Back to Journals » International Journal of General Medicine » Volume 15
Risk Factors for Length of Hospital Stay in Acute Exacerbation Chronic Obstructive Pulmonary Disease: A Multicenter Cross-Sectional Study
Authors Wang H, Yang T , Yu X, Chen Z, Ran Y, Wang J , Dai G, Deng H, Li X, Zhu T
Received 18 December 2021
Accepted for publication 4 March 2022
Published 29 March 2022 Volume 2022:15 Pages 3447—3458
DOI https://doi.org/10.2147/IJGM.S354748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hong Wang,1,* Tao Yang,2,* Xiaodan Yu,3,* Zhihong Chen,4 Yajuan Ran,5 Jiajia Wang,6 Guangming Dai,1 Huojin Deng,7 Xinglong Li,8 Tao Zhu8
1Respiratory Medicine, First People’s Hospital of Suining City, Suining, 629000, Sichuan, People’s Republic of China; 2Thoracic Surgery, First Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China; 3Respiratory Medicine, Fifth People’s Hospital of Chengdu, Chengdu, 610000, Sichuan, People’s Republic of China; 4Respiratory Medicine, Zhongshan Hospital of Fudan University, Shanghai, 20032, People’s Republic of China; 5Pharmacy Department, Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China; 6Rheumatology Medicine, Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China; 7Respiratory Medicine, ZhuJiang Hospital of Southern Medical University, Guangzhou, Guangdong, 510280, People’s Republic of China; 8Respiratory Medicine, Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tao Zhu, Respiratory Medicine, Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China, Tel +86 23 63693094, Email [email protected]
Background/Purpose: A patient’s length of hospital stay (LHS) is associated with the severity and outcome of acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Therefore, identification of patients with prolonged LHS at an early stage can potentially reduce the risk of adverse events and treatment costs in patients with AECOPD. Therefore, this study aimed to explore the independent predictors of prolonged LHS in AECOPD patients.
Patients and Methods: This multicenter cross-sectional study was conducted at two tertiary hospitals between January 2019 and August 2020. Demographic data, underlying diseases, symptoms, and laboratory findings were collected. Univariate analysis was used to identify variables with significant differences. A collinearity diagnostic was applied to the selected variables before the establishment of the regression model. Ordinal logistic regression was performed to explore the independent risk factors for prolonged LHS in patients with AECOPD.
Results: In total, 598 patients with AECOPD were screened. Finally, the LHS of 111, 218, and 100 patients was < 7, 7– 10, and ≥ 11 days, respectively. Significant differences in the 12 variables were found in the univariate analysis. Because collinearities among white blood cells (WBC), neutrophils (NS), and NS% were observed, WBC and NS% were excluded. Subsequently, an ordinal logistic regression model identified that rates of hypertension and chronic cor pulmonale (CCP), neutrophil–lymphocyte ratio (NLR), and erythrocyte sedimentation rate (ESR) were independent predictors of prolonged LHS in AECOPD patients.
Conclusion: Collectively, our results showed that inflammatory status, hypertension, and CCP were independently associated with LHS in patients with AECOPD. These data indicate that early and appropriate administration of antibiotics and anti-inflammatory drugs is essential for reducing LHS. Hypertension and CCP were independent predictors of worse outcomes in patients with AECOPD. Therefore, advanced management and care should be provided to AECOPD patients with hypertension and/or CCP on admission.
Keywords: acute exacerbation of chronic obstructive pulmonary disease, chronic cor pulmonale, erythrocyte sedimentation rate, hypertension, length of hospital stay, neutrophil–lymphocyte ratio
Introduction
Chronic obstructive pulmonary disease (COPD) is a worldwide public health problem and one of the leading causes of mortality and morbidity.1 Acute exacerbation of COPD (AECOPD), a severe status of COPD, is characterized by worsening of respiratory manifestations and is associated with increased mortality.2 Symptoms such as dyspnea, sputum purulence and volume, cough, and wheezing that worsen in short time periods are the major respiratory presentations of AECOPD.3 It was reported that AECOPD accounted for about 13% of all admitted patients.4 Mounting evidence showed that length of hospital stay (LHS) was independently associated with the severity of AECOPD.5–7 Although the risk factors for hospitalization in COPD have been well explored, including heart failure,8 malnutrition,8 and ambient particulate matter (PM) 10,9 the predictors for prolonged LHS in AECOPD patients are still not very clear.
