotopic neobladder: a retrospective cohort study. Int J Surg. 2018;55:9–14. doi:10.1016/j.ijsu.2018.04.044
22. Bochner BH, Montie JE, Lee CT. Follow-up strategies and management of recurrence in urologic oncology bladder cancer: invasive bladder cancer. Urol Clin North Am. 2003;30(4):777–789. doi:10.1016/S0094-0143(03)00061-2
23. Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55(4):815–825. doi:10.1016/j.eururo.2009.01.002
24. Clark PE, Hall MC. Contemporary management of the urethra in patients after radical cystectomy for bladder cancer. Urol Clin North Am. 2005;32(2):199–206. doi:10.1016/j.ucl.2005.01.004
25. Stewart-Merrill SB, Alahdab F, Benkhadra K, et al. Oncologic surveillance in bladder cancer following radical cystectomy: a systematic review and meta-analysis. Urol Oncol. 2016;34(5):236e213–221. doi:10.1016/j.urolonc.2015.11.025
26. Kassouf W, Spiess PE, Brown GA, et al. Prostatic urethral biopsy has limited usefulness in counseling patients regarding final urethral margin status during orthotopic neobladder reconstruction. J Urol. 2008;180(1):
27. Kates M, Ball MW, Chappidi MR, et al. Accuracy of urethral frozen section during radical cystectomy for bladder cancer. Urol Oncol. 2016;34(12):532e531–532 e536. doi:10.1016/j.urolonc.2016.06.014
28. Ghodoussipour S, Ahmadi N, Hartman N, et al. Factors influencing intraoperative conversion from planned orthotopic to non-orthotopic urinary diversion during radical cystectomy. World J Urol. 2019;37(9):1851–1855. doi:10.1007/s00345-018-2582-2
29. Gakis G, Ali-El-Dein B, Babjuk M, et al. Urethral recurrence in women with orthotopic bladder substitutes: a multi-institutional study. Urol Oncol. 2015;33(5):204e217–223. doi:10.1016/j.urolonc.2015.01.020
30. Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol. 2006;24(3):296–304. doi:10.1007/s00345-006-0061-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



