Back to Journals » Cancer Management and Research » Volume 12
Risk Factors for Lymph Node Metastasis and Survival Outcomes in Colorectal Neuroendocrine Tumors
Authors Wu Z , Wang Z, Zheng Z, Bi J, Wang X, Feng Q
Received 7 April 2020
Accepted for publication 14 July 2020
Published 11 August 2020 Volume 2020:12 Pages 7151—7164
DOI https://doi.org/10.2147/CMAR.S256723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Zijian Wu, Zhijie Wang, Zhaoxu Zheng, Jianjun Bi, Xishan Wang, Qiang Feng
Department of Colorectal Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Qiang Feng Department of Colorectal Surgery
National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 17 Panjiayuan South Road, Chaoyang District, Beijing 100021, People’s Republic of China
Email [email protected]
Objective: The aim of our study was to analyze the factors affecting lymph node metastasis (LNM) and the prognosis of colorectal neuroendocrine tumors (NETs).
Patients and Methods: A retrospective analysis was conducted to collect the clinical data of 135 patients with colorectal NETs from January 2000 to December 2018, including clinical manifestations, pathological results, treatment methods, etc. Follow-up was regularly performed to observe the recurrence and metastasis of tumors and to identify the clinical and pathological features of colorectal NETs, risk factors for LNM and survival outcomes.
Results: Among 135 patients, there were 57 (42.2) patients with LNM, and the independent risk factors for LNM in the multivariable analyses were tumor diameter ≥ 2 cm (P= 0.040) and tumor grade G3 (P=0.001). Patients were followed up for 1 to 190 months, and of the 133 patients who were successfully followed up, the 5-year OS was 71.7%, and the 5-year PFS was 69.0%. The multivariate analysis for survival outcomes showed that age ≥ 65 years (P=0.002/< 0.001) and lymph node metastasis (P=0.018/0.025) were independent risk factors affecting 5-year PFS and OS in colorectal neuroendocrine tumors. Tumors in the colon (P=0.022), moderately positive (++) CgA (P=0.010) and strongly positive (+++) CgA (P=0.007) were independent risk factors for poor 5-year PFS in patients with colorectal NETs.
Conclusion: Rectal NETs have a better prognosis than colonic neuroendocrine tumors. Tumor diameter and tumor grade are independent risk factors for LNM in colorectal neuroendocrine tumors. Age, tumor location, lymph node status and a positive level of the neuroendocrine marker CgA are independent risk factors that affect the prognosis of colorectal NETs.
Keywords: colorectal neoplasms, neuroendocrine neoplasms, lymph node metastasis, prognosis, survival analysis
Introduction
Gastroenteropancreatic neuroendocrine neoplasms (GEP NENs) as well as neuroendocrine tumors (GEP NETs) are heterogeneous tumors that originate from peptidergic neurons and neuroendocrine cells, previously described as “carcinoid tumors” in 1907.1 Most NETs are indolent tumors compared with other epithelial malignancies; however, they are reported to have the potential to metastasize even in well-differentiated tumors and are resistant to therapies.1–3 Data from the Surveillance, Epidemiology and End Results (SEER) database indicate that the incidence of NETs has increased significantly, approximately 5 times, reaching 5.25/100.000 cases/year, of which GEP NETs account for approximately 65% to 74% of all NETs, and the incidence and prevalence of colorectal NETs are inferior only to those of colorectal adenocarcinoma.1,4-6 In addition, the tumor sites varied markedly by race, with the incidence of rectal NETs among Asian populations increasing from 0.2 per 100,000 in 1973 to 0.86 per 100,000 in 2004, which was significantly higher than that among white populations.1,4,7 Similarly, the incidence of rectal neuroendocrine tumors (R-NETs) grew fastest among all NETs, by approximately 8 times compared with the incidence in 2001, accounting for 29.6% of all NETs, which makes it the second most common NET in China.8
For localized colorectal NETs, endoscopic resection, including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), and surgery, including transanal excisions as well as surgical resections, are both effective methods. For metastatic tumors, somatostatin analogs (SSAs), radiation, radiofrequency ablation (RFA), chemotherapy, targeted therapy and peptide receptor radionuclide therapy (PRRT) are all alternatives. However, the 5-year survival rate of NETs with lymph node metastasis (LNM) or distant metastasis is still disappointing, with five-year overall survival rates of approximately 54–74% and 28–44.1%, respectively.6,9-11
The prognostic factors of colorectal NETs have been explored by numerous studies. Tumor stage, location, size, grade, lymphovascular invasion, and the status of resection margins are major factors that have been reported to be associated with LNM and poor prognosis.12 However, these studies were highly heterogeneous, which affected the accuracy of the meta-analysis and the effectiveness of these conclusions.
