Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease
Authors Kim KH, Kang HS , Kim JS , Yoon HK , Kim SK, Rhee CK
Received 14 June 2017
Accepted for publication 16 October 2017
Published 4 December 2017 Volume 2017:12 Pages 3449—3456
DOI https://doi.org/10.2147/COPD.S143967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Kyung Hoon Kim,1 Hye Seon Kang,2 Ju Sang Kim,3 Hyoung Kyu Yoon,4 Sung Kyoung Kim,5 Chin Kook Rhee1
1Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Seoul St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, 2Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Bucheon St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, 3Division of Pulmonary Medicine, Department of Internal Medicine, Incheon St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, 4Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Yeouido St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, 5Division of Pulmonary Medicine, Department of Internal Medicine, St Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Republic of Korea
Introduction: Roflumilast is a phosphodiesterase-4 inhibitor, which can decrease exacerbation in patients with chronic obstructive pulmonary disease (COPD). However, adverse effects are a major barrier to medication use, and little is known regarding the risk factors for discontinuation of roflumilast in COPD patients.
Method: A search of the clinical databases identified all patients who were prescribed roflumilast between December 2012 and April 2015 in the four hospitals of The Catholic University of Korea, Korea. The study subjects were limited to patients who had taken 500 µg of roflumilast. We studied the factors associated with drug discontinuation and drug adverse events by univariate and multivariate analyses.
Results: Among 154 eligible patients, 54 (35.1%) discontinued their roflumilast prescription. Most patients were elderly, male, current or former smokers, and had moderate-to-severe airflow limitation. Low–body mass index (BMI) patients were more likely to undergo drug discontinuation (1-unit decrease in BMI: odds ratio [OR] =1.165, p=0.006; BMI <23 kg/m2: OR =2.960, p=0.004). Fifty-five patients (35.7%) had adverse events. Loss of appetite, diarrhea, nausea, headache, and weight loss were the most frequent adverse events. Low-BMI patients were more likely to experience adverse events (1-unit decrease in BMI: OR =1.151, p=0.010; BMI <23 kg/m2: OR =2.644, p=0.009).
Conclusions: The patient discontinuation and adverse events rates in this study were higher than in previous randomized controlled studies. Discontinuation of roflumilast in ethnic Koreans is more likely to occur in low-BMI patients. In a clinical setting, low-BMI patients can more easily discontinue roflumilast; clinicians should, therefore, provide greater care for these patients.
Keywords: phosphodiesterase-4 inhibitor, chronic obstructive pulmonary disease, body mass index, adverse event
Introduction
Chronic obstructive pulmonary disease (COPD) was ranked as the sixth leading cause of death in 1990, and as the fifth leading cause in 2002; it will be the fourth leading cause of death by 2030 globally.1 Acute exacerbation of COPD has an independent negative prognostic impact, with mortality increasing with the frequency of severe exacerbations, particularly if these require admission to the hospital.2
Roflumilast is a highly selective phosphodiesterase-4 inhibitor that received USA Food and Drug Administration approval in March 2011 for maintenance treatment of severe to very severe COPD, with symptoms of chronic bronchitis and a history of exacerbations, to reduce the risk of COPD exacerbations.3 The first Phase III randomized placebo-controlled study for roflumilast was published in 2005.4 In 2007, a randomized, placebo-controlled study on the effect of 1 year of treatment with roflumilast on severe COPD was published.5 Additionally, two articles published in 2009 on roflumilast described four clinical studies (M2–124, M2–125, M2–127, and M2–128).6,7 In 2015, the Roflumilast and Exacerbations in patients receiving Appropriate Combination Therapy (REACT) study was published.8 These papers proved that roflumilast improved lung function and reduced the exacerbation rate; however, similar adverse events profiles and incidence rates were observed in each trial.4–8 Such a high incidence rate for adverse events is a major barrier for prescribing roflumilast in a clinical setting. Pulmonologists who prescribe roflumilast in clinical practice have felt that the proportion of patients who discontinue treatment is much greater than that seen in randomized controlled trials (RCTs). For this reason, it is common for patients to discontinue roflumilast in clinical practice. However, there are few reports on the frequency of adverse events linked to roflumilast in “real-world practice.” To our best knowledge, there has been only one report (a letter to editor and an abstract by same author) regarding discontinuation of roflumilast in real world. However, only 25 patients were enrolled in that study and there were no data regarding risk factors associated with roflumilast discontinuation.9,10 In this study, we aimed to assess the frequency of roflumilast side effects, the proportion of patients who discontinued the drug, and the risk factors associated with discontinuation by reviewing relatively large number of patients from multiple centers.
