Back to Journals » Cancer Management and Research » Volume 10
Risk factors for delayed chemotherapy-induced nausea and vomiting with low-emetic-risk chemotherapy: a prospective, observational, multicenter study
Authors Hayashi T , Shimokawa M, Matsuo K, Miyoshi T, Toriyama Y , Yokota C, Taniguchi J, Hanada K, Tsumagari K, Okubo N, Koutake Y , Sakata K , Kawamata Y, Goto T, Tsurusaki Y, Koyabu M
Received 7 June 2018
Accepted for publication 20 August 2018
Published 4 October 2018 Volume 2018:10 Pages 4249—4255
DOI https://doi.org/10.2147/CMAR.S176574
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Beicheng Sun
Toshinobu Hayashi,1,2 Mototsugu Shimokawa,3 Koichi Matsuo,2 Takanori Miyoshi,4 Yoko Toriyama,4 Chiaki Yokota,5 Jun Taniguchi,6 Kiyonori Hanada,7 Kyouichi Tsumagari,8 Noriko Okubo,9 Yoshimichi Koutake,10 Kohei Sakata,11 Yosei Kawamata,12 Takashi Goto,13 Yasufumi Tsurusaki,14 Makiko Koyabu1
1Department of Pharmacy, Clinical Research Institute, National Kyushu Medical Center, Fukuoka, Japan; 2Department of Pharmaceutical and Health Care Management, Faculty of Pharmaceutical Sciences, Fukuoka University, Fukuoka, Japan; 3Cancer Biostatistics Laboratory, Clinical Research Institute, National Kyushu Cancer Center, Fukuoka, Japan; 4Department of Pharmacy, National Hospital Organization Beppu Medical Center, Oita, Japan; 5Department of Pharmacy, National Hospital Organization Nagasaki Medical Center, Nagasaki, Japan; 6Department of Pharmacy, National Hospital Organization Ureshino Medical Center, Saga, Japan; 7Department of Pharmacy, National Hospital Organization Kumamoto Saishunso National Hospital, Kumamoto, Japan; 8Department of Pharmacy, National Hospital Organization Miyakonojo Medical Center, Miyazaki, Japan; 9Department of Pharmacy, National Hospital Organization Kumamoto Medical Center, Kumamoto, Japan; 10Department of Pharmacy, National Hospital Organization Fukuoka National Hospital, Japan; 11Department of Pharmacy, National Hospital Organization Kumamoto South National Hospital, Kumamoto, Japan; 12Department of Pharmacy, National Hospital Organization Kagoshima Medical Center, Kagoshima, Japan; 13Department of Pharmacy, National Hospital Organization Kokura Medical Center, Kitakyushu, Japan; 14Department of Pharmacy, National Hospital Organization Saga National Hospital, Saga, Japan
Purpose: Improvement in the control of delayed chemotherapy-induced nausea and vomiting (CINV) is needed. There is limited information on antiemetic prophylaxis for patients undergoing low-emetic-risk chemotherapy (LEC), and the optimal antiemetic treatment is not well understood. Therefore, we analyzed the risk factors for delayed CINV to aid in the development of individualized treatments.
Patients and methods: This prospective multicenter study was conducted in 13 hospitals and included patients with solid cancers undergoing LEC. A total of 222 patients were enrolled between September 2013 and November 2014. The participants completed a daily diary for 5 days after the commencement of the first cycle of LEC to describe the daily incidence of CINV (yes/no). Furthermore, the participants described the severity of nausea and the amount of food intake with the help of VAS.
Results: Two hundred and ten patients provided their data that were analyzed using multivariate logistic regression to examine the risk factors for delayed CINV. History of CINV, Eastern Cooperative Oncology Group performance status score ≥1, acute CINV, and single-day antiemetic prophylaxis were identified as independent risk factors for delayed CINV.
