Back to Journals » Infection and Drug Resistance » Volume 13
Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections and Outcomes
Authors Yuan Y, Wang J, Yao Z , Ma B, Li Y, Yan W, Wang S, Ma Q, Zhang J, Xu J, Li L, Wang Y , Fan E
Received 15 July 2019
Accepted for publication 7 January 2020
Published 22 January 2020 Volume 2020:13 Pages 207—215
DOI https://doi.org/10.2147/IDR.S223243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Youhua Yuan, 1,* Junjie Wang, 2,* Zonghui Yao, 1,* Bing Ma, 1 Yi Li, 1 Wenjuan Yan, 1 Shanmei Wang, 1 Qiong Ma, 1 Jiangfeng Zhang, 1 Junhong Xu, 1 Li Li, 1 Yuming Wang, 1 Enguo Fan 1
1Department of Clinical Microbiology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, and People’s Hospital of Henan University, Zhengzhou, Henan 450003, People’s Republic of China; 2Clinical Laboratory, Luyi Zhenyuan Hospital, Zhoukou, Henan 477200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Enguo Fan; Yuming Wang
Department of Clinical Microbiology Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, and People’s Hospital of Henan University, Weiwu Road 5, Zhengzhou, Henan 450003, People’s Republic of China
Tel +8637165580484
Fax +8637187160318
Email [email protected]; [email protected]
Purpose: The incidence of carbapenem-resistant Klebsiella pneumoniae (CRKP) bloodstream infections (BSIs) is increasing globally; however, little has been reported on the risk factors and outcomes of CRKP BSIs in central China. This study aimed to determine the clinical risk factors for CRKP BSIs and the outcomes of CRKP BSIs.
Patients and Methods: We performed a case-control study of 239 patients with K. pneumoniae BSIs who were treated at Henan Provincial People’s Hospital between July 2017 and July 2018. The cases (n=98, 41%) had CRKP BSIs, and the controls (n=141, 59%) had non-carbapenem-resistant K. pneumoniae (non-CRKP) BSIs. Antimicrobial sensitivity was determined using automated broth microdilution and an agar disk diffusion method. Data were obtained from clinical and laboratory records. Multivariate logistic regression and Pearson chi-square tests were used to identify clinical factors and outcomes associated with carbapenem resistance.
Results: Risk factors for carbapenem resistance included recent carbapenem use (odds ratio [OR]: 9.98, 95% confidence interval [CI]: 5.2– 17.1, P< 0.001), invasive procedures (OR: 11.1, 95% CI: 3.3– 37.7, P< 0.001), and pre-existing diseases of the digestive system (OR: 8.22, 95% CI: 1.73– 39.2, P=0.008). Treatment failure was more frequent in the cases (84.7%) than in the controls (32.6%).
Conclusion: Exposure to antibiotics, especially carbapenems, and invasive procedures were the major risk factors for carbapenem resistance among patients with K. pneumoniae BSIs. Strict control measures should be implemented to prevent the emergence and spread of CRKP.
Keywords: antimicrobial resistance, bacteremia, digestive system diseases, treatment failure
Introduction
With the increasing use of carbapenems in hospitals worldwide, carbapenem-resistant Klebsiella pneumoniae (CRKP) has become an important threat to public health, and its management and treatment pose a challenge for clinicians.1 K. pneumoniae is a major opportunistic pathogen that can lead to hospital-acquired infections,2 and the lower respiratory tract and blood are most common sites of infection.3 CRKP was first reported in 1997, and an increasing number of CRKP cases have been reported throughout the world, including in the United States, Europe, and Asia.4–6 However, little has been reported about the risk factors and outcomes of CRKP in persons with bloodstream infections (BSIs) in central China. Therefore, the present study aimed to determine the risk factors for CRKP, and the outcomes of CRKP among people living in Henan Province, China.