Materials and methods
The medical charts of 270 COPD patients who began roflumilast therapy between December 2012 and April 2015, in the four hospitals of the Catholic Medical Center, Korea, were reviewed to identify all those who had discontinued roflumilast. The four centers are Seoul St Mary’s Hospital, Yeouido St Mary’s Hospital, St Vincent Hospital, and Incheon St Mary’s Hospital. All in-patients and out-patients who were prescribed roflumilast at least once were included and had a clinical diagnosis of COPD (confirmed with a postbronchodilator [albuterol 400 μg] forced expiratory volume in 1 s [FEV1]/forced vital capacity [FVC] ratio ≤70%) and chronic cough and sputum production. Patients were excluded from study enrolment if they were diagnosed with asthma or other relevant lung diseases (eg, lung cancer, bronchiectasis).
The data obtained from electronic medical records were sociodemographic information that included age and sex, in addition to clinical information including height, weight, body mass index (BMI), smoking history, comorbidities, and pulmonary function test results. The use of roflumilast was thoroughly recorded including starting dates, dosages, end dates, reasons for discontinuation, adverse events, and the use of concomitant methylxanthine. We selected patients who were initially treated with 500 μg of roflumilast. Treatment discontinuation was defined as treatment interruption during the treatment period. Treatment interruption included a failure to comply with scheduled visits, an inability to tolerate medication side effects, and patients’ refusal to adhere to the treatment.
This study was approved by the Institutional Review Board of The Catholic University of Korea, Korea. The requirement for written informed consent from each patient was waived because of the retrospective nature of the study and lack of identifying information in the patient data. The statistical analysis was performed with SPSS software (ver. 24.0.0; SPSS Inc., Chicago, IL, USA).
Chi-squared tests and independent t-tests were used to compare characteristics between discontinuation and continuation group. Univariate and multivariate logistic regression models were fitted with roflumilast discontinuation as a dependent variable. Multivariate logistic regression analysis was used to evaluate the risk factors associated with roflumilast discontinuation. We also analyzed differences in adverse event rates between the two groups. Two-sided p-values of <0.05 were considered to be statistically significant.
Results
Patient characteristics
A total of 270 patients with new-onset roflumilast prescriptions during the period 2012–2015 were selected. From these patients, 16 dose-unknown patients, 93 patients treated with 250 μg of roflumilast, and 7 BMI-unknown patients were excluded. The mean age of the 154 patients who were included in the final analysis was 70.3±8.8 (mean ± SD) years (range: 41–87 years) (Figure 1). Table 1 shows the demographic and baseline characteristics of the study population.
  | Table 1 Baseline characteristics of the patients who continued or discontinued roflumilast use |
Most participants were elderly, male, ex- or current smokers (more than 88%) with considerable previous tobacco consumption, and had moderate-to-severe airflow limitation (Table 1). The mean BMI was 21.8±3.45. The mean pre-bronchodilator FEV1 was 1.07 L (SD, 0.43), and the mean post-bronchodilator FEV1 was 1.12 L (SD, 0.42). Ninety-one patients (59.1%) had concomitant diseases. Forty patients (26.0%) had diabetes, 10 (6.5%) had cerebrovascular diseases, and 76 (49.4%) had cardiovascular diseases.
Profiles of the drug discontinued group versus continued group
A total of 54 patients discontinued roflumilast and 100 patients continued with the drug. The rate of discontinuation was 35.1%. The two groups were compared to elucidate factors associated with continuation and discontinuation. There was no significant difference between the groups in age, sex, height, weight, pre-bronchodilator FEV1, post-bronchodilator FEV1, pre-bronchodilator FVC, post-bronchodilator FVC, or history of comorbidity. However, when compared with the drug continuation group, those who had discontinued roflumilast had a significantly lower BMI (p=0.004). Moreover, there was a significant difference in the percentage of underweight, normal, overweight, and obese between discontinuation and continuation groups (p=0.035). The normal and underweight patients discontinued roflumilast more often than the overweight and obese patients, as shown in Figure 2. There was no difference in smoking status between discontinuation and continuation groups (p=0.171).