Conclusion: The current use of antiemetic prophylaxis according to the recommended guideline appears to effectively control delayed CINV in patients undergoing LEC. Therefore, patients with the abovementioned risk factors should be carefully observed, and their treatment should be adjusted according to their symptoms. The use of multiple-day dexamethasone may be beneficial for those patients who develop acute CINV, especially when it is accompanied by anorexia.
Keywords: adverse effects, antiemetics, prophylaxis, quality of life
Introduction
CINV is a well-known potential adverse effect of cancer chemotherapy that impairs the patients’ quality of life, including that of patients undergoing LEC.1,2 The control of delayed CINV, a particularly important issue, remains unresolved. In fact, in our previous study, delayed CINV was observed more frequently than that of predicted CINV, and the severity of nausea gradually increased from day 1, peaking on days 4 and 5.3 Both treatment- and patient-related risk factors need to be considered to ensure the optimal control of CINV.4–12 However, until recently, the lack of clinical trials performed in patients treated with LEC has made it difficult to identify patients at risk of CINV. Moreover, antiemetic guidelines recommend the use of a single agent, such as low-dose dexamethasone on the first day of chemotherapy, stating that it is not necessary to administer antiemetics to prevent delayed CINV in patients undergoing LEC; however, this recommendation is not based on the results of clinical trials, rather it reflects a consensus among experts in the field.13–16 Identifying patients at a high risk of delayed CINV while undergoing LEC may enhance the clinical management by health care providers to reduce the incidence and severity of delayed CINV. Therefore, in this study, we aimed to assess both risk factors and a candidate treatment strategy for delayed CINV in patients with solid cancers undergoing LEC.
Patients and methods
Study design
The study design, including patient enrollment, data collection, and treatment, has been described previously.3 Briefly, this prospective, observational, multicenter study was conducted from September 2013 to November 2014 in 13 hospitals affiliated with the National Hospital Organization in Kyushu, Japan. Adult patients beginning LEC were consecutively recruited at the study sites.
This study is registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (protocol ID: UMIN000020800).
Patients
Men and women ≥20 years of age who were LEC-naïve and scheduled to undergo at least 1 cycle of a single-day cytotoxic LEC were eligible for inclusion in this study. The intended cancer treatment comprised at least one of the following injectable agents: docetaxel, paclitaxel, gemcitabine, pemetrexed, liposomal doxorubicin, eribulin, and 5-fluorouracil. Patients were excluded from the study if they had undergone treatment with chronic systemic corticosteroid therapy, concurrent abdominal or pelvic radiation therapy, or had undergone LEC within 120 hours (5 days) of initiating chemotherapy. Patients were also excluded if they had brain metastases or had vomited in the 24-hour period preceding chemotherapy initiation.
Patients were provided with a diary before the commencement of chemotherapy and were asked to record their digestion-related symptoms (development and severity of nausea, frequency of vomiting, food intake, and the number of salvage treatments received) each day during a 5-day period after commencing LEC. The incidence of nausea was identified by patients. A 100 mm linear VAS was used to quantify food intake (100 mm, no oral food intake; 0 mm, eating as usual) and severity of nausea (100 mm, worst nausea; 0 mm, no nausea). Before or at the time of the initial chemotherapy treatment, we recorded the following patient information on a case report form: initials, sex, hospital number, date of birth, treatment history, alcohol consumption, history of motion sickness, ECOG performance status, cancer chemotherapy regimen, and antiemetic as well as salvage antiemetic treatments. The patients were asked to send their completed diaries to the Central Office using the preaddressed return envelopes provided. Likewise, the investigators sent their case reports to the Central Office in such return envelopes.