AECOPD causes a heavy burden on the health care system, particularly in developing countries.10–12 The direct and indirect costs of AECOPD include healthcare resources devoted to diagnosis, illness management, workability loss, premature mortality, and family caregiver costs.3,13,14 Dalal et al found that the average cost was $9745 for standard admission and $33,440 for ICU stay in hospitalized AECOPD patients.15 Chen et al showed that length of ICU stay, non-invasive or invasive ventilation intervention, and use of antibiotics and systemic steroids were the major predictors of hospitalization costs in AECOPD.16 Therefore, LHS is associated with the medical costs of hospitalized AECOPD patients.
LHS is essential for the prediction of AECOPD severity.17–19 However, the definition of prolonged LHS in AECOPD still lacks a unified standard.17,19–22 In a cohort study, Mushlin et al showed that the mean LHS was 6–7 days in AECOPD patients.19 They also found that a longer LHS was associated with increased PCO2 levels, symptom duration > 1 day, and antibiotic treatment at the time of admission. In another prospective study, Crisafulli et al divided AECOPD patients into normal (≤ 7 days) and prolonged LHS (> 7 days) groups. Their results showed that prolonged LHS was independently associated with a modified Medical Research Council (mMRC) dyspnea score ≥ 2 and the presence of acute respiratory acidosis. In a retrospective study, a prolonged LHS in AECOPD was defined by an LHS > 8 days.17 Meanwhile, in another prospective cohort study, 9 days was used as the threshold of prolonged LHS in AECOPD.23 It revealed that baseline dyspnea, physical activity level, and hospital variability were independent predictors of prolonged LHS in hospitalized AECOPD patients. Simultaneously, Wang et al found that an LHS above the 75th percentile was 11 days in AECOPD patients.22 They also identified that admission between Thursday and Saturday, heart failure, diabetes, stroke, high arterial PCO2, and low serum albumin levels were independently associated with prolonged LHS in AECOPD patients. However, a majority of the previous studies focused more on subjective parameters such as symptoms, mMRC score, and physical activity level rather than objective parameters, especially laboratory results.
Comprehensive laboratory results such as arterial blood gas, routine blood tests, blood electrolytes, renal function tests, and liver function tests were included in our study. The aim of this cross-sectional study was to identify the independent predictors, particularly the lab parameters, of prolonged LHS in AECOPD patients, which can assist physicians in identifying AECOPD patients with potentially worse outcomes at an early stage.
Materials and Methods
Study Design and Population
This multicenter cross-sectional study was performed at the respiratory departments of the Second Affiliated Hospital of Chongqing Medical University and the First People’s Hospital of Suining City between January 2019 and August 2020. This study was approved by the Research Ethics Committees of the Second Affiliated Hospital of Chongqing Medical University (No. 2019–23) and the First People’s Hospital of Suining City (NO. 2020–37) in accordance with the Declaration of Helsinki.24 The heights of the two hospitals were 305 meters and 801 meters above sea level, respectively. All AECOPD patients had no plateau living history. Informed consent was obtained from all patients by the responsible physician or an appropriately trained staff member. Standard care and treatment were provided in our study according to current clinical guidelines.3,25,26
Sample Size Determinations
The sample size was calculated using G-power (version 3.1.9.2). A minimum of 246 participants (82 in each group) was required to detect at least a 20% difference in effect size with a power of 80%, assuming α = 0.05 and an allocation ratio of 1:1:1. Furthermore, 20% more patients (98 patients in each group) were recruited.