Factors associated with roflumilast discontinuation
Univariate Pearson’s chi-squared analysis was used to evaluate the risk factors associated with roflumilast discontinuation. On univariate analysis, there was a significant difference between the low-BMI group and high-BMI group. The odds ratio (OR) of patients with a BMI below 23 was 2.911 (95% CI 1.393–6.083, p=0.004). However, other factors, including the severity of COPD, GOLD classification, smoking history, age, concomitant methylxanthine treatment, comorbidities, change of dose, and sex, were not significantly associated with roflumilast discontinuation. Table 2 shows the detailed results.
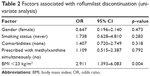  | Table 2 Factors associated with roflumilast discontinuation (univariate analysis) |
Multivariate logistic regression analysis was used to evaluate the risk factors associated with roflumilast discontinuation. On multivariate analysis, when adjusted by age, sex, and post-bronchodilator FEV1 (%), the OR was 1.165 for a 1-unit decrease in BMI (p=0.006). Additionally, the OR of a BMI of <23 was 2.960 (p=0.004; Table 3).
Adverse events of roflumilast
Adverse events were reported by 55 (35.7%) patients among the total population. Adverse events associated with roflumilast were more common in the discontinuation group than in the continuation group (40 [74.1%] versus 15 [15.0%], p<0.001). The most frequent adverse events were loss of appetite, diarrhea, nausea, headache, and weight loss. There was a significant difference in the incidence of loss of appetite, diarrhea, nausea, and headache between the two groups. However, there was no significant difference in the incidence of weight loss, itching, anxiety, insomnia, or facial flushing (Table 4).
  | Table 4 Types of adverse events in patients who continued or discontinued roflumilast use |
We compared the differences between the group that experienced adverse events and the group that did not. There was no significant difference between the groups in age, sex, height, weight, smoking status, COPD severity, pre-bronchodilator FEV1, post-bronchodilator FEV1, pre-bronchodilator FVC, post-bronchodilator FVC, and history of comorbidities. However, when compared with the group that did not experience any adverse event, the group that had experienced adverse events had a significantly lower BMI (p=0.004). The normal and underweight patients experience adverse event more often than the overweight and obese patients, as shown in Figure 3. Table 5 shows the detailed results. On univariate analysis, there was a significant difference between the low-BMI group and high-BMI group. The OR of patients with a BMI of <23 was 2.647 (95% CI 1.284–5.458, p=0.007; Table 6).
  | Table 5 Comparison of patient characteristics by number of adverse events |
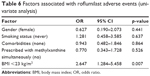  | Table 6 Factors associated with roflumilast adverse events (univariate analysis) |
Furthermore, we compared the differences between those who experienced weight loss and those who did not. The group that had weight-loss side effect had significantly lower BMI than the group that had not experienced weight loss, 21.9 versus 18.5, p=0.033. Chi-squared test was used to compare the difference in the incidence of weight loss between underweight/normal group and overweight/obese group. There was no significant difference in incidence of weight loss between two groups, p=0.077. There were no weight-loss patients in overweight/obese patient group and all weight-loss patients belonged to underweight/normal patient group.
Multivariate logistic regression analysis was used to evaluate the risk factors associated with roflumilast adverse events. On multivariate analysis, when adjusted by age, sex, and post-bronchodilator FEV1 (%), the OR was 1.151 for a 1-unit decrease in BMI (p=0.010). The OR of a BMI of <23 was 2.644 (p=0.009; Table 7).
Discussion
Among 154 Korean patients prescribed 500 μg of roflumilast, the drug discontinuation rate was 35.1%. A lower BMI was independently associated with drug discontinuation. On multivariate analysis, a low BMI was associated with drug discontinuation.
The major side effects of roflumilast were loss of appetite (9.1%), diarrhea (6.5%), nausea (5.2%), and headache (3.9%), and all of these adverse events were associated with drug discontinuation (all p<0.05).
In the roflumilast clinical development trials, the rate of patient withdrawal because of adverse events was similar, at around 10%–15%. In the first RCT on roflumilast in 2005, the discontinuation rates because of adverse events in 500 and 250 μg roflumilast groups were 15.1% and 9.3%, respectively.4 In a 2007 RCT by Calverley et al, the drug discontinuation rate because of adverse events was 13.5% in the 500 μg group.5 In a 2009 RCT, the M2–127 trial (roflumilast + salmeterol), the discontinuation rate because of adverse events was 16.5% and that of M2–128 (roflumilast + tiotropium) was 8.9%.7 In 2009, another two RCTs were performed, both with dosage of 500 μg of roflumilast, and the discontinuation rates because of adverse events were 15.5% (M2–124) and 13.1% (M2–125).6 In the 2015 REACT trial, the 500 μg roflumilast (once daily) plus inhaled corticosteroid and long-acting beta2 agonist combination group discontinuation rate (because of adverse events) was 8.5%.8 Unlike the above RCT results, the patient discontinuation rate because of adverse events in the present study was 33.8%; this was much higher than those of the above RCTs.