Statistical analyses
Patient demographics, clinical characteristics, and antiemetic treatments prescribed for the acute and delayed phases were summarized using contingency tables. Independent risk factors for delayed CINV incidence (dependent variable) were evaluated using logistic regression analysis with backward elimination method. The following independent factors were included in the model: sex, CINV history, development of acute CINV, opioid use, motion sickness, morning sickness, alcohol consumption, ECOG performance status, antiemetic prophylaxis, LEC other than taxane, and age. The severity patterns of nausea and food intake in relation to the occurrence of acute CINV were evaluated by the transition of the VAS score (without conducting further statistical analysis). All reported P-values corresponded to two-sided tests; and P-values <0.05 were considered statistically significant. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethics approval and informed consent
All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committees of each participating institution, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from individual participants, prior to their inclusion in the study.
Results
Participants
In this study, 222 patients were enrolled who were undergoing LEC for the first time. After excluding patients who withdrew within 5 days of undergoing LEC or who did not submit a diary, the data of 210 patients (94.6% of all patients enrolled) were finally analyzed (Figure 1). Table 1 summarizes the demographic and clinical characteristics of the 210 patients.
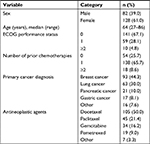  | Table 1 Patient demographics and clinical characteristics (n=210) Abbreviation: ECOG, Eastern Cooperative Oncology Group. |
Antiemetic treatment
The CINV guidelines recommend the use of single-day antiemetic agents (e.g., dexamethasone) for patients undergoing LEC.13–16 Such patients should not undergo routine antiemetic prophylaxis during the delayed phase. However, 78 patients (37.1%) received antiemetic agents on multiple days. Table 2 summarizes the antiemetic treatments prescribed for the acute and delayed phases in the first cycle of LEC.
Incidence of delayed CINV
Delayed CINV (of any grade) was reported by 27.3% of the patients who underwent the single-day antiemetic prophylaxis and by 11.5% of the patients who underwent the multiple-day antiemetic prophylaxis. Among patients who developed acute CINV, 68.8% and 33.3% who underwent the single- and multiple-day prophylaxis, respectively, developed delayed CINV (Table 2). The antiemetic most commonly used on day 2 or later, for multiple-day prophylaxis was dexamethasone.
Analysis of risk factors
Univariate and multivariate logistic regression analyses were performed to determine the degree of delayed CINV risk associated with various CINV-related factors. The multivariate analysis identified the history of nausea and/or vomiting, ECOG performance status score ≥1, acute CINV, and single-day antiemetic prophylaxis as independent risk factors for delayed CINV (Table 3).
Severity of nausea and amount of food intake
Figures 2 and 3 shows the daily mean VAS scores for severity of nausea and the amount of food intake, respectively, on days 1–5 postchemotherapy. Because the low incidence of vomiting precluded the observation of a visible difference in vomiting, the incidence of nausea alone was assessed. In patients who developed acute CINV, those who underwent multiple-day antiemetic prophylaxis had a lesser reduction in food intake than those who underwent the single-day antiemetic prophylaxis.
Discussion
This study demonstrated both risk factors and a candidate treatment strategy for delayed CINV in patients with solid cancers undergoing LEC. History of CINV, ECOG performance status score ≥1, CINV in the acute phase, and undergoing single-day prophylactic antiemetics were found to be the independent risk factors for delayed CINV in patients undergoing LEC.
We found that younger age was not a risk factor for CINV; this finding is consistent with previous reports.17,18 However, it differs from the widely accepted clinical view that younger patients are more prone to CINV than that of older patients. It is possible that age was not identified as a risk factor in this study because of the age strata bias—the median age of the participants in this study was found to be 64 years.
Single-day antiemetic prophylaxis was identified as a risk factor for delayed CINV. Patients undergoing multiple-day antiemetic prophylaxis experienced delayed CINV less frequently than those undergoing single-day antiemetic prophylaxis. However, the VAS-based evaluation of the severity of nausea revealed that patients experienced only mild nausea; hence, the change of antiemetic treatment, from the single-day to the routine multiple-day prophylaxis, was unnecessary for all patients undergoing LEC. “No significant nausea” has historically been defined as a VAS score <25.18,19 However, a more robust and modern approach would be to use “no nausea” as the primary endpoint, especially in the context of LEC. This is the most patient-centered clinical outcome; this parameter was also used in a recent study.20 In this study, we relied on the information provided by the patients when assessing the incidence of nausea. Therefore, we defined a VAS score of 0 mm as “no nausea.”
