Back to Journals » Cancer Management and Research » Volume 12
Risk Factors for Postoperative Infectious Complications in Elderly Patients with Gastric Cancer
Authors Liu X , Xue Z, Yu J, Li Z, Ma Z, Kang W, Ye X, Jiang L
Received 12 March 2020
Accepted for publication 16 May 2020
Published 9 June 2020 Volume 2020:12 Pages 4391—4398
DOI https://doi.org/10.2147/CMAR.S253649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Xiao Liu,1 Zhigang Xue,2,3 Jianchun Yu,2 Zijian Li,2,3 Zhiqiang Ma,2 Weiming Kang,2 Xin Ye,2 Lin Jiang2,3
1Department of Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 3Graduate School, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
Correspondence: Jianchun Yu Email [email protected]
Background: Elderly patients with gastric cancer (GC) are at increased risk of infectious complications following gastrectomy. A limited set of risk factors has been identified to predict complications in these patients. To improve the safety of gastrectomy in this population, we investigated the incidence of infectious complications and associated clinicopathologic, nutritional and surgical risk factors in a cohort of elderly patients with GC.
Methods: Elderly GC patients (≥ 70 years) who underwent gastrectomy between January 2013 and December 2017 in Peking Union Medical College Hospital were included in the study. Clinicopathologic data were collected retrospectively. Severity of complications was classified using the Clavien–Dindo system. Infectious complications were assessed based on clinical diagnosis of health care-associated infection as defined by the US Centers for Disease Control and Prevention. Multivariate logistic regression analyses were performed to determine the risk factors for infectious complications.
Results: Three hundred thirty-one consecutive patients were included, with a median age of 74 years (range 70– 88). The rate of surgical morbidity was 37.5% and the mortality rate was 1.2%. The incidence of infectious complications was 19.6%, with the most common infectious complication being pulmonary infection (11.5%). Preoperative weight loss ≥ 5% (odds ratio [OR] = 2.21; 95% CI, 1.15– 4.28; p = 0.018), Charlson comorbidity index score ≥ 3 (OR = 2.83; 95% CI, 1.30– 6.16; p = 0.009) and preoperative hsCRP level ≥ 10 mg/L (OR = 2.48; 95% CI, 1.14– 5.38; p = 0.022) were independently associated with infectious complications.
Conclusion: Preoperative weight loss, elevated hsCRP level and comorbidity burden can be used to predict postoperative infectious complications in elderly GC patients. It is recommended to pay more attention to the treatment of elderly GC patients with these risk factors.
Keywords: gastric cancer, elderly, complications, risk factors, weight loss
Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed malignancy and the third leading cause of cancer death globally.1 In China, both incidence and mortality of GC rank second among malignancies and are significantly higher in the elderly population compared to the young.2 In 2015, there were an estimated 679,100 new cases and 498,000 deaths from GC in China; more than two-thirds of cases and more than three-fourths of GC deaths were accounted for by patients over 60 years old, a group that is growing as the Chinese population ages.3
The predominant curative therapy for GC is surgery, which achieved great success thanks to advances in minimally invasive surgical techniques.4,5 However, numerous challenges are involved in managing elderly GC patients, including greater incidence of postoperative complications.6–8 Specifically, owing to a greater comorbidity burden and lower functional reserve capacity, elderly patients are more susceptible to infectious complications, such as pneumonia,9,10 which can be fatal. There is no consistent definition of “elderly” patients achieved, but several studies indicated significance of surgical outcomes in GC patients when comparing patients older than 70 years with those ≤70 years.11–14
Recent studies show that postoperative complications are associated with poor short- and long-term outcomes in GC patients, such as reduced tolerance of adjuvant therapy15 and lower disease-specific survival.16,17 Surgeons must weigh the benefits of tumor resection as a potential curative therapy against the risk of postoperative complications in elderly GC patients in light of their limited life expectancy. Elderly patients with prolonged inflammatory response and increased comorbid possibly leads to high risk of infections after surgery.16 Limited studies implied that infectious complications were over 20% in older GC patients with prolonged the length of hospitalization and delayed recovery after surgery.17–19 However, evidence focused on infectious complications after gastrectomy and associated risk factors in elderly GC patients are quite little. Accordingly, we aimed to identify risk factors of infectious complications following gastrectomy in a cohort of elderly GC patients at an academic medical center in China.