Inclusion and Exclusion Criteria
The inclusion criterion was an acute exacerbation of COPD requiring hospitalization in subjects aged ≥ 40 years.3 The exclusion criteria were as follows: death during hospitalization, non-respiratory failure patients without lung function test data, active pulmonary tuberculosis (TB), asthma, bronchiectasis, pneumoconiosis, interstitial lung diseases (ILDs), pulmonary edema, pulmonary thromboembolism (PTE), other chronic lung diseases, dysphagia and aspiration, dementia, hospital-acquired pneumonia (HAP), antibiotic administration within the last 2 weeks, immunosuppressive status (immunosuppressive drugs administered in the previous 2 weeks, organ transplant recipient, and/or present HIV infection), systemic steroid use within the last 2 weeks, history of malignant diseases, renal failure, and liver failure. Currently, no optimum standard for prolonged LHS has been consistently described.20 Meanwhile, based on previous studies,21,22 7 and 11 days were used as the cutoffs for mild prolonged LHS and severe prolonged LHS in the AECOPD patients in this study. A total of 598 hospitalized AECOPD patients were enrolled, and 169 were excluded. In the end, 111 patients had LHS < 7 days (normal LHS, N-LHS), 218 patients had LHS between 7 and 10 days (mild prolonged LHS, MP-LHS), and 100 patients had LHS ≥ 11 days (severe prolonged LHS, SP-LHS) (Figure 1).
Definitions
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations, the diagnosis of COPD was confirmed by pulmonologists based on criteria such as noxious stimuli exposure history, risk elements, clinical symptoms, and spirometry results (FEV1/FVC% < 0.7 after bronchodilator inhalation).3 AECOPD was defined as an event in the natural course of the disease that was characterized by acute changes in clinical symptoms beyond the normal day-to-day variations that resulted in the need for additional therapy.3,27 According to community-acquired pneumonia (CAP) guidelines25,26,28 and previous studies,29,30 CAP was defined by the typical symptoms and signs of systemic or acute lower respiratory tract infection and with evidence compatible with a diagnosis of CAP on chest computed tomography (CT). The radiological features of pneumonia include new-onset patchy infiltrates, consolidation, ground-glass opacities, or lung interstitial changes without other explanations.25,26,29,30 Chronic cor pulmonale (CCP) was defined as right ventricular hypertrophy resulting from diseases affecting the function and/or structure of the lungs, except when these pulmonary alterations were the result of diseases that primarily affect the left side of the heart.9,31,32 The diagnosis of CCP was based on the clinical presentation, echocardiography, and electrocardiography findings.9,31,32 Participants were defined as ex-smokers if they had abstained from smoking for ≥ 6 months. The neutrophil–lymphocyte ratio (NLR) was defined as the number of neutrophils divided by the lymphocytes in the blood.27
Data Collection
Demographic data, underlying diseases, comorbidities, symptoms, and LHS were recorded and collected. Blood samples for laboratory tests and lung function tests were collected within 24 h after admission. However, because of safety and cooperation concerns, a spirometer test was not performed in patients with respiratory failure. All patients underwent CT within 48 h after admission. The results were reviewed by an independent radiologist and pulmonologist in each hospital, who were blinded to the study. Discrepancies were resolved by consensus.
Statistical Analysis
Data were analyzed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA). Bar graphs were plotted using GraphPad Prism 7.0. Continuous variables were expressed as mean ± standard deviation (SD), and categorical data were expressed as frequencies. The data distribution was examined using the Kolmogorov–Smirnov test. Continuous variables with normal distributions were analyzed using one-way analysis of variance with Least Significant Difference (LSD) and Student-Newman–Keuls (SNK) post hoc tests. Continuous variables with abnormal distributions and ordinal variables were measured using the Kruskal–Wallis H-test. The chi-square test was used to analyze categorical variables. A collinearity diagnostic was applied to the selected variables before the establishment of the regression model. Ordinal logistic regression was performed to investigate the independent risk factors associated with LHS in patients with AECOPD. Statistical significance was set at p < 0.05.
Results
Demographic Characteristics of AECOPD Patients
This cross-sectional study enrolled 598 patients with AECOPD. Ultimately, 111 (26%) patients had LHS < 7 days (N-LHS), 218 (51%) patients had LHS of 7–10 days (MP-LHS), and 100 (23%) patients had LHS ≥ 11 days (SP-LHS) (Figure 1). The demographic data of the patients are presented in Table 1. Age and rates of CAP, CCP, and hypertension were significantly different among the three groups.
 |
Table 1 Demographic Data of the Patients with AECOPD (n=429) |
Clinical Presentations and Laboratory Data of AECOPD Patients
As shown in Table 2, white blood cell (WBC), neutrophil (NS), NS%, lymphocyte%, NLR, procalcitonin (PCT), ESR, and albumin (ALB) levels were significantly different among the three groups.