Currently, controversies remain in the treatment of colorectal NETs. Experts from the Chinese Society of Clinical Oncology (CSCO) agreed that colonic NETs greater than 1 cm and less than 2 cm could be completely resected endoscopically when the T stage was less than T2, but the National Comprehensive Cancer Network (NCCN) recommends that these tumors be treated by surgery in accordance with the guidelines for colon adenocarcinoma.13–15
The aim of this study was to evaluate the outcomes of colorectal NETs, explore the risk factors for lymph node metastasis in colorectal NETs and identify the prognostic factors for survival outcomes.
Methods
Clinical Data Collection
Between 2000 and 2018, a total of 161 consecutive patients received treatments for colorectal NETs in our center. We constructed a database of retrospectively collected data from patients’ medical records, including clinical characteristics, pathological reports, recurrence and survival during the follow-up period.
For radical resection with lymph node dissection, lymph node metastasis was detected by pathological evaluation. For local excision, such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) or transanal excision (TAE), LNM was evaluated through computed tomography (CT) or magnetic resonance imaging (MRI) before the treatment and during the follow-up periods. The diagnosis of a metastatic lymph node was based on the following criteria: 1) Size criteria: short axis diameter of lymph nodes was greater than 8 mm for round lymph nodes and greater than 10 mm for ovoid lymph nodes. 2) Morphological abnormalities: irregular contour and margin, unclear border, heterogeneous internal echoes or signal intensity.16,17 The tumor diameter refers to the longest diameter of the tumor according to pathology reports. For patients with distant metastases, tumor diameter was determined by endoscopic findings before treatment.
Pathological Diagnosis
The tumor stage was classified according to the American Joint Committee on Cancer (AJCC) cancer staging manual, 7th edition and 8th edition,18,19 and the tumor grade was classified according to the 2010 classification.20 For 13 patients before 2010, we revised their pathology results and found that they were all neuroendocrine carcinomas (NECs); therefore, we classified them as having G3 grade tumors. The mitosis count (N=23) was expressed as the number of mitotic cells in ten high-power fields (HPFs) from hematoxylin and eosin (H&E)-stained slides examined with microscopy. According to ENETS/WHO guidelines, G1 grade: mitotic image <2/10 HPFs; G2 grade: mitotic image (2~20)/10 HPFs; and G3 grade: mitotic images>20/10 HPFs. The Ki-67 index was calculated as the percentage of cells labeled by immunohistochemistry. According to ENETS/WHO guidelines, G1 grade: Ki-67 positive index≤2%; G2 grade: Ki-67 positive index 3% to 20%; and G3 grade: Ki-67 positive index>20%. The expression levels of chromogranin A (CgA) (N=101) and synaptophysin (Syn) (N=109) were scored according to the percentage of positive cells and the intensity of cell staining. The positive cell percentage score was based on the following system: 0 points, no positive cells; 1 point, positive cells accounting for 1% to 10%; 2 points, positive cells accounting for 11% to 50%; 3 points, positive cells accounting for 51% to 80%; and 4 points, positive cells accounting for 81% to 100%. The positive cell staining intensity score was based on the following system: 0 points, negative; 1 point, weakly positive; 2 points, moderate positive; and 3 points, strong positive. The two scores were multiplied together: 0 points for negative; 1 to 4 points for weak positive (+); 5 to 8 points for moderate positive (++); and 9 to 12 points for strong positive (+++).
Inclusion Criteria
Patients who were treated in our center for localized and metastatic colorectal NETs from 2000 to 2018.
Exclusion Criteria
- Patients who had colorectal NETs combined with other malignancies.
- Patients for whom the pathological diagnosis was mixed adenoneuroendocrine carcinoma.
