Back to Journals » Infection and Drug Resistance » Volume 15
Risk Factors for Mortality and Outcomes in Hematological Malignancy Patients with Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections
Authors Meng H , Han L, Niu M, Xu L, Xu M, An Q, Lu J
Received 24 May 2022
Accepted for publication 28 July 2022
Published 4 August 2022 Volume 2022:15 Pages 4241—4251
DOI https://doi.org/10.2147/IDR.S374904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Haiyang Meng,1 Lu Han,2 Mengxia Niu,3 Lu Xu,4 Min Xu,5 Qi An,1 Jingli Lu1
1Department of Pharmacy, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Pharmacy, Zhengzhou Second People’s Hospital, Zhengzhou, People’s Republic of China; 3Department of Pharmacy, Zhengzhou Western Hospital of Traditional Chinese Medicine, Zhengzhou, People’s Republic of China; 4Department of Clinical Laboratory, Henan Children’s Hospital, Zhengzhou, People’s Republic of China; 5Department of Clinical Laboratory, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Correspondence: Jingli Lu, Department of Pharmacy, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China, Tel +86-371-66913047, Email [email protected]
Background: This study aimed to identify risk factors for mortality and outcomes in hematological malignancy (HM) patients with bloodstream infection (BSI) caused by carbapenem-resistant Klebsiella pneumoniae (CRKP).
Methods: A retrospective study was conducted at a tertiary teaching hospital in Henan Province, China, between January 2018 and December 2021. All BSIs caused by CRKP in hospitalized HM patients were identified. Data on patient demographics, disease, laboratory tests, treatment regimens, outcomes of infection, and the antimicrobial susceptibility of each isolate were collected from medical records.
Results: A total of 129 patients with CRKP BSI were included in the study, and the 28-day mortality rate was 80.6% (104/129). In Cox analysis an absolute neutrophil count < 500 at discharge (hazard ratio [HR] 6.386, 95% confidence interval [CI] 3.074– 13.266, p < 0.001), intensive care unit admission (HR 1.834, 95% CI 1.065– 3.157, p = 0.029), and higher Pitt bacteremia score (HR 1.185, 95% CI 1.118– 1.255, p < 0.001) were independent risk factors associated with 28-day mortality. Survival curve analysis indicated that compared with ceftazidime-avibactam-based therapy, both polymyxin b (HR 8.175, 95% CI 1.099– 60.804, p = 0.040) and tigecycline (HR 14.527, 95% CI 2.000– 105.541, p =0.008) were associated with a higher risk of mortality.
Conclusion: In HM patients CRKP BSI resulted in high mortality. Intensive care unit admission, higher Pitt bacteremia score, and absolute neutrophil count < 500 at discharge were independently associated with higher mortality. Early initiation of new agents such as ceftazidime-avibactam may improve outcomes.
Keywords: bloodstream infection, carbapenem-resistant Klebsiella pneumonia, hematological malignancy, risk factor
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE), especially Klebsiella pneumonia (KP), has emerged as a global threat over the past decade. According to data from the China Antimicrobial Surveillance Network, KP resistance to meropenem and imipenem increased to 25.0% and 26.3% in 2018 respectively from 3.0% and 2.9% in 2005, and the resistance rate increased by more than 8 times.1 Due to primary immunodeficiency, neutropenia caused by hematopoietic stem cell transplantation or chemotherapy, and frequent exposure to broad-spectrum antibiotics, patients with a hematological malignancy (HM) appear to be at higher risk of carbapenem-resistant KP (CRKP) infection.2–5
The mortality rate of CRKP bloodstream infection (BSI) is > 50% worldwide, higher than that of other Gram-negative BSIs in patients with HM.6–8 Trecarichi et al9 conducted a prospective cohort study of 278 episodes of KP BSI in 13 Italian hematological units. They reported 21-day mortality rates of 52.2% in patients with CRKP BSI, and 14.5% in patients with carbapenem-susceptible KP (CSKP) BSI. Therefore, it is necessary to identify patients with CRKP early and accurately. However, studies on risk factors for mortality in HM patients with CRKP BSI have yielded inconsistent results. Septic shock, acute respiratory failure, inadequate initial antimicrobial therapy, rectal CRKP colonization, severe neutropenia, and invasive mechanical ventilation are reportedly independently associated with mortality.9,10 Considering the poor outcomes of HM patients with CRKP BSI, early initiation of an active therapy is critical. Nevertheless, antimicrobial therapy has largely relied on the use of older agents such as polymyxins, tigecycline, aminoglycosides, and fosfomycin, which have a high risk of toxicity and poor efficacy.11,12
The aim of the current study was to identify risk factors for mortality and outcomes in HM patients with CRKP BSIs.