Methods
Patients and Study Design
All patients ≥70 years old diagnosed with GC and undergoing surgery between January 2013 and December 2017 at Peking Union Medical College Hospital were evaluated for inclusion. Exclusion criteria were: (1) patients whose pathological diagnosis were not gastric adenocarcinoma, (2) surgical treatment was not gastrectomy, (3) study data were missing, and (4) patients underwent emergency surgery or had concomitant malignancy. Currently, there is no universally common definition of “elderly” patients.14 Previous studies found GC patients ≥70 years had significantly higher postoperative morbidity and mortality than those <70 years.11–14 The study protocol was reviewed and approved by the Institutional Review Board of Peking Union Medical College Hospital. Given the retrospective nature of the study, a rapid approval was granted by the PUMCH-IRB. Each patient provided written informed consent.
Data Collection and Evaluation
All data were collected retrospectively from electronical medical records. Variables collected included age and body mass index (BMI) at the time of surgery, comorbidities as evaluated using the Charlson comorbidity index (CCI, Supplementary Table 1),20,21 preoperative nutritional status as defined by nutritional risk screening (NRS 2002) score22 and prognostic nutritional index (PNI),23 preoperative weight loss of ≥5% total body weight within 12 months before surgery, and GC stage based on the 7th edition of American Joint Committee on Cancer TNM Classification System.24 Surgical data included type of operation, operation duration and intraoperative blood loss. We used 240 minutes25 and 400ml26 as cutoffs of operation duration and intraoperative blood loss, respectively.
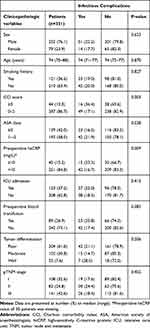 |
Table 1 Clinicopathologic Variables and Postoperative Infectious Complications |
The principal outcome was postoperative infectious complications at 30 days following surgery. Severity of postoperative complications was graded according to the Clavien–Dindo (CD) classification system.27 Complications were defined as CD grade II or more severe. Infectious complications were assessed based on a clinical diagnosis of health care-associated infection as defined by the US Centers for Disease Control and Prevention.28 Signs, symptoms, changed laboratory results and/or imaging evidence are used when confirming each incidence of infectious complications (Supplementary Table 2), such as fever (>38.0°C) and elevated white blood cell (≥12,000/mm3) and X-ray indicating new or progressive infiltrate. In patients with multiple complications, the most severe complication based on the CD classification was used in calculating the severity of complications. When calculating infectious complications, all infectious complications were included regardless of severity.
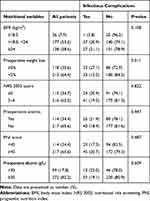 |
Table 2 Nutritional Variables and Postoperative Infectious Complications |
Statistical Analysis
Categorical data are presented as number (percentage, %) and continuous data as median (range). Incidence of infectious complications was compared based on clinicopathologic, nutritional, and surgical variables using the Mann–Whitney test, Pearson’s chi-square test, or Fisher’s exact test as appropriate. Multivariate analyses were then performed using logistic regression. Risk factors with a P-value close to 0.1 in univariate analyses were included in the multivariate model. Multivariate analysis was performed in a stepwise fashion, with a P-value of <0.05 considered statistically significant. All statistical analyses were performed using SPSS for Windows (Version 20, IBM, Chicago, IL, USA).
Results
Patient Characteristics
A total of 1446 GC patients underwent surgery during the study period. After applying exclusion criteria, 331 elderly patients who underwent gastrectomy for gastric adenocarcinoma were included in the study (Figure 1). The characteristics of the included patients including clinicopathologic, nutritional and surgical variables are summarized in Tables 1, 2 and 3, respectively. Among the 331 elderly GC patients, 76.1% were male and the median age was 74 years (range, 70–88 years). Two-thirds (67.4%, 223/331) of patients had advanced GC, 13.3% (44/331) had CCI score ≥3, and 15.2% (45/296) had preoperative high-sensitivity C-reactive protein (hsCRP) level ≥10 mg/L (Table 1). A total of 35.6% (118/331) of patients had preoperative weight loss ≥5%, 34.7% (115/331) had NRS 2002 score ≥5, and 34.4% (114/331) had PNI <45 (Table 2). More than half of patients (55.6%, 184/331) underwent traditional open surgery, 39.0% (139/331) underwent laparoscopic surgery and 5.4% (18/331) began with laparoscopic surgery which was then converted to an open procedure. Total gastrectomy was performed on 28.4% (94/331) of patients (Table 3).