- Patients for whom there was insufficient clinical information or inappropriate pathology reports from outside hospitals.
Risk factors for lymph node metastasis and prognostic factors related to survival were investigated in all patients.
The primary endpoint was progression-free survival (PFS), which was defined as the interval between initial treatment and the first documentation of disease progression or death.
Statistical Analysis
Statistical analysis was performed using SPSS for Mac, version 24.0 (SPSS, Chicago, IL, USA). Continuous data are described as means±SDs in this study. The risk factors for LNM were assessed using Pearson’s χ2 test in univariable analysis and logistic regression analyses in multivariable analysis. The 5-year overall survival (OS) and progression-free survival (PFS) were analyzed with the Kaplan–Meier method. Variables were compared with the Log rank test, and the multivariable analysis for survival outcomes was conducted using the Cox proportional hazards model. Statistical significance was accepted for p values < 0.05.
Results
Clinical and Histopathological Characteristics (Figure 1)
A total of 135 patients were included in our study (Figure 1). The age of the patients was 53.3±13.0 years, and the male:female ratio was 89 (65.9%): 46 (34.1%). The frequencies of grade G1, G2, and G3 NETs were 69 (51.1%), 13 (9.6%) and 53 (39.3%), respectively. Of the 135 patients, 65 (48.1%) patients were resected locally, 18 (13.3%) by EMR, 35 (25.9%) by ESD and 12 (8.9%) by transanal excision. In addition, 52 (38.5%) NETs were surgically resected, including 34 (25.2%) radical resections, 7 (5.2%) multivisceral resections and 11 (8.1%) palliative resections due to distant metastasis. The remaining 18 (13.3%) patients were treated by systemic treatment due to distant metastasis. The most commonly used chemotherapeutic regimen in our center was the EP regimen (etoposide and cisplatin) as the first-line chemotherapy, and the second-line chemotherapy was variable and included the XELOX regimen (oxaliplatin and capecitabine); the FOLFOX regimen (oxaliplatin, calcium folate and 5-FU); and everolimus, temozolomide, and tegafur/gimeracil/oteracil and combinations between them. The tumor diameter was less than 2 cm in 74 (54.8%) patients, and the distance from the anal verge was less than 10 cm in 102 (75.6%) patients. LNM was found in 57 (42.2%) cases, and distant metastasis occurred in 36 (26.7%) patients. Two patients had radiologically determined LNM after TAE in the follow-up period, and one of them went on to undergo radical surgery; the other patient was also found to have liver metastasis; therefore, he was treated with chemotherapy. The clinical and histopathological characteristics are summarized in Table 1.
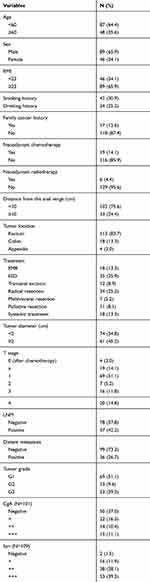 |
Table 1 Clinical and Histopathological Characteristics of Colorectal NETs (N=135) |
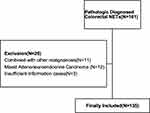 |
Figure 1 Flowchart of the selection of patients. |
Risk Factors for LNM
The risk factors for LNM through univariate analysis were tumor location in the colon (p=0.001), tumor diameter ≥2 cm (p<0.001), T stage (p<0.001), tumor grade (p<0.001) and the positive degree of Syn (p=0.012) and CgA (p=0.049) (Table 2). In multivariable analyses, tumor diameter ≥2 cm (OR 36.515, 95% CI 1.175~1134.341, p=0.040) and tumor grade G3 (OR 133.495, 95% CI 7.686~2318.690, p=0.001) were independent risk factors for LNM in colorectal NTEs. Tumor location in the colon (OR 0.718, 95% CI 0.033~15.676, p=0.083) and tumor grade G2 (OR 14.788, 95% CI 0.835~262.038, p=0.066) might be independent risk factors for LNM, even though the p value was greater than 0.05 (Table 3).