Materials and Methods
Study Design and Patients
This retrospective study was conducted at the First Affiliated Hospital of Zhengzhou University in Zhengzhou, Henan Province, China. All episodes of BSI caused by CRKP that occurred between January 2018 and December 2021 in hospitalized HM patients were identified. The characteristics collected from the hospital electronic medical records system included patient demographics, disease, laboratory data, treatment regimens, outcome of infection, and antimicrobial susceptibilities of each isolate. In patients with recurrent infections, only the first episode was included in the analysis. Patients aged < 18 years were excluded from the study. The primary outcome was 28-day mortality after BSI onset. The study was reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2022-KY-0306-002). Because patient data were analyzed anonymously and confidentiality was maintained, the requirement for patient consent was waived. The study was conducted in accordance with the Declaration of Helsinki.
Definitions
The following terms were defined prior to data analysis. CRKP BSI was defined as an infection with a positive blood culture for CRKP. BSI onset was defined as the collection date of the first positive blood culture. Neutropenia and severe neutropenia were respectively defined as absolute neutrophil counts (ANCs) < 500 cells/mm3 and < 100 cells/mm3. Carbapenem exposure was defined as any carbapenem administered within 30 days before BSI. Appropriate early antimicrobial treatment was defined as the administration of active agents during the first 72 h of BSI onset. Pitt bacteremia score (PBS) was calculated within 48 h of the day of the first positive blood culture, and the highest score was recorded. Septic shock was defined as sepsis associated with hypotension and perfusion abnormalities refractory to organ dysfunction.13
Antibiotic Susceptibility Test
CRKP refers to KP isolates that were resistant to at least one carbapenem. The VITEK 2 Compact system (bioMérieux, Marcy l’Etoile, France) and the Phoenix100 automated system (Becton Dickinson, Spark, MD, USA) were used for isolate identification. The results were interpreted in accordance with the Clinical and Laboratory Standards Institute Document M100 edition (2020). For colistin, the results were interpreted in accordance with European Committee on Antimicrobial Susceptibility Testing clinical breakpoints (version 6.0). The combined modified carbapenem inactivation method and EDTA-CIM was used for phenotypic detection of carbapenemase-producing Enterobacteriaceae.
Statistical Analysis
Continuous variables with normal distributions are presented as means ± the standard deviation (SD), and those with non-normal distributions are presented as medians and interquartile ranges. Categorical variables are presented as frequencies and proportions. Cox regression analysis was conducted to identify independent risk factors for 28-day mortality. Variables with p values < 0.01 in univariate analysis were included in the Cox regression model. Optimal cutoffs for continuous variables were evaluated using receiver operating characteristic (ROC) curve analysis. The Kaplan–Meier method was used for survival analysis. All statistical analyses were performed using SPSS version 26.0 for Windows.