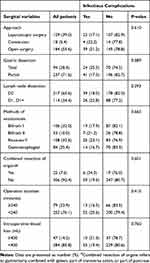 |
Table 3 Surgical Variables and Postoperative Infectious Complications |
Postoperative Complications
A total of 124 patients had complications, equating to a morbidity rate of 37.5% (Table 4). Complications categorized as CD grade II were observed in 23.3% (77/331) of patients. Grade III, complications were observed in 11.8% (n=39) of patients, and grades IV and V complications were each observed in 1.2% (n=4) of patients, respectively. The overall mortality rate was 1.2% (n=4); all four patients died of bleeding. Pulmonary infection was the most frequent complication, with an incidence of 11.5% (38/331); two patients with pulmonary infection were reintubated. The second most frequently-occurring complication was delayed gastric emptying, which was observed in 6.0% of patients (n=20). 4.2% of patients (n=14) had gastrointestinal (GI) fistula, including 11 with anastomotic fistula, two with pancreatic fistula and one with duodenal dump fistula. 3.3% of patients (n=11) had bleeding complications (5 intraabdominal bleeding and 6 GI bleeding). Infectious complications were observed in 19.6% of patients (n=65), including 48 patients (14.5%) with grade II complications, 15 patients (4.5%) with grade III and two patients (0.6%) with grade IV (Table 5).
 |
Table 4 Incidence of Any Complications and Infectious Complications by Clavien–Dindo Classification Grade (Total N=331) |
 |
Table 5 Types of Infectious Complications by Clavien–Dindo Classification Grade (Total N=331) |
Risk Factors for Infectious Complications
In multivariate analysis, we included CCI score, hsCRP, blood transfusion, BMI, weight loss and gastric dissection that were identified by the univariate analysis. We found that preoperative weight loss ≥5% (OR=2.21; 95% CI, 1.15–4.28, p=0.018), CCI score ≥3 (OR=2.83; 95% CI, 1.30–6.16, p=0.009) and preoperative hsCRP level ≥10mg/L (OR=2.48; 95% CI, 1.14–5.38, p=0.022) were independently associated with incidence of infectious complications (Table 6).
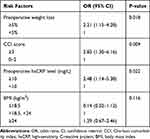 |
Table 6 Results from Multivariate Logistic Regression Analysis of Risk Factors for Infectious Complications |
Discussion
In the present study, we found that preoperative weight loss ≥5% had over 2-folded increased risk of postoperative infectious complications in elderly GC patients, and CCI score ≥ 3 (OR=2.83; 95% CI, 1.30–6.16) and elevated hsCRP ≥ 10 mg/L (OR=2.48; 95% CI, 1.14–5.38) were associated with significantly higher risks of infectious complications as well. These identified risk factors for postoperative infectious complications can be helpful when we had gastrectomy in elderly GC patients, and perioperative management may be improved. Preoperative nutrition supplement, better evaluation and control of comorbidities, and more attention to preoperative elevated hsCRP might be helpful to optimally reduce postoperative infectious complications in these patients based on our findings.