The main reasons for discontinuation of roflumilast in the above studies were diarrhea, nausea, or headache.4–8,11 Similarly, the most prevalent side effects in the present study were gastrointestinal symptoms including diarrhea, weight loss, and nausea.4–8,11 The average rate of withdrawal in RCTs was 12.6%.2,6–8,12 However, in our clinical practice-based study, the rate of discontinuation of roflumilast was higher (33.8%) than this. The possible cause of the significant difference in withdrawal between the five previous RCTs and our study is the difference in baseline characteristics of the participants in those studies versus our study. In our study, the lower the BMI, the higher the probability of discontinuity. In particular, among the baseline characteristics, the BMI of our Korean patients was significantly lower than that of western patients, suggesting that the adverse events were greater, and thus withdrawal was more frequent, in this group. This suggests that 500 μg of roflumilast may be too much for Koreans. Also, there may be other possible reasons for discontinuation of roflumilast. There were some patients who did not feel any beneficial effect of roflumilast and some patients who just did not want administration of roflumilast. These were the minor reasons for discontinuation of roflumilast.
There is currently little information on the optimal management of roflumilast treatment in patients with COPD in a clinical setting. However, it was certain that most patients who discontinued their drug prescriptions were associated with side effects in clinical setting.
As far as we are aware, our study is the largest evaluation ever conducted on the discontinuation profiles and incidence of adverse events of roflumilast in patients treated in real-world clinical settings. There have been few studies of roflumilast discontinuation and adverse events outside of drugs trials, and there are currently no studies on its risk factors. Clinical trials for roflumilast are not based on the clinical practice setting and their programs differ greatly from our clinical setting. We captured data in clinical practice instead of under research conditions, so that our findings are more reflective of the real world. We, therefore, believe that our study demonstrates real clinical practice-based rates of discontinuation and side effects. Moreover, our study is the first to clarify the risk factors for the discontinuation of roflumilast in patients with COPD in a clinical setting via univariate and multivariate logistic regression analyses. Our study suggests that in a clinical setting, low-BMI patients can more easily discontinue roflumilast; thus, clinicians should take greater care of these patients.
Our study had several limitations. First, the retrospective design is associated with potential bias, including regarding the encoding of data from existing charts. Adverse events of roflumilast have been checked by a pulmonologist who prescribed it in the outpatient clinic. However, there was a limit in revealing the reason for every discontinuation of roflumilast by retrospective chart review. Second, other risk factors may also be present that were not measured. Finally, although this was a multicenter study, our study population was not large and was confined to patients visiting the pulmonology clinics of four tertiary referral hospitals.
In conclusion, this study showed that the patients more likely to discontinue medication were those with a low BMI. This information would, therefore, be useful for planning pretreatment consultations, and for improving treatment compliance in patients with COPD who require roflumilast.
Acknowledgment
This study was presented orally at the 118th Korean Society of Tuberculosis and Respiratory Society Fall Conference 2014; October 24, 2014; Seoul, Korea.
Disclosure
CKR received consulting and/or lecture fees from MSD, AstraZeneca, Novartis, GSK, Takeda, Mundipharma, Sandoz, Boehringer-Ingelheim, and Teva-Handok. The authors report no other conflicts of interest in this work.
References
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. | ||
Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. | ||
Tashkin DP. Roflumilast: the new orally active, selective phophodiesterase-4 inhibitor, for the treatment of COPD. Expert Opin Pharmacother. 2014;15:85–96. | ||
Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast–an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. | ||
Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. | ||
Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. | ||
Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. | ||
Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. | ||
Worndl E, Hunt EB, Kennedy MK, Plant BJ, Henry MT, Murphy DM. Real-life experience with roflumilast therapy in COPD patients at Cork University Hospital. Irish J Med Sci. 2014;183:S506–S507. | ||
Worndl E, Hunt EB, Kennedy MP, Henry MT, Plant BJ, Murphy DM. Roflumilast in COPD. Chest. 2015;148:e31. | ||
Giembycz MA, Field SK. Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des Dev Ther. 2010;4:147–158. | ||
Kim MK, Lee WY, Kang JH, et al. Clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul). 2014;29: (4)405–409. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.