Results
Patient Characteristics
The clinical characteristics of 129 HM patients with CRKP BSI are summarized in Table 1. The average age of the population was 46.1 ± 13.8 years, and 65.9% of the patients were male. Seventy-seven patients were diagnosed with acute myeloid leukemia, which accounted for the majority (59.7%) of HM. At the onset of BSI, 10 (7.8%) patients had hematopoietic stem cell transplantation, 120 (93%) had neutropenia, and 98 (76%) had severe neutropenia. Only 31 (24%) patients with neutropenia had recovered prior to discharge. A total of 93 (72.1%) patients needed to be admitted to the intensive care unit (ICU), 86 (66.7%) patients had septic shock, and 70 (54.3%) patients needed mechanical ventilation. More than 90% of patients had been exposed to carbapenems prior to the onset of BSI, and more than 70% of patients received appropriate early antimicrobial therapy after the onset of BSI. Carbapenems and tigecycline were the most commonly used antimicrobial drugs, with respective frequencies of 82.9% and 66.7%.
 |
Table 1 Clinical Characteristics of 129 Patients with CRKP Bloodstream Infection Carbapenem-Resistant Klebsiella Pneumonia |
Microbiological Characteristics
A total of 129 isolates were identified as CRKP. The isolation rate of CRKP ranged from 28.7% to 48.6% during the study period (Figure 1). Only 22 (17%) of isolates were tested for carbapenemase, and all were KP carbapenemase. Colistin and tigecycline were the most active drugs, whereas ciprofloxacin and aztreonam were the least active (Table 2). Ceftazidime-avibactam (CAZ/AVI) susceptibility was not routinely tested at our hospital.
 |
Table 2 Antibiotic Susceptibility Characteristics of Klebsiella Pneumonia Isolates |
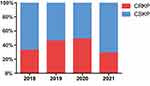 |
Figure 1 The isolation rate of CRKP during the study period. |
Risk Factors for 28-Day Mortality
The univariate analysis of Cox regression indicated that ANC < 500 at discharge, ICU admission, higher PBS, mechanical ventilation, septic shock, and length of hospitalization were associated with 28-day mortality, whereas CAZ/AVI treatment and appropriate early antimicrobial treatment were significantly associated with survival (p < 0.05). In the multivariate analysis of Cox regression, ANC < 500 at discharge (hazard ratio [HR] 6.386, 95% confidence interval [CI] 3.074–13.266, p < 0.001), ICU admission (HR 1.834, 95% CI 1.065–3.157, p = 0.029), and higher PBS (HR 1.185, 95% CI 1.118–1.255, p < 0.001) were identified as independent risk factors (Table 3). ROC curve analysis indicated that a PBS cut-off of 2 was a good predictor of mortality in HM patients with CRKP BSI, with an area under curve of 0.846 (95% CI 0.772–0903, p < 0.001), sensitivity of 72.1%, and specificity of 84% (Figure 2). In Kaplan-Meier curve analysis PBS ≥ 2 was associated with a higher risk of mortality (p < 0.001) (Figure 3A).
 |
Table 3 Univariate and Multivariate Cox Regression Analysis of Mortality of Patients with Carbapenem-Resistant Klebsiella Pneumonia Bloodstream Infection |
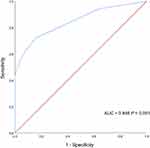 |
Figure 2 Receiver operating characteristic (ROC) curve of Pitt bacteremia score (PBS). |
 |
Figure 3 Kaplan-Meier survival estimates among patients with (A) PBS≥2 and PBS<2, (B) different antimicrobial regimen. |
Therapy and Outcomes
The overall 14-day mortality rate was 77.5% (100/129), and the 28-day mortality rate was 80.6% (104/129). Among patients who received active drugs as targeted therapy, 30 received polymyxin b-based therapy, 86 received tigecycline-based therapy, one received levofloxacin, and seven patients received CAZ/AVI-based therapy which was considered a potential therapeutic alternative. Survival curve analysis indicated that compared with CAZ/AVI-based therapy, both polymyxin b (HR 8.175, 95% CI 1.099–60.804, p = 0.040) and tigecycline (HR 14.527, 95% CI 2.000–105.541, p = 0.008) were associated with a higher risk of mortality (Figure 3B).