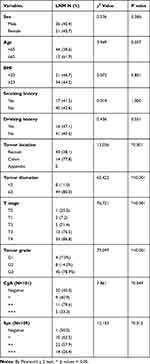 |
Table 2 Univariable Analysis for Risk Factors for Lymph Node Metastasis (N = 135) |
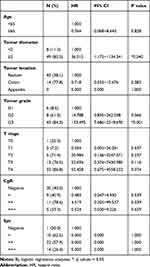 |
Table 3 Multivariable Analysis for Risk Factors for Lymph Node Metastasis (N=135) |
Risk Factors for Survival Outcomes
The median follow-up period was 38.0 months (range, 1~190 months). A total of 30 (22.2%) patients died in this cohort. In 36 patients with distant metastasis before treatment, 13 patients died during chemotherapy, 2 patients died after multivisceral resections, and 4 patients died after palliative resections due to tumor progression. In 99 patients without distant metastasis before treatment, 10 patients died due to the recurrence of distant metastasis at the liver (3), peritoneum (2), lung (2), pleura (2), and brain (1), and 1 patient died of severe lung infections. The 5-year progression-free survival (PFS) and overall survival (OS) rates of all patients were 67.9% and 71.6%, respectively. The prognostic factors for the 5-year PFS and OS rate in all patients were age, neoadjuvant chemotherapy, tumor diameter, tumor location, tumor grade, LNM, CgA level and treatment method (Table 4). In the multivariable analysis, age (≥65, HR, 5.728/11.456, 95% CI, 1.881~17.442/3.203~40.974, p=0.002/0.001) and LNM (yes, HR 20.548/17.189, 95% CI 1.684~250.757/1.427~207.117, p=0.018/0.025) were independent risk factors for 5-year PFS and OS. The CgA level [moderate positive (++), HR 8.094, 95% CI, 1.650~39.280, p=0.010 and strong positive (+++), HR, 12.422, 95% CI, 2.012~76.696, p=0.007] were independent risk factors for 5-year PFS. Tumor diameter ≥2 cm (HR 16.274, 95% CI, 0.858~308.738, p=0.063) and tumor grade G3 (HR 0.064, 95% CI, 0.003~1.528, p=0.090) were independent risk factors for 5-year PFS, even though the p value was greater than 0.05 (Table 5).
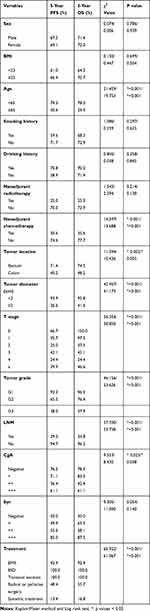 |
Table 4 Prognostic Factors for Survival Outcomes |
 |
Table 5 Multivariable Analysis for Survival Outcomes |
Comparison of Two Age Groups
In univariable analyses, 114 patients were in the <65-year group, and 21 patients were in the ≥65-year group. The proportion of tumors with a diameter ≥2 cm was significantly higher in the ≥65-year group than in the <65-year group (71.4% vs 40.7%, p=0.016). There were also significantly more patients with LNM in the ≥65-year group (61.9% vs 38.6%, p=0.041). For T stage, the proportion of early-stage (T1) patients in the ≥65-year group was significantly less than that in the <65-year group, although p was >0.05 (33.3% vs 54.4%, p=0.086). For treatments, there were significantly more patients who were treated with systematic chemotherapy in the ≥65-year group (33.3% vs 9.6%, p=0.040).
Different Operative Methods for T1N0M0 Colorectal NETs
There was no significant difference in tumor grade, tumor location, surgical margin, relapse or 5-year OS%, except for tumor diameter (p=0.012). The diameters of tumors resected by EMR, ESD and transanal excision were 0.56±0.46 cm, 0.79±0.40 cm and 1.10±0.50 cm, respectively (Table 6).
 |
Table 6 Comparison of Different Treatment Methods for T1N0M0 Colorectal NETs |
Kaplan–Meier Survival Curves
Kaplan–Meier survival curves for OS and PFS according to tumor grade, diameter, location, and CgA level are shown in Figures 2–6.