Of the patients who developed acute CINV, the incidence of delayed CINV was found to be higher in those undergoing single-day antiemetic prophylaxis than those undergoing multiple-day antiemetic prophylaxis. Therefore, acute CINV is a possible predictor of delayed CINV. Patients who underwent multiple-day antiemetic prophylaxis had less severe nausea and lesser reduction in food intake than patients who underwent single-day antiemetic prophylaxis. Molassiotis et al reported the symptom cluster related to nausea as loss of appetite, dry mouth, feeling drowsy and bloated, and vomiting; nausea, rather than vomiting, was associated with the loss of appetite and dry mouth.21 Olver et al reported that fatigue often accompany chemotherapy-induced nausea.22 In this study, dexamethasone was the most common antiemetic used to prevent delayed CINV. It improves anorexia and fatigue; thus, it may be useful in patients experiencing these adverse effects. Ito et al suggested that administration of dexamethasone on days 1–3, compared to only day 1, reduces the incidences of nausea, anorexia, depression, and fatigue during the delayed phase.23 Thus, in patients who develop acute CINV, dexamethasone may be useful in preventing nausea and anorexia in the delayed phase. However, because dexamethasone has several side effects, including insomnia, indigestion/epigastric discomfort, agitation, and increased appetite,24,25 we need to be mindful of these side effects when using dexamethasone for delayed antiemetic prophylaxis.
In a randomized controlled trial comparing the efficacy of palonosetron plus dexamethasone on day 1 with or without dexamethasone on days 2 and 3 for the prevention of CINV, patients receiving dexamethasone on multiple days experienced a significantly higher incidence of insomnia than that of patients receiving single-day dexamethasone. In another study, the incidence of adverse events potentially attributable to dexamethasone, such as abdominal pain and hiccups, tended to be lower in the single-day than in the multiple-day dexamethasone group, but this difference was not statistically significant.26 Therefore, multiple-day dexamethasone could be a candidate alternative prophylactic antiemetic regimen for patients undergoing LEC. However, randomized comparative studies with large patient populations are needed to confirm the efficacy of the salvage treatment. Hesketh et al demonstrated that palonosetron was well tolerated and effectively prevented CINV in both acute and delayed phases in patients with the history of CINV.27 Although palonosetron is a useful antiemetic treatment option, it is expensive and does not increase the appetite or improve fatigue.
Breakthrough CINV is also an important issue. In this study, rescue antiemetics were administered to 28.9% of the patients with breakthrough CINV during the delayed phase, while metoclopramide was prescribed most frequently in this study (data not shown). Guidelines on breakthrough CINV recommend the use of antiemetics with a different mechanism of action from those used as initial prophylaxis (e.g., dopamine receptor antagonists, glucocorticoids, and antipsychotic or antianxiety agents) or a first generation 5-hydroxytryptamine 3 receptor antagonist different from that used as initial prophylaxis.13–16 However, the recommended treatment is unable to effectively control the breakthrough CINV.28 Navari et al reported that olanzapine was significantly better than metoclopramide in controlling breakthrough CINV in patients undergoing HEC.29 As reported, the repeated use of rescue palonosetron is useful in controlling breakthrough CINV in HEC or moderate-emetic-risk chemotherapy, even when it was already used as prophylaxis.30 However, the repeated use of palonosetron does not appear to be reasonable in LEC from an economic point of view. The antiemetic treatment for breakthrough CINV is not well established, and optimal antiemetic regimen for breakthrough CINV in LEC is still unclear. Further studies are needed to establish the strategy to prevent and suppress breakthrough CINV. However, the novel strategy, multiple-day dexamethasone, as shown in this study, can be expected to reduce the incidence of breakthrough CINV in the delayed phase.