Discussion
CRKP is an emerging global health problem and poses a significant risk to immunocompromised patients. Few studies conducted in HM patients with carbapenem-resistant Gram-negative bacteria (including KP) BSI have reported independent risk factors for mortality, such as septic shock, acute respiratory failure, inadequate initial antimicrobial therapy, CRKP isolation, Acute Physiology and Chronic Health Evaluation score, prolonged neutropenia, and treatment with one active drug.9,14–17 In the current study ICU admission, higher PBS, and ANC < 500 at discharge were associated with 28-day mortality. It is not surprising that ICU admission was a risk factor for mortality, and previous studies have suggested that ICU admission increased the risk of mortality in general populations infected with CRKP.18,19 Mechanical ventilation and septic shock requiring vasopressors were the two most common indications for ICU admission in the current study, and they were also identified as independent predictors of mortality in HM patients in previous studies.20–25 Liu et al26 conducted a retrospective chart review of 121 sequential ICU admissions with HM over a 5-year period, with a 1-month mortality rate of 85.9%. In the present study more than 70% of patients were admitted to ICU, with a mortality rate of 92.4%, slightly higher than that reported by Liu et al,26 which could be related to the fact that all our patients were infected with CRKP.
The PBS is a severity of acute illness index widely used in infectious disease research, with a score ≥ 4 commonly used as an indicator of critical illness and increased risk of mortality.27–30 In recent studies higher PBS was an independent risk factor for mortality in CRE BSI, as well as in CRKP BSI, confirming the value of the PBS in such infections.31–36 However, reports on the use of PBS in HM patients with CRKP BSI are scarce. In the current study higher PBS was independently associated with mortality. Consistent with this finding, higher PBS has been associated with increased risk of mortality in HM patients with BSI.10 In the present study a PBS cutoff of 2 was a better predictor than the scores of 3 or 4 reported in previous studies, which could be a result of differences in patient characteristics.10,33,37 Overall, all these findings validated the predictive significance of the severity of illness with respect to mortality in HM patients with CRKP BSI.
Neutropenia is a common adverse effect of chemotherapy or hematopoietic stem cell transplantation in HM patients. Neutropenic HM patients with intestinal mucositis, central venous catheter, and gastrointestinal bacterial colonization are at high risk of BSI due to immunosuppression. In previous studies neutropenia and HM were independent risk factors for CRKP BSI.3,10,14,38 In addition, neutropenia is associated with the risk of mortality. Li et al39 identified neutropenia as an independent predictor of mortality in CRE BSI patients. Moreover, in the neutropenic patients with hematological diseases, unresolved neutropenia (> 14 days) was an independent predictor of death.16,17 Consistent with these findings, in the current study ANC < 500 at discharge was an independent risk factor for mortality in HM patients with CRKP BSI. Neutrophils are essential in the acute inflammatory response, as well as host defenses against bacterial infections. Neutropenia reduces the inflammatory response to developing infections, which promotes bacterial growth and invasion. The majority of patients in the present study had neutropenia or severe neutropenia at BSI onset, and remained unrecovered from neutropenia at discharge, resulting in a poor outcome. Therefore neutropenic patients require more attention, and new treatment strategies are urgently needed for these patients.
The most effective treatment strategy for CRKP BSI remains controversial. Polymyxins, tigecycline, and aminoglycosides were the most regularly used antibiotics, with at least one of them active in vitro. Tigecycline and polymyxin b are the two most commonly used active drugs at our center, but in the current study neither significantly affected patient outcome in univariate analysis, which was consistent with previous studies. In retrospective studies tigecycline has exhibited poor efficacy in patients with CRKP BSI, possibly due to suboptimal doses and blood concentrations.36,38 In addition, in a systematic review of 3195 CRKP-infected patients there were no statistically significant differences in the pooled likelihood of mortality between combination-containing and combination-sparing regimens of polymyxins, tigecycline, aminoglycosides, and carbapenems.40 In the present study CAZ/AVI-based therapy was associated with a lower risk of mortality than polymyxin b. Similar findings were obtained in the CRACKLE study, in which using CAZ/AVI was associated with lower all-cause hospital mortality and better benefit-risk outcomes than colistin.41 Furthermore, several previous retrospective studies have supported the superiority of CAZ/AVI in the treatment of CRKP infection.42–44 Given the modest number of patients who received CAZ in the current study, the efficacy of CAZ/AVI in patients with CRKP BSI needs to be confirmed in larger-scale trials.