Materials and Methods
Study Subjects
This cross-sectional case-control study used clinical and microbiological data of patients who had been treated for K. pneumoniae BSIs in the Henan provincial hospital, a tertiary care teaching hospital with 5000 beds, between July 2017 and July 2018. Henan Province has the second highest population in China with about 100 million people, and the hospital has 13 intensive care unit (ICU) wards with about 300 beds that provide care to about 7000 inpatients annually. The ICUs are staffed by 100 professional doctors and 300 nurses. The study group comprised all patients with a first episode of CRKP BSI diagnosed during the study period. Accordingly, the patients in the control group were recruited from among non-CRKP patients of the same age and from the same source population, following the principle of matching, in a simple and random manner. Calculation of the sample size is based on analysis of the difference in incidence between patients with and those without CRKP BSIs.
Definition of Cases and Controls
The Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation (EBMT),7,8 defined a K. pneumoniae BSI as the presence of at least one positive blood culture with concomitant signs and symptoms of infection. Patients with K. pneumoniae BSIs were identified from the microbiology laboratory database. CRKP was defined as minimal inhibition concentration (MIC) for imipenem or meropenem ≥4 μg/mL. Patients with CRKP BSIs were assigned to the case group, and patients with non-carbapenem-resistant K. pneumoniae (non-CRKP) BSIs were randomly assigned to the control group.
Definition of Outcomes
The EBMT defines successful treatment of CRKP BSIs as recovery from BSI following treatment, with resolution of patients with CRKP BSI who remained alive or therapy patients without the signs of infection.7,8 Treatment failure is defined as either death or a recurrence of K. pneumoniae infection before discharge from the hospital. However, we were unable to ascertain all K. pneumoniae-related deaths because some patients were discharged from the hospital without completing treatment. In accordance with Chinese tradition, especially in some rural areas, family members are not willing to let patients die outside of their homes, and seriously ill patients sometimes request to be discharged from the hospital and to discontinue treatment so that they can die at home. Therefore, we extended the definition of treatment failure to include patients who were discharged from the hospital without completing the treatment.
K. pneumoniae BSI-related death or recurrence was defined as follows: BSI was considered as the primary cause of death or recurrence if the patient died after admission, within 120 hrs after the last positive blood culture and K. pneumonia was identified as the cause of death, with no other cause (including underlying disease, persistent neutropenia, other infections, and hemorrhage). K. pneumoniae BSI was considered an associated cause of death or recurrence when another cause was also present (uncontrolled underlying disease, persistent neutropenia, and/or graft versus host disease). Deaths or recurrences that were not related to K. pneumoniae BSI were defined as such when K. pneumoniae BSI had cleared by the time of death (as indicated by an absence of signs of infection or a positive culture).
Variables
Clinical data were collected from medical charts and/or hospital computer system databases. The following data were collected on each patient: age, sex, inpatient time, admission diagnosis, hospitalization, ICU admission, antibiotic use, intravascular catheter use, number of previous transfers between hospitals and departments, and history of blood transfusion. Data on the length of hospitalization, microbiological data, antimicrobial therapies, and outcomes were also collected.
The definition of admission diagnosis was based on the patient’s diagnosis on admission, classified according to the International Classification of Diseases-11 (ICD-11).9
Ethical Approval
The Ethics Committee of the Henan Provincial People’s Hospital approved this study. The requirement for informed consent was waived because this was a retrospective study that used routinely collected data and inclusion of these data in the study was not associated with any risks to the patients. Additionally, we stated to confirm the patient data confidentiality.