For patients with GC undergoing gastrectomy, the detailed nature of the association between age and postoperative complications is unclear. Some studies indicated that age was not a risk factor for postoperative complications when comparing elderly group (≥70 years) with non-elderly group (<70 years).11,12 However, studies with large cohort of patients proved that increased risks of postoperative complications in older patients. Hamilton et al demonstrated that age ≥75 years was independently associated with incidence of postoperative complications in a cohort of 3637 GC patients.8 Yang et al found that age ≥80 years was an independent risk factor for severe complications.7 Chronological age, as an irreversible factor, might be a potential risk factor for adverse outcomes after surgery, which leads to the significance of elderly population. However, there is no common and universal definition of “elderly” patients.14
Postoperative infectious complications are among the most important concerns in elderly patients undergoing surgery. Infectious complications can lead to prolonged length of stay and delayed adjuvant chemotherapy, resulting in adverse impacts on prognosis. Previous study found that the incidence of infectious complications increased with age in GC patients, as 19.6% in patients of 71–75 years, 21.4% in 76–80 years and 23.6% in patients older than 80 years.8 However, specific infections, such as pneumonia were not reported in this study. Takama et al demonstrated infectious complications occurred in 24.2% patients and pneumonia in 11.1% patients in a cohort of 190 elderly GC patients (≥75 years).18 Similarly, we reported that the incidence of infectious complications was 19.6% and pneumonia in 11.5% in a cohort of 331 elderly GC patients. We had relatively lower infection rate, probably because the definition of “elderly” patients is over 70 years, which is lower than that in these studies. Pneumonia is the most common infectious complications in GC patients.17–19 Tu et al and Miki et al found that 4.3–7.2% experienced postoperative pneumonia in surgical GC patients, which is lower than that we reported.17,19 One of the main reasons could be the younger populations (60–68 years) in these studies had relatively less proportion of impaired respiratory function. Consistent with these results, pneumonia was the most common postoperative infectious complication but a higher incidence was reported in our study.
In our analysis, we excluded fistula from postoperative infectious complications. Fistula, including anastomosis leakage, duodenal stump leakage and pancreatic leakage, is highly related to surgical procedures. In cases of anastomotic leakage, sufficient blood supply29 and adequate tension on the anastomosis site are the most important factors affecting healing. Anastomosis procedures have also been reported as risk factors for fistula.30,31 In contrast, postoperative infectious complications such as pneumonia are more related to patients’ physiological status, which is the focus of our study.
Nutrition is an especially crucial issue in elderly GC patients, and malnutrition is well recognized as a risk factor for complications after major abdominal surgery.32,33 We found that preoperative weight loss ≥5% was an independent risk factor for postoperative infectious complications. Weight loss may indicate not only malnutrition but also increased incidence of sarcopenia, which is defined as loss of lean body mass and is associated with physical dysfunction.34–36 BMI level was not related to postoperative infectious disease. Ejaz et al had similar findings that low BMI (<18.5 kg/m2) was not associated with postoperative infectious diseases but indicated decreased overall survival after gastrectomy for cancer.37 Compared to younger patients, elderly GC patients had a higher prevalence of malnutrition and in turn experienced significantly higher rates of postoperative infectious complications. Accordingly, we urge a greater focus on preoperative nutritional assessment and intervention in this population.
The CCI was originally developed to evaluate comorbid conditions with the goal of determining mortality risks20 and was used in our study to evaluate comorbidities. Some previous studies showed that CCI score predicted poor outcomes including morbidity.38 Consistent with these results, we found that elderly GC patients with a CCI score ≥3 had significantly higher risk of postoperative infectious complications.
Findings from our study and others suggest that preoperative hsCRP may play a significant role in the occurrence of infectious complications. CRP is one of the most important acute phase reactants in humans and is a widely used biomarker of inflammation. CRP has been recognized as a biomarker of acute infection, though the optimal cutoff point to predict infection has yet to be determined.39 Higher CRP levels indicate more severe systemic inflammation, which is associated with worse underlying disease and elevated risks of adverse outcomes, as seen in several previous studies. For example, Shimizu et al found that preoperative CRP >65 mg/L predicted surgical site infection after appendectomy (OR = 3.80; 95% CI, 1.31–11.04; p = 0.014).40 Zhou et al demonstrated that elevated preoperative CRP level (>1 mg/L) was significantly correlated with infectious complications after GC surgery (multivariate OR = 1.62; 95% CI, 1.22–2.15, p < 0.001).41 Additionally, Ishino et al found that preoperative CRP >5 mg/L was significantly associated with poor survival in GC patients following gastrectomy (hazard ratio = 6.26; 95% CI, 1.11–35.28, p = 0.038).42 We use the cutoff, 10 mg/L, of hsCRP to identify the significant risk of infection in our clinical practice. Indeed, we found that preoperative hsCRP level ≥10mg/L (OR 2.48; 95% CI, 1.14–5.38, p = 0.022) were independently associated with incidence of postoperative infectious complications.