This study has some limitations. First, its design was neither randomized nor blinded, and the sample size was not very large. Second, more than half of the patients in this study had undergone prior chemotherapy treatment. Compared with chemotherapy-naïve patients, previous chemotherapy might have affected the incidence of CINV among patients in this study. Finally, patients who underwent taxane therapy (e.g., docetaxel or paclitaxel) accounted for ~70% of the study population. Despite these limitations, we believe that the results show the risk factors for delayed CINV in routine clinical practice, as opposed to a controlled trial design, and may therefore, be more realistic.
Conclusion
The current use of antiemetic prophylaxis, according to the recommended guideline, controls the delayed CINV in patients undergoing LEC. However, patients with the identified risk factors (i.e., the history of nausea and/or vomiting, ECOG performance status score ≥1, acute CINV, and single-day antiemetic prophylaxis) should be carefully observed, and treatment should be adjusted according to their symptoms. The use of multiple-day dexamethasone may be beneficial in patients who develop acute CINV, especially when it is accompanied by anorexia.
Abbreviations
CINV, chemotherapy-induced nausea and vomiting
ECOG, Eastern Cooperative Oncology Group
HEC, high-emetic-risk chemotherapy
LEC, low-emetic-risk chemotherapy
VAS, visual analog scale
Data availability
All datasets supporting the results reported in this article are kept by the corresponding author and can be provided upon reasonable request.
Acknowledgments
We thank the study participants and their families as well as the support personnel. This study included the following 13 medical institutions: the National Kyushu Medical Center (Fukuoka, Fukuoka), National Kyushu Cancer Center (Fukuoka, Fukuoka), National Hospital Organization (NHO) Fukuoka National Hospital (Fukuoka, Fukuoka), NHO Kokura Medical Center (Kitakyushu, Fukuoka), NHO Saga National Hospital (Saga, Saga), NHO Ureshino Medical Center (Ureshino, Saga), NHO Nagasaki Medical Center (Omura, Nagasaki), NHO Kumamoto Medical Center (Kumamoto, Kumamoto), NHO Kumamoto Saishunso National Hospital (Koushi, Kumamoto), NHO Kumamoto Minami National Hospital (Uki, Kumamoto), NHO Beppu Medical Center (Beppu, Oita), NHO Miyakonojo Medical Center (Miyakonojo, Miyazaki), and NHO Kagoshima Medical Center (Kagoshima, Kagoshima). Funding for this study was provided by the Policy-Based Medical Service Foundation. The Foundation was not involved in the research design or manuscript writing.
Disclosure
The authors report no conflicts of interest in this work.
References
Grunberg SM, Osoba D, Hesketh PJ, et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity--an update. Support Care Cancer. 2005;13(2):80–84. | ||
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478. | ||
Hayashi T, Shimokawa M, Miyoshi T, et al. A prospective, observational, multicenter study on risk factors and prophylaxis for low emetic risk chemotherapy-induced nausea and vomiting. Support Care Cancer. 2017;25(9):2707–2714. | ||
du Bois A, Meerpohl HG, Vach W, Kommoss FG, Fenzl E, Pfleiderer A. Course, patterns, and risk-factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetron. Eur J Cancer. 1992;28(2-3):450–457. | ||
Hesketh P, Navari R, Grote T, et al. Double-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. Dolasetron Comparative Chemotherapy-induced Emesis Prevention Group. J Clin Oncol. 1996;14(8):2242–2249. | ||
Osoba D, Zee B, Pater J, Warr D, Latreille J, Kaizer L. Determinants of postchemotherapy nausea and vomiting in patients with cancer. Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1997;15(1):116–123. | ||
Pater J, Slamet L, Zee B, Osoba D, Warr D, Rusthoven J. Inconsistency of prognostic factors for post-chemotherapy nausea and vomiting. Support Care Cancer. 1994;2(3):161–166. | ||
Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64(5):1117–1122. | ||