Microbiological Methods
The Vitek 2 system (bioMérieux, Marcy l’Étoile, France) and the Phoenix100 automated system (Becton Dickinson Co., Sparks, MD, USA) were used for K pneumoniae isolate identification. Matrix-assisted lasers desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) (Bruker Corporation, Karlsruhe, Germany) was used to confirm the identity of the isolate. MIC values for antimicrobial agents were determined by an automated broth microdilution method (Becton Dickinson Co., Sparks, MD, USA). Antibiotic susceptibility testing results were compared using the agar disk diffusion method (Becton Dickenson Co., Sparks, MD, USA), in accordance with the Clinical and Laboratory Standards Institute (CLSI) breakpoints. Results were interpreted according to CLSI criteria (CLSI2018).10,11
Statistical Analyses
Categorical variables were reported as frequencies and percentages, and continuous variables were reported as means and standard deviations (SDs) if they were normally distributed or as medians and interquartile ranges (IQRs) if they were non-normally distributed. Categorical variables were compared using chi-square or Fisher’s exact tests, and continuous variables were compared using Student’s t test or the Mann–Whitney U-test, according to their distribution. For univariate analyses, results were reported as odds ratios (ORs) with 95% confidence intervals (CIs) and P values. Variables with P values<0.1 in the univariate analyses were selected for possible inclusion in multivariate logistic regression. We used backward stepwise logistic regression to select variables for inclusion in the final multivariate logistic regression model to evaluate risk factors for CRKP BSIs. The discrimination ability of the logistic regression model was assessed by estimating the area under the receiver operating characteristic (ROC) curve. Model calibration was assessed using the Hosmer–Lemeshow test for goodness of fit. Five hundred-day survival curves were constructed using the Kaplan–Meier method. Log-rank tests were performed with Prism7.0 (GraphPad) software. Other statistical analyses were performed with SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). Two-tailed P values <0.05 were considered statistically significant.
Results
Characteristics of Study Participants
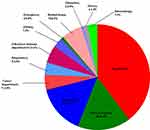
We identified 239 patients who were diagnosed with K. pneumoniae BSIs during the study period. Of the 239 patients, 98 had CRKP BSIs (cases), and 141 had Non-CRKP BSIs (controls). Patient characteristics are shown in Table 1. The mean ages of the cases and controls were 55±17 years and 56±17 years, respectively (P=0.66). The mean length of hospital stay was also similar in cases and controls (55±7 days and 51±5 days, respectively; P=0.67). As shown in Figure 1, the main admission diagnoses were infectious diseases, diseases of the digestive system, hematologic diseases, and tumors. As shown in Figure 2, the largest number of study patients were from the ICU, the surgical department, and the hematology department.
 |
Table 1 Univariate Analysis of Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections |
Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections
The results of the univariate analysis to identify risk factors associated with CRKP BSIs are shown in Table 1. The following factors were found to be associated with CRKP BSIs: underlying disease, injury, prior presence of intravascular catheter, number of previous transfers between hospitals and departments, blood transfusion, and hospitalization in ICUs. Prior exposure to cephalosporin, carbapenem, beta-lactam-beta-lactamase inhibitors, antifungal drugs and other antibiotics were also significant risk factors.
The results of the multivariate analysis are shown in Table 2. The independent risk factors for CRKP BSIs were diseases of the digestive system, diagnostic punctures, transfer between departments, and carbapenem exposure.
 |
Table 2 Multivariate Analysis of Factors Associated with Carbapenem Resistance Among Patients with Klebsiella pneumonia Bloodstream Infections |
Comparison of the Incidence of Treatment Failure Among the Cases and the Controls
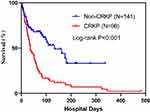
Of the 98 cases, 7 died, 76 discontinued treatment or asked to be discharged from the hospital, and 15 were cured. Of the 141 controls, 2 died, 44 discontinued treatment or asked to be discharged from the hospital, and 95 were cured. The rate of treatment failure among cases (84.7%) was considerably higher than that among controls (32.6%). The survival curve analysis confirmed that the cases had a lower survival rate than the controls (Figure 3).
Risk Factors for Treatment Failure
The results of the univariate analysis are shown in Table 3, Patients with treatment failure were hospitalized for a significantly shorter period than those with treatment success (mean: 44±5 days versus 62±1 days; p=0.01). In the univariate analysis, the following variables were identified as risk factors for treatment failure: underlying diseases, such as cardiovascular or respiratory diseases; days in hospital; prior presence of intravascular catheter; number of previous transfers between hospitals and departments; blood transfusion; and hospitalization in ICUs. Prior use of carbapenem, linezolid, antifungal agents, vancomycin, and beta-lactam-beta-lactamase inhibitors were also significant risk factors. Independent risk factors for CRKP treatment failure included malignancy, diagnostic punctures, tracheal intubation, transfer between hospital departments, and prolonged hospitalization (Table 4).