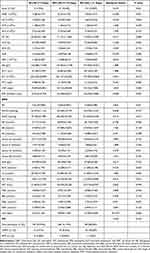 |
Table 2 Clinical Features and Laboratory Data of the Patients with AECOPD (n=429) |
Ordinal Logistic Regression Analysis Based on LHS in AECOPD Patients
To explore the independent factors associated with LHS in patients with AECOPD, ordinal logistic regression was performed. Since collinearities among WBC, NS, and NS% were observed (eigenvalue = 0.012, condition index = 15.674), WBC and NS% were excluded. In the ordinal logistic regression model, we included 10 factors that were found to be significantly associated with LHS in univariate analysis, including age, the rates of CAP, CCP, hypertension, NS, lymphocyte%, NLR, PCT, ESR, and ALB. Subsequently, our data identified that NLR, ESR, and the rates of hypertension and CCP were independently associated with LHS in patients with AECOPD (Table 3).
 |
Table 3 Ordinal Logistics Regression Analysis of Independent Factors Associated with LHS in AECOPD (N= 429) |
NLR and ESR Were Markedly Increased in AECOPD Patients with Prolonged LHS
NLR and ESR were significantly higher in the MP-LHS and SP-LHS groups than in the N-LHS group (Table 2 and Figure 2). However, no differences in NLR and ESR were observed between the MP-LHS and SP-LHS groups.
 |
Figure 2 NLR and ESR in AECOPD patients. (A) NLR; (B) ESR. Abbreviations: NLR, neutrophil–lymphocyte ratio; ESR, erythrocyte sedimentation rate; LHS, length of hospital stay. |
Discussion
This multicenter cross-sectional study enrolled 598 patients with AECOPD. Finally, 111 (26%) patients with LHS < 7 days (N-LHS), 218 (51%) patients with LHS of 7–10 days (MP-LHS), and 100 (23%) patients with LHS ≥ 11 days (SP-LHS) were included. Significant differences in 12 factors, including age, rate of CAP, hypertension, CCP, WBC, NS, NS%, lymphocyte%, NLR, PCT, ESR, and ALB were identified among the three groups in the univariate analysis. Since collinearity among WBC, NS%, and NS was observed, WBC and NS% were excluded from the regression model. Subsequently, an ordinal logistic regression model revealed that NLR, ESR, and rates of hypertension and CCP were independently associated with LHS in patients with AECOPD. Furthermore, the NLR, ESR, and rates of hypertension and CCP in AECOPD patients with prolonged LHS were significantly higher than those in patients with normal LHS.
According to the GOLD recommendations, the prevalence of COPD is 11.7% (95% CI 8.4% to 15.0%), indicating that approximately 384 million people suffer from COPD globally.3,33 AECOPD is also a major cause of admission in COPD patients. Mounting evidence showed that LHS was independently associated with the severity, cost burden, in-hospital mortality, and readmission rates of COPD.20 Some studies reported that comorbidities were independently associated with LHS in AECOPD patients.22,34 In a retrospective study, Wang et al showed that heart failure, diabetes, stroke, increased PaCO2, and reduced ALB were independent risk factors for prolonged LHS in AECOPD patients.22 Meanwhile, in a longitudinal retrospective observational study, Inabnit et al revealed that LHS was significantly correlated with the number of comorbidities in COPD patients.34 Furthermore, they also noticed that congestive heart failure, fluid and electrolyte disorders, and renal failure were associated with 28%, 20%, and 50% greater LHS in COPD, respectively. However, the variables obtained in these studies were not comprehensive. Hence, some potentially important risk factors and predictors were not identified in these studies. Furthermore, to date, the risk factors associated with prolonged LHS have not been well explored in Chinese patients with AECOPD. In the present study, comprehensive data including demographic data, underlying diseases, comorbidities, symptoms, lung function (GOLD stages), laboratory parameters, and CT scans were collected.