Several limitations to our study should be acknowledged. First and most significantly, the retrospective nature of the study design limits the ability to draw conclusions regarding causality, and the sample size of our study is relatively small. Secondly, our study included patients from one single academic medical center, and treatment practices may be different in other hospitals. It should be cautious when extrapolating our findings to the broader population. Finally, postoperative complications were assessed by various clinicians and may not have been recorded consistently by the different providers. Nevertheless, to the best of our knowledge, this study is the first to determine potential risk factors for infectious complications after gastrectomy in elderly GC patients. Future investigations are needed with prospective, multi-center designs and larger sample sizes to verify potential risk factors for postoperative infectious complications in elderly GC patients and to determine the optimal cutoff point for defining “elderly” surgical patients.
Conclusions
In conclusion, we found that preoperative weight loss ≥5%, CCI score ≥3, and preoperative hsCRP level ≥10 mg/L independently predicted postoperative infectious complications in elderly GC patients undergoing gastrectomy. Clinicians are recommended to treatment elderly GC patients who had these risk factors with more attention.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; LX and ZGX drafted the article; all authors revised the draft critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Chen WQ, Zheng RS, Zhang SW, et al. [Analysis of cancer incidence and mortality in elderly population in China, 2013]. Zhonghua Zhong Liu Za Zhi [Chin J Oncol]. 2017;39(1):60–66. Chinese.
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Jeong GA, Cho GS, Kim HH, et al. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146(3):469–474.
5. Kim W, Kim HH, Han SU, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer. Ann Surg. 2016;263(1):28–35.
6. Takeuchi D, Koide N, Suzuki A, et al. Postoperative complications in elderly patients with gastric cancer. J Surg Res. 2015;198(2):317–326.
7. Yang JY, Lee HJ, Kim TH, et al. Short-and long-term outcomes after gastrectomy in elderly gastric cancer patients. Ann Surg Oncol. 2017;24(2):469–477.
8. Hamilton TD, Mahar AL, Haas B, et al. The impact of advanced age on short-term outcomes following gastric cancer resection: an ACS-NSQIP analysis. Gastric Cancer. 2018;21(4):710–719.
9. Kunisaki C, Akiyama H, Nomura M, et al. Comparison of surgical outcomes of gastric cancer in elderly and middle-aged patients. Am J Surg. 2006;191(2):216–224.
10. Yamada H, Shinohara T, Takeshita M, et al. Postoperative complications in the oldest old gastric cancer patients. Int J Surg. 2013;11(6):467–471. doi:10.1016/j.ijsu.2013.04.005
11. Cho GS, Kim W, Kim HH, et al. Multicentre study of the safety of laparoscopic subtotal gastrectomy for gastric cancer in the elderly. Br J Surg. 2009;96(12):1437–1442. doi:10.1002/bjs.6777
12. Biondi A, Cananzi FCM, Persiani R, et al. The road to curative surgery in gastric cancer treatment: a different path in the elderly? J Am Coll Surg. 2012;215(6):858–867. doi:10.1016/j.jamcollsurg.2012.08.021
13. Hartgrink HH, Van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22(11):2069–2077. doi:10.1200/JCO.2004.08.026
14. Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643. doi:10.7326/0003-4819-134-8-200104170-00008
15. Jin LX, Sanford DE, Squires MH, et al. Interaction of postoperative morbidity and receipt of adjuvant therapy on long-term survival after resection for gastric adenocarcinoma: results from the U.S. Gastric cancer collaborative. Ann Surg Oncol. 2016;23(8):2398–2408. doi:10.1245/s10434-016-5121-7
16. Kubota T, Hiki N, Sano T, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21(3):891–898. doi:10.1245/s10434-013-3384-9
17. Tu R H, Lin J X, Li P, et al. Prognostic significance of postoperative pneumonia after curative resection for patients with gastric cancer. Cancer Med. 2017;6(12):2757–2765. doi:10.1002/cam4.1163
18. Takama T, Okano K, Kondo A, et al. Predictors of postoperative complications in elderly and oldest old patients with gastric cancer. Gastric Cancer. 2015;18(3):653–661. doi:10.1007/s10120-014-0387-6
19. Miki Y, Makuuchi R, Tokunaga M, et al. Risk factors for postoperative pneumonia after gastrectomy for gastric cancer. Surg Today. 2016;46(5):552–556. doi:10.1007/s00595-015-1201-8
20. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
21. Hall WH, Ramachandran R, Narayan S, et al. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4(1):94. doi:10.1186/1471-2407-4-94
22. Kondrup J, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–336. doi:10.1016/S0261-5614(02)00214-5
23. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005.