 |
Table 3 Univariate Analysis of Risk Factors for Treatment Failure of Klebsiella pneumonia Bloodstream Infections |
 |
Table 4 Multivariate Analysis of Risk Factors for Treatment Failure Among Patients with Klebsiella pneumonia Bloodstream Infections |
Discussion
Among the 239 patients with K. pneumoniae BSIs, 98 were infected with CRKP. The risk factors for CRKP BSIs and treatment failure included the prior use of antibiotics, invasive procedures, and digestive system diseases. This study is the first to report these risk factors for CRKP BSIs.
K. pneumoniae is one of the most common Gram-negative bacterial infections, and it is associated with serious BSIs.12 In hospitals, BSIs caused by K. pneumoniae rank second among Gram-negative bacterial infections and are associated with serious conditions and a poor prognosis.13,14 CRKP BSIs are common among hospital inpatients but are especially common among patients admitted to ICUs.15 This may be because of several factors. Patients in ICUs might have received multiple antibiotics, have had severe primary diseases, have undergone major surgery, or have experienced trauma. In addition, patients in ICUs are subjected to a variety of invasive surgical procedures. In our hospital, the emergence of CRKP BSIs is a challenge for medical staff. The high frequency of CRKP BSIs in the general surgery department is likely to be largely due to the high frequency of invasive procedures such as catheter insertion.
Previously published reviews have reported several risk factors for CRKP, including severe underlying diseases, use of broad-spectrum antibiotics, use of carbapenem antibiotics, a long hospitalization period, malignancies, hematopoietic stem cell transplantation, tracheotomy, mechanical ventilation, and indwelling catheters.5,16,17 In our study, univariate analysis results showed that ICU admission, respiratory disease, cardiovascular disease, nervous system disease, digestive disease, invasive operations, and carbapenem exposure were all risk factors for CRKP BSIs. These results are similar to those of previous studies in other parts of the world.18 Multivariate regression analysis showed that digestive disease, invasive procedures, and the length of hospitalization were independent risk factors for CRKP BSIs. Notably, in the present study, digestive diseases were found to be a risk factor for CRKP BSIs. Digestive diseases have not been reported as a risk factor for CRKP BSIs in published studies and thus is an important finding with clinical implications. K. pneumoniae is part of the normal commensal flora of the digestive tract. If digestive system diseases such as bleeding within the digestive tract, gastric ulcer, and pancreatitis occur, K. pneumoniae may enter the bloodstream and cause BSIs, including CRKP BSIs.
The overuse of broad-spectrum antimicrobial agents and invasive procedures has led to an increase in the prevalence of drug-resistant K. pneumoniae and in the incidence of CRKP infection.19,20 Our findings are consistent with those of other studies, which have reported a strong association between exposure to carbapenem agents and CRKP infection.21,22 Carbapenem use may cause selective pressure on resistant microorganisms, thereby increasing the risk of infection. Although a few studies have shown that avoiding unnecessary changes in the use of antimicrobials can reduce the incidence of CRKP infections,23 the results of our study suggest that the use of carbapenems should be minimized. Moreover, the control measures for hospital infection include appropriate and strict isolation precautions, such as hand hygiene of medical staff and isolation of each patient in a separate, walled room, and surgery for only CRKP infection should be implemented to prevent the emergence and spread of CRKP.
This study showed that risk factors for CRKP include repeated, long-term hospitalization, and multiple hospital and department transfers during hospitalization. These results are similar to those of other studies.18,24 More attention should be given to the risks posed by these factors in hospital settings.