The mortality rate of CRE BSI in immunocompromised hosts is surprisingly high, ranging from 45% to 100% in HM patients,9,10,14,45,46 25% to 82% in solid organ transplant recipients,47–49 and 63.7% to 72.7% in solid tumor patients.50,51 There was a high mortality rate in the present study (80.6%). The high proportion of patients with shock, acute myeloid leukemia, neutropenia, and ICU admission, and the low number of patients receiving active antibiotics such as polymyxin, aminoglycosides, and CAZ/AVI as targeted treatment may be the key reasons for the high mortality rate. The majority of patients died within 14 days. Preemptive detection of CRKP colonization, early identification of at-risk patients, and initiation of antibiotics active against CRKP isolates including new drugs such as CAZ/AVI may help to reduce mortality in patients with HM.52,53
The current study had several limitations. It was conducted in a hospital with a high prevalence of CRKP, and only included HM patients. The findings may not be applicable to other settings or patients. Furthermore, not all patients underwent phenotypic screening and detection. Lastly, the small number of patients in the study may have influenced the capacity of the analysis to identify risk factors and outcomes.
Conclusions
HM patients with CRKP BSI exhibited a high mortality rate. ICU admission, higher PBS, and ANC < 500 at discharge were independently associated with higher mortality. Early initiation of new agents such as CAZ/AVI may improve outcomes in these patients.
Abbreviations
ANC, absolute neutrophil count; BSI, bloodstream infection; CAZ/AVI, ceftazidime/avibactam; CRKP, carbapenem-resistant Klebsiella pneumoniae; HM, hematological malignancy; KP, Klebsiella pneumoniae; PBS, Pitt bacteremia score; ROC, receiver operating characteristic; SD, standard deviation.
Data Sharing Statement
All data analyzed during this study are included in this published article.
Consent for Publication
Not applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82073860).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281.
2. Zhang Y, Guo LY, Song WQ, Wang Y, Dong F, Liu G. Risk factors for carbapenem-resistant K. pneumoniae bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis. 2018;18(1):248.
3. Giacobbe DR, Del Bono V, Trecarichi EM, et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect. 2015;21(12):1106 e1101–1108.
4. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106.
5. Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;58(9):1274–1283.
6. Girmenia C, Rossolini GM, Piciocchi A, et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a nationwide retrospective survey from Italy. Bone Marrow Transplant. 2015;50(2):282–288.
7. Tang Y, Xu C, Xiao H, Wang L, Cheng Q, Li X. Gram-Negative Bacteria Bloodstream Infections in Patients with Hematological Malignancies - The Impact of Pathogen Type and Patterns of Antibiotic Resistance: a Retrospective Cohort Study. Infect Drug Resist. 2021;14:3115–3124.
8. Andria N, Henig O, Kotler O, et al. Mortality burden related to infection with carbapenem-resistant Gram-negative bacteria among haematological cancer patients: a retrospective cohort study. J Antimicrob Chemother. 2015;70(11):3146–3153.
9. Trecarichi EM, Pagano L, Martino B, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. 2016;91(11):1076–1081.
10. Zhang P, Wang J, Hu H, et al. Clinical Characteristics and Risk Factors for Bloodstream Infection Due to Carbapenem-Resistant Klebsiella pneumoniae in Patients with Hematologic Malignancies. Infect Drug Resist. 2020;13:3233–3242.
11. Pouch SM, Satlin MJ. Carbapenem-resistant Enterobacteriaceae in special populations: solid organ transplant recipients, stem cell transplant recipients, and patients with hematologic malignancies. Virulence. 2017;8(4):391–402.
12. Giannella M, Bartoletti M, Conti M, Righi E. Carbapenemase-producing Enterobacteriaceae in transplant patients. J Antimicrob Chemother. 2021;76(Suppl 1):i27–i39.
13. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
14. Micozzi A, Gentile G, Minotti C, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis. 2017;17(1):203.
15. Huang ST, Chiang MC, Kuo SC, et al. Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. 2012;45(5):356–362.
16. Wang L, Wang Y, Fan X, Tang W, Hu J. Prevalence of Resistant Gram-Negative Bacilli in Bloodstream Infection in Febrile Neutropenia Patients Undergoing Hematopoietic Stem Cell Transplantation: a Single Center Retrospective Cohort Study. Medicine. 2015;94(45):e1931.
17. Tofas P, Skiada A, Angelopoulou M, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: analysis of 50 cases. Int J Antimicrob Agents. 2016;47(4):335–339.
18. Dai G, Xu Y, Kong H, Xie W, Wang H. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection and associated clinical outcomes. Am J Transl Res. 2021;13(6):7276–7281.
19. Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52(3):1028–1033.
20. Grgic Medic M, Gornik I, Gasparovic V. Hematologic malignancies in the medical intensive care unit–Outcomes and prognostic factors. Hematology. 2015;20(5):247–253.
21. Yeo CD, Kim JW, Kim SC, et al. Prognostic factors in critically ill patients with hematologic malignancies admitted to the intensive care unit. J Crit Care. 2012;27(6):739 e731–736.
22. Judickas S, Stasiunaitis R, Zucenka A, Zvirblis T, Serpytis M, Sipylaite J. Outcomes and Risk Factors of Critically Ill Patients with Hematological Malignancy. Prospective Single-Centre Observational Study. Medicina. 2021;57(12):87.
23. Bird GT, Farquhar-Smith P, Wigmore T, Potter M, Gruber PC. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 yr study. Br J Anaesth. 2012;108(3):452–459.
24. Kroschinsky F, Weise M, Illmer T, et al. Outcome and prognostic features of intensive care unit treatment in patients with hematological malignancies. Intensive Care Med. 2002;28(9):1294–1300.
25. Namendys-Silva SA, Gonzalez-Herrera MO, Garcia-Guillen FJ, Texcocano-Becerra J, Herrera-Gomez A. Outcome of critically ill patients with hematological malignancies. Ann Hematol. 2013;92(5):699–705.
26. Liu J, Cheng Q, Yang Q, et al. Prognosis-related factors in intensive care unit (ICU) patients with hematological malignancies: a retrospective cohort analysis in a Chinese population. Hematology. 2015;20(9):494–503.
27. Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11(1):7–12.
28. Lee CC, Wang JL, Lee CH, et al. Age-Related Trends in Adults with Community-Onset Bacteremia. Antimicrob Agents Chemother. 2017;61:12.
29. Yu VL, Chiou CC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37(2):230–237.
30. Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009;31(2):146–150.
31. Liu KS, Tong YS, Lee MT, Lin HY, Lu MC. Risk Factors of 30-Day All-Cause Mortality in Patients with Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infection. J Pers Med. 2021;11:7.
32. Wang X, Wang Q, Cao B, et al. Retrospective Observational Study from a Chinese Network of the Impact of Combination Therapy versus Monotherapy on Mortality from Carbapenem-Resistant Enterobacteriaceae Bacteremia. Antimicrob Agents Chemother. 2019;63:1.
33. Gomez-Simmonds A, Nelson B, Eiras DP, et al. Combination Regimens for Treatment of Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections. Antimicrob Agents Chemother. 2016;60(6):3601–3607.
34. Chen L, Han X, Li Y, Li M. Assessment of Mortality-Related Risk Factors and Effective Antimicrobial Regimens for Treatment of Bloodstream Infections Caused by Carbapenem-Resistant Enterobacterales. Antimicrob Agents Chemother. 2021;65(9):e0069821.
35. Shen L, Lian C, Zhu B, et al. Bloodstream Infections due to Carbapenem-Resistant Klebsiella pneumoniae: a Single-Center Retrospective Study on Risk Factors and Therapy Options. Microb Drug Resist. 2021;27(2):227–233.