 |
Figure 2 (A) PFS curves according to tumor grade. (B) OS curves according to tumor grade. |
 |
Figure 3 (A) PFS curves according to tumor diameter. (B) OS curves according to tumor diameter. |
 |
Figure 4 (A) PFS curves according to tumor location. (B) OS curves according to tumor location. |
 |
Figure 5 (A) PFS curves according to age. (B) OS curves according to age. |
 |
Figure 6 (A) PFS curves according to CgA level. (B) OS curves according to CgA level. |
Discussion
NETs have a relatively good prognosis, with a median OS time reported to be 9.3 years (112 months) and a 5-year OS rate of 60~84.4%.3,11,21 However, the survival outcomes varied significantly at different stages of NETs. The 5-year OS rate of stage I and II tumors was reported to be as high as 90% and 77% but dropped to 50% and 15% for stage III and IV tumors, respectively.22 According to epidemiological data from the SEER database and GKR (Joint Cancer Registry), the 5-year OS rates of lymph node metastases (stage IIIb) and distant metastases (stage IV) are 54~74% and 15–37%, respectively.1,6,10 The 5-year OS rates of all patients and patients without distant metastasis were 71.6% and 90.4%, respectively, and the 5-year PFS rates were 67.9% and 87.7%, respectively. Lymph node metastasis is the most important factor that determines the prognosis of NETs, and the prediction of lymph node metastasis is necessary for clinicians to choose a suitable treatment. The aim of our research was to explore the predictive factors for lymph node metastasis of colorectal NETs and assess the current therapeutic algorithm.
Most NETs are at the G1 phase, and G2 or G3 phases account for only 2% to 13% of all NETs and have been reported to be risk factors for LNM by numerous studies.12,23 A multicenter clinical study in China showed that pathological type G3 NEC is an independent risk factor affecting the prognosis of patients with rectal NETs (p= 0.005).24 Similarly, Sohn et al3 found that the LNM rate of G1 phase rectal NETs was only 6%, but it increased remarkably to 75% at the G2 phase. In our study, our results showed that histological tumor grades G2 (p= 0.066) and G3 (p=0.001) were independent risk factors for lymph node metastasis. Lymph node metastasis occurred in 84.3% of patients with G3 tumors and 61.5% with G2 tumors but only 8.5% with G1 tumors. The 5-year OS rate decreased sharply from 92.2% to 39.4% when the tumor grade increased from G3 to G1. However, tumor grade was not significant in survival outcomes, possibly because tumor grade affects survival outcomes indirectly by directly affecting lymph node status.
Tumor size has been reported to be a strong predictive factor for lymph node metastasis. Previous studies have shown that a tumor less than 10 mm is usually limited to the submucosa with a low metastasis risk of less than 3%, and the 5-year survival rate can reach approximately 95% to 100%.10,12,25 According to the ENETS guidelines, surgical treatment is recommended if R-NETs are greater than 2 cm (G1~G3) or are G3 phase with or without metastasis; endoscopic resection is feasible when the tumors are less than 1 cm, G1~2 phase and T1 stage.15 The treatment of R-NETs in Western countries and in China is similar, but there have been controversies regarding the treatment of 1 ~ 2-cm-sized C-NETs. Chinese experts agree that endoscopic resection can be considered for C-NETs less than 2 cm; however, there is no explicit mention of treatment in the ENETS, guidelines, and experts from the NCCN recommended surgical resection instead of endoscopic resection.13,15 In our study, survival curves were significantly better (p<0.001) among patients with tumors less than 2 cm (Figure 2). A tumor diameter greater than 2 cm was an independent risk factor for LNM (p=0.040) (Table 3), and we believe this was due to the small sample size. However, 8 (11.0) patients with tumors less than 2 cm had LNM, and 3 (7.0) patients with tumors less than 1 cm also developed LNM. The LNM in small NETs might be due to the tumor cells extending to the submucosal layer, which has abundant lymphatic vessels for them to spread through. Previous studies have reported that small NETs also have malignant potential.26 Therefore, even if tumor size was a strong predictive factor for LNM, LNM should not be predicted only by tumor size, and further examinations such as EUS or CT can help us to evaluate the status of lymph nodes more specifically.