However, it should be noted that this study has several limitations. First, the retrospective study design could have led to information and selection biases. Results of the multivariate analysis showed that baseline disease aggravates CRKP BSIs. This has not been reported previously25,26 and may be because of sampling bias caused by the small number of patients with underlying diseases in this study. Second, being a retrospective study, it also does not provide sufficient strength of evidence to establish a causal relationship between possible risk factors for CRKP infection. Additionally, there may have been unmeasured confounders associated with CRKP infection. Third, we did not measure the overall incidence of CRKP BSIs and thus the patients in our study may not represent the range of severity of CRKP, and this may be a source of bias.27
In conclusion, our results confirm that CRKP BSIs are relatively common in hospital settings, which poses an important challenge to clinicians. This retrospective analysis of risk factors for CRKP infection suggests that the misuse of antibiotics such as carbapenems as well as invasive procedures contribute to the spread of CRKP. Therefore, clinicians should strictly monitor antibiotic application and ensure rational use of carbapenems.
Ethics Approval and Informed Consent
The Ethics Committee of the Henan Provincial People’s Hospital approved this study. Informed consent was waived because it was a retrospective study that used routinely collected data and inclusion in the study was not associated with any risk for patients. Additionally, we stated to confirm patient data confidentiality.
Data Sharing Statement
The datasets generated for this study are available from the corresponding author on request.
Acknowledgments
We gratefully acknowledge Dr Francoise Jacob-Dubuisson working at Institute Pasteur Lille, France for a critical reading of our manuscript. We thank the patients who participated in this study for providing health information for research purposes.
Funding
This work was supported by Henan Provincial Key Programs in Science and Technology (182102310576, 182102310097) and Joint Program of Henan Province and Chinese Health Committee (SB201903018).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Amit S, Mishali H, Kotlovsky T, Schwaber MJ, Carmeli Y. Bloodstream infections among carriers of carbapenem-resistant Klebsiella pneumoniae: etiology, incidence and predictors. Clin Microbiol Infect. 2015;21(1):30–34. doi:10.1016/j.cmi.2014.08.001
2. Hsu JF, Chu SM, Huang YC, et al. Predictors of clinical and microbiological treatment failure in neonatal bloodstream infections. Clin Microbiol Infect. 2015;21(5):482 e489–417. doi:10.1016/j.cmi.2015.01.009
3. Jiao Y, Qin Y, Liu J, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health. 2015;109(2):68–74. doi:10.1179/2047773215Y.0000000004
4. Kohler PP, Volling C, Green K, Uleryk EM, Shah PS, McGeer A. Carbapenem resistance, initial antibiotic therapy, and mortality in Klebsiella pneumoniae bacteremia: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2017;38(11):1319–1328. doi:10.1017/ice.2017.197
5. Righi E, Peri AM, Harris PN, et al. Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(3):668–677. doi:10.1093/jac/dkw459
6. Kiddee A, Assawatheptawee K, Na-Udom A, et al. Risk factors for gastrointestinal colonization and acquisition of carbapenem-resistant gram-negative bacteria among patients in intensive care units in Thailand. Antimicrob Agents Chemother. 2018;62:8. doi:10.1128/AAC.00341-18
7. Frere P, Hermanne JP, Debouge MH, de Mol P, Fillet G, Beguin Y. Bacteremia after hematopoietic stem cell transplantation: incidence and predictive value of surveillance cultures. Bone Marrow Transplant. 2004;33(7):745–749. doi:10.1038/sj.bmt.1704414
8. Cappellano P, Viscoli C, Bruzzi P, Van Lint MT, Pereira CA, Bacigalupo A. Epidemiology and risk factors for bloodstream infections after allogeneic hematopoietic stem cell transplantation. New Microbiol. 2007;30(2):89–99.