36. Xiao T, Zhu Y, Zhang S, et al. A Retrospective Analysis of Risk Factors and Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Bacteremia in Nontransplant Patients. J Infect Dis. 2020;221(Suppl 2):S174–S183.
37. Jorgensen SCJ, Trinh TD, Zasowski EJ, et al. Evaluation of the INCREMENT-CPE, Pitt Bacteremia and qPitt Scores in Patients with Carbapenem-Resistant Enterobacteriaceae Infections Treated with Ceftazidime-Avibactam. Infect Dis Ther. 2020;9(2):291–304.
38. Chang H, Wei J, Zhou W, et al. Risk factors and mortality for patients with Bloodstream infections of Klebsiella pneumoniae during 2014-2018: clinical impact of carbapenem resistance in a large tertiary hospital of China. J Infect Public Health. 2020;13(5):784–790.
39. Li C, Li Y, Zhao Z, Liu Q, Li B. Treatment options and clinical outcomes for carbapenem-resistant Enterobacteriaceae bloodstream infection in a Chinese university hospital. J Infect Public Health. 2019;12(1):26–31.
40. Agyeman AA, Bergen PJ, Rao GG, Nation RL, Landersdorfer CB. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int J Antimicrob Agents. 2020;55(1):105833.
41. van Duin D, Lok JJ, Earley M, et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171.
42. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob Agents Chemother. 2017;61:8.
43. Hakeam HA, Alsahli H, Albabtain L, Alassaf S, Al Duhailib Z, Althawadi S. Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant Enterobacteriaceae bacteremia. Int J Infect Dis. 2021;109:1–7.
44. Fang J, Li H, Zhang M, et al. Efficacy of Ceftazidime-Avibactam Versus Polymyxin B and Risk Factors Affecting Clinical Outcomes in Patients With Carbapenem-Resistant Klebsiella pneumoniae Infections a Retrospective Study. Front Pharmacol. 2021;12:780940.
45. Pagano L, Caira M, Trecarichi EM, et al. Carbapenemase-producing Klebsiella pneumoniae and hematologic malignancies. Emerg Infect Dis. 2014;20(7):1235–1236.
46. Lalaoui R, Javelle E, Bakour S, Ubeda C, Rolain JM. Infections Due to Carbapenem-Resistant Bacteria in Patients With Hematologic Malignancies. Front Microbiol. 2020;11:1422.
47. Bergamasco MD, Barroso Barbosa M, de Oliveira Garcia D, et al. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl Infect Dis. 2012;14(2):198–205.
48. Raviv Y, Shitrit D, Amital A, et al. Multidrug-resistant Klebsiella pneumoniae acquisition in lung transplant recipients. Clin Transplant. 2012;26(4):E388–394.
49. Mouloudi E, Massa E, Papadopoulos S, et al. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae among intensive care unit patients after orthotopic liver transplantation: risk factors for infection and impact of resistance on outcomes. Transplant Proc. 2014;46(9):3216–3218.
50. Ghafur AK, Vidyalakshmi PR, Kannaian P, Balasubramaniam R. Clinical study of carbapenem sensitive and resistant Gram-negative bacteremia in neutropenic and nonneutropenic patients: the first series from India. Indian J Cancer. 2014;51(4):453–455.
51. Freire MP, Pierrotti LC, Filho HH, et al. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur J Clin Microbiol Infect Dis. 2015;34(2):277–286.
52. Sahitya DSK, Jandiyal A, Jain A, et al. Prevention and management of carbapenem-resistant Enterobacteriaceae in haematopoietic cell transplantation. Ther Adv Infect Dis. 2021;8:20499361211053480.
53. Micozzi A, Gentile G, Santilli S, et al. Reduced mortality from KPC-K.pneumoniae bloodstream infection in high-risk patients with hematological malignancies colonized by KPC-K.pneumoniae. BMC Infect Dis. 2021;21(1):1079.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