The acknowledged definition of prolonged LHS has not yet been unified. Various definitions of prolonged LHS in COPD have been used in different studies.21,22,35 In a retrospective longitudinal study, COPD patients registered by London general practitioners and patients admitted to the emergency room with COPD from 2006 to 2010 were screened.35 It was found that the average LHS was 7 days in COPD patients. Meanwhile, in a prospective study at the Hospital Clinic of Barcelona, 7 days was also used as the cutoff for prolonged LHS in AECOPD patients.21 However, in another retrospective study, 11 days was used to define prolonged LHS in AECOPD patients.22 Therefore, two cutoffs of prolonged LHS, both 7 and 11 days, were considered in our study. Our ordinal logistic regression revealed that NLR, ESR, and rates of hypertension and CCP were independently associated with prolonged LHS in patients with AECOPD.
Hypertension is considered to be the most common comorbidity of COPD and is potentially associated with the prognosis of COPD patients.3,36,37 In a retrospective cohort study, 314 AECOPD patients in Switzerland were screened.36 They found that new or worsening hypertension was an independent risk factor for re-exacerbation in AECOPD patients. Meanwhile, in a cross-sectional study, the association between COPD and comorbidities (represented by Charlson comorbidity scores) was explored.37 The results revealed that Charlson comorbidity scores in COPD patients were higher than in non-COPD patients. Furthermore, more than 40% of patients with COPD had cardiovascular disease, hypertension, and hyperlipidemia. Several studies have found that low-grade systemic inflammation substantially contributes to the pathogenesis of both hypertension and COPD. Barnes et al showed that arterial constriction, which resulted from COPD-induced airway inflammation, lung hyperinflation, systemic inflammation, endothelial dysfunction, and oxidative stress, was essential for hypertension in COPD patients.38 Furthermore, several studies also revealed the benefits of blood pressure control in AECOPD patients with hypertension.39 It was found that angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) were negatively associated with LHS in AECOPD combined with hypertension. In a retrospective national cohort study, Mortensen et al showed that ARBs and ACE inhibitors were associated with decreased mortality in hospitalized AECOPD patients with hypertension.39 Furthermore, some studies revealed that diastolic dysfunction as a result of hypertension led to exercise intolerance and increased LHSs in AECOPD patients.3,40,41 Consistently, our results also identified that hypertension was an independent predictor of prolonged LHS, indicating the importance and value of optimal blood pressure control in hospitalized AECOPD patients with hypertension. Further studies focusing on blood pressure management in AECOPD combined with hypertension are warranted.
The effect of CCP on COPD prognosis has not been well explored. CCP was defined as right ventricle hypertrophy resulting from diseases affecting the function and/or structure of the lungs, except when these pulmonary alterations are the result of diseases that primarily affect the left side of the heart.9,31 In this study, the diagnosis of CCP was based on the clinical presentation, echocardiography, and electrocardiography findings.9,31,32 In advanced COPD, endothelial dysfunction, pulmonary arteriole constriction, and vascular remodeling, which are characterized by intimal hyperplasia and vascular smooth muscle hypertrophy/hyperplasia, are induced by hypoxia and persistent chronic pulmonary inflammation, eventually leading to pulmonary hypertension (PH). In chronic pulmonary diseases such as COPD and idiopathic pulmonary fibrosis, PH and CCP are considered to be a single disease in different stages.31 Progressive PH can cause right ventricular hypertrophy and eventually lead to right cardiac failure. Lung disease-associated PH is defined as a mean pulmonary arterial pressure greater than 20 mmHg at rest. Additionally, it was reported that the diameter of the pulmonary artery was independently associated with acute exacerbation in COPD.42 In this study, our data found that CCP was independently associated with prolonged LHS in patients with AECOPD. This result indicates that CCP represents more severe illnesses and worse outcomes in patients with AECOPD. Advanced treatment and intensive care should be provided early on admission.