Chromogranin A (CgA) and synaptophysin (Syn) are two neuroendocrine differentiation (NED) immunohistochemistry markers frequently used in NETs. In univariable analysis, there was a significant difference for colorectal NETs with different expression levels of CgA in terms of both risk factors for LNM and survival outcomes (both p<0.05). In multivariable analysis, moderate positive (++) (p=0.010) and strong positive (+++) CgA (p=0.007) were independent risk factors for 5-year PFS, which has been proven in a wide variety of retrospective analyses.27–30 In addition, prospective clinical trials RADIANT-1, −2 and −3 have been performed to assess the prognostic value of CgA in advanced NETs, and the results showed shorter OS for patients with elevated CgA.31–34 However, the increase in the expression level of CgA was not proportional to the increase in the LNM rate or 5-year OS rate, and CgA was negative in 20 (35.1%) patients with LNM, which may affect the accuracy of the prognostic value of CgA. Lindholm et al35 also found that a relevant portion of NETs, 30~50%, do not show elevated CgA levels. The major problem is that several confounding factors can affect CgA levels, including gastrointestinal and cardiovascular disorders or proton pump inhibitor (PPI) consumption and a variety of other nontumor reasons.36 Regarding Syn, there was only a significant difference in the univariable analysis for LNM. Previous studies have shown that patients with a low level of synaptophysin had a better OS rate than those with a high level; however, the small sample size limited the accuracy of the results.37 Based on the findings of this research and previous studies,38 it can be suggested that NED might affect the survival outcomes of colorectal NET patients, and markers, especially CgA, might be suitable for clinicians to predict the prognosis of patients.
The location of the tumor is also an important factor affecting the prognosis and treatment of NETs. Tumors in the colon are more common in NECs and generally have a worse prognosis with higher metastatic potential than tumors in the rectum. The outcomes of neuroendocrine tumors from the right hemi-colon of the midgut and from the left hemi-colon from the hindgut are not the same. The well-differentiated biological behavior of the left hemi-colon is closer to that of the rectum. A recent Chinese multicenter study found that more than 94% of colonic NETs of midgut origin are NECs or mixed adenoneuroendocrine carcinomas (MANECs).39 According to statistics from the SEER database, the 5-year survival rate of patients with R-NETs is 75.2~83.3%, which is significantly higher than that of colonic NETs (40%~70%).4,39,40 In our study, the lymph node metastasis rate in the colon was 77.8%, which was significantly higher than that in the rectum (38.1%). The 5-year OS rate and PFS rate of individuals with LNM in the appendix and in the rectum were significantly better than those of individuals with LNM in the colon (p=0.005 and p=0.003, respectively), which was consistent with previous studies.1 Based on the findings of this research and previous studies, it can be suggested that colonic NETs should be completely resected.
In our research, the survival outcomes of patients 65 years and older in our study were worse than those of patients younger than 65 years (p<0.001). We also found that tumors from elderly patients (≥65 years) were larger and more advanced than those from younger patients (<65 years) (both p<0.001). The reason for the poor prognosis in elderly patients may be that elderly patients have lower tolerance to surgical trauma and side effects of chemotherapy because of their weakened organ physiological functions, which leads to multiple complications.41,42 Therefore, when we encounter elderly patients, minimally invasive therapies such as laparoscopic surgery could help reduce surgical trauma, and Chinese herbs can relieve and reduce the adverse events of chemotherapy.43
For NETs in the colon, the recommended treatment varies among different guidelines, but surgical resection is generally recommended because of the greater likelihood of malignant behavior than with rectal NETs. Endoscopic resection may be considered if the tumor diameter is less than 2 cm and does not reach the muscularis propria. For NETs in the rectum, EUS is required before surgery. Surgical resection is recommended when the tumor diameter is more than 2 cm, G3 grade, T3 to T4 stage or when there is peripheral lymph node metastasis; when the tumor diameter is less than 1 cm, G1 or 2 grade and T1 stage, endoscopic resection is feasible; in other cases, the treatment method is determined according to the depth of tumor invasion assessed by EUS.21
This study has some limitations, including its retrospective design and the relatively small number of patients included. Although LNM should be evaluated after radical resection with lymph node dissection, we analyzed the risk factors for LNM by CT or MRI in those who underwent local excision before the treatment and during the follow-up periods, and we believe the results are reliable. Because this study lasted more than 15 years, we could investigate the long-term survival outcomes and prognostic factors after different treatments, even with the small number of patients. Finally, further studies should be performed to validate our main conclusions.