Persistence of efficacy of three antiemetic regimens and prognostic factors in patients undergoing moderately emetogenic chemotherapy. Italian Group for Antiemetic Research. J Clin Oncol. 1995;13(9):2417–2426. | ||
Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14(3):238–242. | ||
Roila F, Tonato M, Basurto C, et al. Antiemetic activity of high doses of metoclopramide combined with methylprednisolone versus metoclopramide alone in cisplatin-treated cancer patients: a randomized double-blind trial of the Italian Oncology Group for Clinical Research. J Clin Oncol. 1987;5(1):141–149. | ||
Tamura K, Aiba K, Saeki, et al; CINV Study Group of Japan. Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol. 2015;20(5):855–865. | ||
Olver I, Ruhlmann CH, Jahn F, et al. 2016 Updated MASCC/ESMO Consensus Recommendations: Controlling nausea and vomiting with chemotherapy of low or minimal emetic potential. Support Care Cancer. 2017;25(1):297–301. | ||
Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(28):3240–3261. | ||
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (2017) Antiemetics. Version 2; 2017. Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed April 30, 2018. | ||
Japan Society of Clinical Oncology. [Guidelines for Antiemetics in Oncology 2015]. Tokyo: Kanehara & Co, Ltd; 2015. Available from: https://www.kanehara-shuppan.co.jp/books/detail.html?isbn=9784307101745. Accessed September 26, 2018. Japanese. | ||
Pirri C, Katris P, Trotter J, Bayliss E, Bennett R, Drummond P. Risk factors at pretreatment predicting treatment-induced nausea and vomiting in Australian cancer patients: a prospective, longitudinal, observational study. Support Care Cancer. 2011;19(10):1549–1563. | ||
Molassiotis A, Aapro M, Dicato M, et al. Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: results from a European prospective observational study. J Pain Symptom Manage. 2014;47(5):839–848.e4. | ||
Hesketh PJ, Grunberg SM, Gralla RJ, et al; Aprepitant Protocol 052 Study Group. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112–4119. | ||
Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134–142. | ||
Molassiotis A, Farrell C, Bourne K, Brearley SG, Pilling M. An exploratory study to clarify the cluster of symptoms predictive of chemotherapy-related nausea using random forest modeling. J Pain Symptom Manage. 2012;44(5):692–703. | ||
Olver IN, Eliott JA, Koczwara B. A qualitative study investigating chemotherapy-induced nausea as a symptom cluster. Support Care Cancer. 2014;22(10):2749–2756. | ||
Ito Y, Tsuda T, Minatogawa H, et al. Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol. 2018;36(10):1000–1006. | ||
Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006;94(7):1011–1015. | ||
Nakamura M, Ishiguro A, Muranaka T, et al. A prospective observational study on effect of short-term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO-01). Oncologist. 2017;22(5):592–600. | ||
Komatsu Y, Okita K, Yuki S, et al. Open-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with Palonosetron. Cancer Sci. 2015;106(7):891–895. | ||
Hesketh PJ, Morrow G, Komorowski AW, Ahmed R, Cox D. Efficacy and safety of palonosetron as salvage treatment in the prevention of chemotherapy-induced nausea and vomiting in patients receiving low emetogenic chemotherapy (LEC). Support Care Cancer. 2012;20(10):2633–2637. | ||
Tamura K, Aiba K, Saeki T, et al; CINV Study Group of Japan. Breakthrough chemotherapy-induced nausea and vomiting: report of a nationwide survey by the CINV Study Group of Japan. Int J Clin Oncol. 2017;22(2):405–412. | ||
Navari RM, Nagy CK, Gray SE. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2013;21(6):1655–1663. | ||
Musso M, Scalone R, Bonanno V, et al. Palonosetron (Aloxi) and dexamethasone for the prevention of acute and delayed nausea and vomiting in patients receiving multiple-day chemotherapy. Support Care Cancer. 2009;17(2):205–209. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