Some studies have shown that increased neutrophils and decreased lymphocytes, particularly CD3+ cells and CCR5+CD3+ cells, were observed in the submucosa of the airway in COPD.43 Meanwhile, these pathological alterations were more obvious in patients with severe stage COPD.43 Therefore, a high NLR represented severe inflammation and less immunity.44 It was found that the NLR was associated with worse prognosis, such as mortality, in hospitalized AECOPD patients.45 Karauda et al found that the NLR showed high sensitivity and specificity in the prediction of in-hospital death in AECOPD patients.46 Lu et al revealed that the NLR was higher in frequent exacerbators than in non-frequent exacerbators with COPD.47 Meanwhile, they also noticed that increased NLR was associated with worse outcomes. Collectively, the NLR was considered a good predictor of the severity and outcomes of AECOPD.46,47 Additionally, the ESR is one of the most commonly used inflammatory parameters in clinical practice. Taylan et al showed that the ESR was significantly higher in AECOPD patients than in stable COPD and healthy control subjects.48 In this study, our findings also showed that the NLR and ESR were independent predictors of prolonged LHS in AECOPD patients. Furthermore, the NLR and ESR in the MP-LHS and SP-LHS groups were noticeably higher than those in the N-LHS group (Figure 2). Collectively, these results support that the NLR and ESR are promising predictors of LHS and its severity in hospitalized AECOPD patients. These results also indicate that systemic inflammatory status is independently associated with the prognosis and outcomes of AECOPD. Additional studies with larger populations are required to validate these findings.
To our knowledge, this is the first multicenter cross-sectional study to explore the risk factors for prolonged LHS in Chinese AECOPD patients. Meanwhile, two cutoffs of prolonged LHS, 7 and 11 days, were considered, making our data more convincing, which was one of the major strengths of this study. Additionally, comprehensive data such as demographic data, underlying diseases, comorbidities, symptoms, lung function, and laboratory data were collected. In particular, chest CT was performed in each patient, which effectively promoted and improved diagnostic accuracy and reduced the number of confounders.
This study has several limitations. First, the etiologies of acute exacerbation, which are potentially associated with LHS and the prognosis of AECOPD, were not explored in this study. Data on the treatment and therapies applied to AECOPD patients in stable phases were not analyzed. This study was performed only in tertiary general hospitals in China. Therefore, the results cannot be generalized to primary health care facilities. Additionally, only Chinese patients with AECOPD were included in this study. Thus, the results cannot be generalized to other ethnic groups.
Taken together, our results identified that NLR, ESR, hypertension, and CCP were independently associated with LHS in patients with AECOPD. These results indicate that AECOPD in patients with hypertension and/or CCP are more severe, requiring advanced treatment and care. In addition, we found that a patient’s inflammatory status was associated with their prognosis and severity of AECOPD, and that antibiotics and anti-inflammatory drugs are critical in the treatment of AECOPD. However, these findings should be validated in primary healthcare settings and in other ethnic groups in the future.
Acknowledgments
We want to express our sincere appreciation to all the patients who participated in the study. We would like to thank Chi Pham from California National Primate Research Center, University of California, Davis, who help us with drafting the manuscript. Meanwhile, we would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by the Chongqing Health Joint Medical Research Project (NO. 2020MSXM112) and Chongqing Natural Science Foundation (NO. cstc2018jcyjAX0245).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang H, Naghavi M, Allen C. A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi:10.1016/S0140-6736(16)31012-1
2. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD Executive Summary. Eur Respir J. 2017;49(3):1700214. doi:10.1183/13993003.00214-2017
3. (GOLD). GIfCLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (Revised 2020). Available from: https://goldcopdorg/gold-reports/2020.