24. Sobin L, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours.
25. Park H A, Park S H, Cho S I, et al. Impact of age and comorbidity on the short-term surgical outcome after laparoscopy-assisted distal gastrectomy for adenocarcinoma. Am Surg. 2013;79(1):40–48.
26. Mizuno A, Kanda M, Kobayashi D, et al. Adverse effects of intraoperative blood loss on long-term outcomes after curative gastrectomy of patients with stage II/III gastric cancer. Dig Surg. 2016;33(2):121–128. doi:10.1159/000443219
27. Dindo D, Demartines N, Clavien P A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
28. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi:10.1016/j.ajic.2008.03.002
29. Schietroma M, Cecilia EM, Carlei F, et al. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: a prospective randomized, double-blind, controlled, single-center trial. Ann Surg Oncol. 2013;20(5):1584–1590. doi:10.1245/s10434-012-2714-7
30. Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201(2):253–262. doi:10.1016/j.jamcollsurg.2005.02.002
31. Ri M, Hiki N, Ishizuka N, et al. Duodenal stump reinforcement might reduce both incidence and severity of duodenal stump leakage after laparoscopic gastrectomy with Roux-en-Y reconstruction for gastric cancer. Gastric Cancer. 2019;22(5):1053–1059. doi:10.1007/s10120-019-00946-8
32. Kuzu MA, Terzioğlu H, Genç V, et al. Preoperative nutritional risk assessment in predicting postoperative outcome in patients undergoing major surgery. World J Surg. 2006;30(3):378–390. doi:10.1007/s00268-005-0163-1
33. Schiesser M, Kirchhoff P, Müller MK, et al. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery. 2009;145(5):519–526. doi:10.1016/j.surg.2009.02.001
34. Ongaro E, Buoro V, Cinausero M, et al. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer. 2017;20(4):563–572.
35. Kim TN. Elderly obesity: is it harmful or beneficial? J Obes Metab Syndr. 2018;27(2):84–92.
36. Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22(1):309–323.
37. Ejaz A, Spolverato G, Kim Y, et al. Impact of body mass index on perioperative outcomes and survival after resection for gastric cancer. J Surg Res. 2015;195(1):74–82.
38. Hsu JT, Liu MS, Wang F, et al. Standard radical gastrectomy in octogenarians and nonagenarians with gastric cancer: are short-term surgical results and long-term survival substantial? J Gastrointest Surg. 2012;16(4):728–737.
39. Ticinesi A, Lauretani F, Nouvenne A, et al. C-reactive protein (CRP) measurement in geriatric patients hospitalized for acute infection. Eur J Intern Med. 2017;37:7–12.
40. Shimizu T, Ishizuka M, Kubota K. The preoperative serum C-reactive protein level is a useful predictor of surgical site infections in patients undergoing appendectomy. Surg Today. 2015;45(11):1404–1410.
41. Zhou J, Hiki N, Mine S, et al. Role of prealbumin as a powerful and simple index for predicting postoperative complications after gastric cancer surgery. Ann Surg Oncol. 2017;24(2):510–517.
42. Ishino Y, Saigusa S, Ohi M, et al. Preoperative C‐reactive protein and operative blood loss predict poor prognosis in patients with gastric cancer after laparoscopy‐assisted gastrectomy. Asian J Endosc Surg. 2014;7(4):287–294.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