9. Kazlauskas E, Gegieckaite G, Eimontas J, Zelviene P, Maercker A. A brief measure of the international classification of diseases-11 adjustment disorder: investigation of psychometric properties in an adult help-seeking sample. Psychopathology. 2018;51(1):10–15. doi:10.1159/000484415
10. Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
11. Zhang F, Li Y, Lv Y, Zheng B, Xue F. Bacterial susceptibility in bloodstream infections: results from China Antimicrobial Resistance Surveillance Trial (CARST) program, 2015–2016. J Glob Antimicrob Resist. 2019;17:276–282. doi:10.1016/j.jgar.2018.12.016
12. Wang C, Yuan Z, Huang W, Yan L, Tang J, Liu CW. Epidemiologic analysis and control strategy of Klebsiella pneumoniae infection in intensive care units in a teaching hospital of People’s Republic of China. Infect Drug Resist. 2019;12:391–398. doi:10.2147/IDR.S189154
13. Zhang Y, Guo LY, Song WQ, Wang Y, Dong F, Liu G. Risk factors for carbapenem-resistant K. pneumoniae bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis. 2018;18(1):248. doi:10.1186/s12879-018-3160-3
14. Li M, Wang X, Wang J, et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: a 4-year quasi-experimental before-and-after study. Antimicrob Resist Infect Control. 2019;8(1):8. doi:10.1186/s13756-018-0453-7
15. Zheng X, Wang JF, Xu WL, Xu J, Hu J. Clinical and molecular characteristics, risk factors and outcomes of Carbapenem-resistant Klebsiella pneumoniae bloodstream infections in the intensive care unit. Antimicrob Resist Infect Control. 2017;6(1):102. doi:10.1186/s13756-017-0256-2
16. Katchanov J, Asar L, Klupp EM, et al. Carbapenem-resistant Gram-negative pathogens in a German university medical center: prevalence, clinical implications and the role of novel beta-lactam/beta-lactamase inhibitor combinations. PLoS ONE. 2018;13(4):e0195757. doi:10.1371/journal.pone.0195757
17. Zheng B, Dai Y, Liu Y, et al. Molecular epidemiology and risk factors of carbapenem-resistant Klebsiella pneumoniae infections in Eastern China. Front Microbiol. 2017;8:1061. doi:10.3389/fmicb.2017.01061
18. Zhao D, Zuo Y, Wang Z, Li J. Characterize carbapenem-resistant Klebsiella pneumoniae isolates for nosocomial pneumonia and their Gram-negative bacteria neighbors in the respiratory tract. Mol Biol Rep. 2019;46(1):609–616. doi:10.1007/s11033-018-4515-y
19. Micozzi A, Gentile G, Minotti C, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis. 2017;17(1):203. doi:10.1186/s12879-017-2297-9
20. Mills JP, Talati NJ, Alby K, Han JH. The epidemiology of carbapenem-resistant Klebsiella pneumoniae colonization and infection among long-term acute care hospital residents. Infect Control Hosp Epidemiol. 2016;37(1):55–60. doi:10.1017/ice.2015.254
21. Giacobbe DR, Del Bono V, Bruzzi P, et al. Previous bloodstream infections due to other pathogens as predictors of carbapenem-resistant Klebsiella pneumoniae bacteraemia in colonized patients: results from a retrospective multicentre study. Eur J Clin Microbiol Infect Dis. 2017;36(4):663–669. doi:10.1007/s10096-016-2843-1
22. Candevir Ulu A, Kurtaran B, Inal AS, et al. Risk factors of carbapenem-resistant Klebsiella pneumoniae infection: a serious threat in ICUs. Med Sci Monit. 2015;21:219–224. doi:10.12659/MSM.892516
23. Wang Z, Qin RR, Huang L, Sun LY. Risk factors for carbapenem‑resistant Klebsiella pneumoniae infection and mortality of Klebsiella pneumoniae infection. Chin Med J. 2018;131:
24. Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5(1):48. doi:10.1186/s13756-016-0145-0
25. Liu P, Li X, Luo M, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. Microb Drug Resist. 2018;24(2):190–198. doi:10.1089/mdr.2017.0061
26. Harada S, Aoki K, Yamamoto S, et al. Clinical and molecular characteristics of Klebsiella pneumoniae causing bloodstream infections in Japan: occurrence of hypervirulent infections in healthcare. J Clin Microbiol. 2019. JCM.01206-19. doi:10.1128/JCM.01206-19
27. Hu Y, Ping Y, Li L, Xu H, Yan X, Dai H. A retrospective study of risk factors for carbapenem-resistant Klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries. 2016;10(3):208–213. doi:10.3855/jidc.6697
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.