4. Ozkaya S, Findik S, Atici AG. The costs of hospitalization in patients with acute exacerbation of chronic obstructive pulmonary disease. Clinicoecon Outcomes Res. 2011;3:15–18. doi:10.2147/CEOR.S14820
5. Alshabanat A, Otterstatter MC, Sin DD, et al. Impact of a COPD comprehensive case management program on hospital length of stay and readmission rates. Int J Chron Obstruct Pulmon Dis. 2017;12:961–971. doi:10.2147/COPD.S124385
6. Ko FWS, Chan KP, Ngai J, et al. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology. 2020;25(3):259–266. doi:10.1111/resp.13660
7. Milan S, Bondalapati P, Megally M, et al. Positive expiratory pressure therapy with and without oscillation and hospital length of stay for acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2553–2561. doi:10.2147/copd.S213546
8. Genao L, Durheim MT, Mi X, Todd JL, Whitson HE, Curtis LH. Early and long-term outcomes of older adults after acute care encounters for chronic obstructive pulmonary disease exacerbation. Ann Am Thorac Soc. 2015;12(12):1805–1812. doi:10.1513/AnnalsATS.201504-250OC
9. You L, Niu H, Huang K, Dong F, Yang T, Wang C. Clinical features and outcomes of acute exacerbation in chronic obstructive pulmonary disease patients with pulmonary heart disease: a multicenter observational study. Int J Chron Obstruct Pulmon Dis. 2021;16:2901–2910. doi:10.2147/copd.S325925
10. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of Chronic Obstructive Pulmonary Disease (COPD): a Systematic Literature Review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460. doi:10.2147/copd.S234942
11. Torabipour A, Hakim A, Ahmadi Angali K, Dolatshah M, Yusofzadeh M. Cost analysis of hospitalized patients with chronic obstructive pulmonary disease: a State-Level Cross-Sectional Study. Tanaffos. 2016;15(2):75–82.
12. Li F, Sun Z, Li H, Yang T, Shi Z. Factors associated with hospitalisation costs in patients with chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2018;22(4):458–463. doi:10.5588/ijtld.17.0430
13. Sin DD, Stafinski T, Ng YC, Bell NR, Jacobs P. The impact of chronic obstructive pulmonary disease on work loss in the United States. Am J Respir Crit Care Med. 2002;165(5):704–707. doi:10.1164/ajrccm.165.5.2104055
14. Koul PA, Nowshehr AA, Khan UH, Jan RA, Shah SU. Cost of severe chronic obstructive pulmonary disease exacerbations in a High Burden Region in North India. Ann Glob Health. 2019;85(1). doi:10.5334/aogh.2423
15. Dalal AA, Christensen L, Liu F, Riedel AA. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341–349. doi:10.2147/COPD.S13771
16. Chen YH, Yao WZ, Cai BQ, et al. Economic analysis in admitted patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J (Engl). 2008;121(7):587–591. doi:10.1097/00029330-200804010-00003
17. Tsimogianni AM, Papiris SA, Stathopoulos GT, Manali ED, Roussos C, Kotanidou A. Predictors of outcome after exacerbation of chronic obstructive pulmonary disease. J Gen Intern Med. 2009;24(9):1043–1048. doi:10.1007/s11606-009-1061-2
18. Limsuwat C, Mankongpaisarnrung C, Dumrongmongcolgul N, Nugent K. Factors influencing the length of hospital stay in patients with acute exacerbations of chronic obstructive pulmonary disease admitted to intensive care units. Qual Manag Health Care. 2014;23(2):86–93. doi:10.1097/qmh.0000000000000024
19. Mushlin AI, Black ER, Connolly CA, Buonaccorso KM, Eberly SW. The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991;266(1):80–83. doi:10.1001/jama.1991.03470010084035
20. Agboado G, Peters J, Donkin L. Factors influencing the length of hospital stay among patients resident in Blackpool admitted with COPD: a cross-sectional study. BMJ Open. 2012;2(5):e000869. doi:10.1136/bmjopen-2012-000869
21. Crisafulli E, Ielpo A, Barbeta E, et al. Clinical variables predicting the risk of a hospital stay for longer than 7 days in patients with severe acute exacerbations of chronic obstructive pulmonary disease: a prospective study. Respir Res. 2018;19(1):261. doi:10.1186/s12931-018-0951-4\]
22. Wang Y, Stavem K, Dahl FA, Humerfelt S, Haugen T. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int J Chron Obstruct Pulmon Dis. 2014;9:99–105. doi:10.2147/COPD.S51467
23. Quintana JM, Unzurrunzaga A, Garcia-Gutierrez S, et al. Predictors of hospital length of stay in patients with exacerbations of COPD: a Cohort Study. J Gen Intern Med. 2015;30(6):824–831. doi:10.1007/s11606-014-3129-x.