Conclusion
The clinical and pathological characteristics of rectal and colon neuroendocrine tumors are different. Rectal neuroendocrine tumors have a better prognosis than colonic neuroendocrine tumors. Tumor diameter and tumor grade are independent risk factors for lymph node metastasis in colorectal neuroendocrine tumors. Age, tumor location, lymph node status and positive levels of the neuroendocrine marker CgA are independent risk factors that affect the prognosis of colorectal neuroendocrine tumors.
Ethical Statement
The study protocol was approved by the Institutional Review Board (NCC2013-093) of the Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). The purpose and procedures of the study was explained to all patients before data collection. Written and informed consent from every participant was obtained in this study. The investigation conforms with principles of the Declaration of Helsinki.
Acknowledgment
The authors thank the medical records department of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College for clinical data providing.
Author Contributions
All authors made substantial contributions to conception and design, acquisition and analysis of data, drafting and revising of the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi:10.1200/JCO.2007.15.4377
2. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707–712. doi:10.1097/MPA.0b013e3181ec124e
3. Sohn B, Kwon Y, Ryoo SB, et al. Predictive factors for lymph node metastasis and prognostic factors for survival in rectal neuroendocrine tumors. J Gastrointest Surg. 2017;21(12):2066–2074. doi:10.1007/s11605-017-3603-y
4. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi:10.1002/cncr.11105
5. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(1):1–18, vii. doi:10.1016/j.ecl.2010.12.005
6. Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer. 2012;3:292–302. doi:10.7150/jca.4502
7. Jiang MJ, Hu HG, Zheng S, et al. Progress in diagnosis and treatment of colorectal neuroendocrine tumors. Chin J Colorec Dis. 2014;3(5):641–647.
8. Fan JH, Zhang YQ, Shi SS, et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget. 2017;8(42):71699–71708. doi:10.18632/oncotarget.17599
9. Scherubl H, Streller B, Stabenow R, et al. Clinically detected gastroenteropancreatic neuroendocrine tumors are on the rise: epidemiological changes in Germany. World J Gastroenterol. 2013;19:9012–9019. doi:10.3748/wjg.v19.i47.9012
10. Bertani E, Ravizza D, Milione M, et al. Neuroendocrine neoplasms of rectum: a management update. Cancer Treat Rev. 2018;66:45–55. doi:10.1016/j.ctrv.2018.04.003
11. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi:10.1001/jamaoncol.2017.0589
12. de Mestier L, Lorenzo D, Fine C, et al. Endoscopic, transanal, laparoscopic, and transabdominal management of rectal neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2019;33(5):101293. doi:10.1016/j.beem.2019.101293
13. Expert Committee on Neuroendocrine Tumors of Chinese Society of Clinical Oncology. Expert consensus on gastroenteropancreatic neuroendocrine tumors in China (2016 edition). Chin Clin Oncol. 2016;21(10):927–946.
14. Chen HS, Chen Y. Consensus and controversy of endoscopic diagnosis and treatment of gastroenteropancreatic neuroendocrine tumors. Chin J Gastrointest Surg. 2017;20(9):982–986.
15. Ramage JK, De Herder WW, Delle Fave G, et al. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):139–143. doi:10.1159/000443166
16. Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227(2):371–377. doi:10.1148/radiol.2272011747
17. Zhang G, Cai YZ, Xu GH. Diagnostic accuracy of MRI for assessment of T category and circumferential resection margin involvement in patients with rectal cancer: a meta-analysis. Dis Colon Rectum. 2016;59(8):789–799. doi:10.1097/DCR.0000000000000611
18. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
19. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of theAJCC cancer staging manualand the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
20. Carneiro F, Hruban RH, Theise N. WHO Classification of Tumors of the Digestive System. Lyon: International Agency for Research on Cancer; 2009:417.
21. Mandair D, Caplin ME. Colonic and rectal NET’s. Best Pract Res Clin Gastroenterol. 2012;26(6):775–789. doi:10.1016/j.bpg.2013.01.007
22. Chagpar R, Chiang YJ, Xing Y, et al. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013;20(4):1170–1178. doi:10.1245/s10434-012-2746-z
23. Li P, Wu F, Zhao H, et al. Analysis of the factors affecting lymph node metastasis and the prognosis of rectal neuroendocrine tumors. Int J Clin Exp Pathol. 2015;8(10):13331–13338.