24. World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
25. Bartlett JG, Dowell SF, Mandell LA, File TM
26. Lim WS, Woodhead M. British Thoracic Society adult community acquired pneumonia audit 2009/10. Thorax. 2011;66(6):548–549. doi:10.1136/thoraxjnl-2011-200081
27. Dai G, Ran Y, Wang J, et al. Clinical differences between eosinophilic and noneosinophilic acute exacerbation of chronic obstructive pulmonary disease: a Multicenter Cross-Sectional Study. Mediators of Inflammation. 2020;2020:1059079. doi:10.1155/2020/1059079
28. Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. 2018;12(4):1320–1360. doi:10.1111/crj.12674
29. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974–982. doi:10.1164/rccm.201501-0017OC
30. Flateau C, Le Bel J, Tubiana S, et al. High heterogeneity in community-acquired pneumonia inclusion criteria: does this impact on the validity of the results of randomized controlled trials? BMC Infect Dis. 2018;18(1):607. doi:10.1186/s12879-018-3515-9
31. Shujaat A, Minkin R, Eden E. Pulmonary hypertension and chronic cor pulmonale in COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(3):273–282.
32. Park SY, Lee CY, Kim C, et al. One-year prognosis and the role of brain natriuretic peptide levels in patients with chronic cor pulmonale. J Korean Med Sci. 2015;30(4):442–449. doi:10.3346/jkms.2015.30.4.442
33. Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD. 2010;7(5):375–382. doi:10.3109/15412555.2010.510160
34. Inabnit LS, Blanchette C, Ruban C. Comorbidities and length of stay in chronic obstructive pulmonary disease patients. COPD. 2018;15(4):355–360. doi:10.1080/15412555.2018.1513470
35. Harries TH, Thornton HV, Crichton S, Schofield P, Gilkes A, White PT. Length of stay of COPD hospital admissions between 2006 and 2010: a retrospective longitudinal study. Int J Chron Obstruct Pulmon Dis. 2015;10:603–611. doi:10.2147/copd.S77092
36. Engel B, Schindler C, Leuppi JD, Rutishauser J. Predictors of re-exacerbation after an index exacerbation of chronic obstructive pulmonary disease in the REDUCE randomised clinical trial. Swiss Med Wkly. 2017;147:w14439. doi:10.4414/smw.2017.14439
37. Li LS, Caughey G, Johnston K. Comorbidity associated with referral to pulmonary rehabilitation in people hospitalized with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2014;34(6):430–436. doi:10.1097/HCR.0000000000000080
38. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi:10.1183/09031936.00128008
39. Mortensen EM, Copeland LA, Pugh MJ, et al. Impact of statins and ACE inhibitors on mortality after COPD exacerbations. Respir Res. 2009;10(1):45. doi:10.1186/1465-9921-10-45
40. Abusaid GH, Barbagelata A, Tuero E, Mahmood A, Sharma G. Diastolic dysfunction and COPD exacerbation. Postgrad Med. 2009;121(4):76–81. doi:10.3810/pgm.2009.07.2033
41. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
42. Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi:10.1056/NEJMoa1203830
43. Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, Adcock I. COPD immunopathology. Semin Immunopathol. 2016;38(4):497–515. doi:10.1007/s00281-016-0561-5
44. Rahimirad S, Ghaffary MR, Rahimirad MH, Rashidi F. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. Tuberk Toraks. 2017;65(1):25–31. doi:10.5578/tt.27626
45. Gómez-Rosero JA, Cáceres-Galvis C, Ascuntar J, Atencia C, Vallejo CE, Jaimes F. Biomarkers as a prognostic factor in COPD exacerbation: a Cohort Study. COPD. 2021;18(3):325–332. doi:10.1080/15412555.2021.1922370
46. Aksoy E, Karakurt Z, Gungor S, et al. Neutrophil to lymphocyte ratio is a better indicator of COPD exacerbation severity in neutrophilic endotypes than eosinophilic endotypes. Int J Chron Obstruct Pulmon Dis. 2018;13:2721–2730. doi:10.2147/copd.S170353
47. Lu FY, Chen R, Li N, et al. Neutrophil-to-lymphocyte ratio predicts clinical outcome of severe acute exacerbation of COPD in frequent exacerbators. Int J Chron Obstruct Pulmon Dis. 2021;16:341–349. doi:10.2147/copd.S290422
48. Taylan M, Demir M, Kaya H, et al. Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients. Clin Respir J. 2017;11(3):311–317. doi:10.1111/crj.12336
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