24. Wei G, Feng X, Wang W, et al. Analysis of risk factors of lymph node metastasis in rectal neuroendocrine neoplasms using multicenter data. Future Oncol. 2018;14(18):1817–1823. doi:10.2217/fon-2018-0059
25. Shields CJ, Tiret E, Winter DC. Carcinoid tumors of the rectum: a multi-institutional international collaboration. Ann Surg. 2010;252(5):750–755. doi:10.1097/SLA.0b013e3181fb8df6
26. Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103(8):1587–1595. doi:10.1002/cncr.20939
27. Nanno Y, Toyama H, Matsumoto I, et al. Baseline plasma chromogranin A levels in patients with well-differentiated neuroendocrine tumors of the pancreas: a potential predictor of postoperative recurrence. Pancreatology. 2017;17(2):291–294. doi:10.1016/j.pan.2016.12.012
28. Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14(23):7798–7803. doi:10.1158/1078-0432.CCR-08-0734
29. Arnold R, Wilke A, Rinke A, et al. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol. 2008;6(7):820–827. doi:10.1016/j.cgh.2008.02.052
30. Nehar D, Lombard-Bohas C, Olivieri S, et al. Interest of Chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol (Oxf). 2004;60(5):644–652. doi:10.1111/j.1365-2265.2004.02030.x
31. Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, Phase 3 study. Lancet. 2011;378(9808):2005–2012. doi:10.1016/S0140-6736(11)61742-X
32. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi:10.1016/S0140-6736(15)00817-X
33. Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a Phase II trial. J Clin Oncol. 2010;28:69–76. doi:10.1200/JCO.2009.24.2669
34. Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26(26):4311–4318. doi:10.1200/JCO.2008.16.7858
35. Lindholm DP, Oberg K. Biomarkers and molecular imaging in gastroenteropancreatic neuroendocrine tumors. Horm Metab Res. 2011;43(12):832–837. doi:10.1055/s-0031-1287794
36. Marotta V, Zatelli MC, Sciammarella C, et al. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer. 2018;25(1):R11–r29. doi:10.1530/ERC-17-0269
37. Tudorascu DR, Pirici D, Tartea EA, et al. Synaptophysin expression as prognostic factor for survival in colorectal carcinomas. Rom J Morphol Embryol. 2017;58(4):1409–1415.
38. Zeng YJ, Lai W, Fau-Liu L, et al. Prognostic significance of neuroendocrine differentiation in colorectal adenocarcinoma after radical operation: a meta-analysis. Journal of Gastrointestinal Surgery. 2014;18(5):968–976. doi:10.1007/s11605-014-2480-x
39. Zhang Y, Peng XJ, Jin KZ, et al. Clinicopathological characteristics and prognosis analysis of colorectal neuroendocrine neoplasms based on the data from domestic six medical centers. Chin J Gastrointest Surg. 2016;19(11):1235–1240.
40. Huang YT, Jia R, Xu Q, et al. Prognostic analysis of colon and rectal neuroendocrine neoplasm in different stages. Chin J Oncol. 2019;41(2):146–151.
41. Yamano T, Yamauchi S, Kimura K, et al. Influence of age and comorbidity on prognosis and application of adjuvant chemotherapy in elderly Japanese patients with colorectal cancer: a retrospective multicentre study. Eur J Cancer. 2017;81:90–101. doi:10.1016/j.ejca.2017.05.024
42. Menegozzo CA-O, Teixeira-Júnior F, Couto-Netto SDD, Martins-Júnior O, Bernini CO, Utiyama EA-O. Outcomes of elderly patients undergoing emergency surgery for complicated colorectal cancer: a retrospective cohort study. Clinics. 2019;74. doi:10.6061/clinics/2019/e1074
43. Zhang SA-O, Shi L, Mao D, et al. Use of Jianpi Jiedu Herbs in patients with advanced colorectal cancer: a systematic review and meta-analysis. Evid Based Compl Altern Med. 2018;2018:1–13. doi:10.1155/2018/6180810
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
