Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Risk Factors for Asymptomatic and Symptomatic Intracranial Atherosclerosis Determined by Magnetic Resonance Vessel Wall Imaging in Chinese Population: A Case–Control Study
Authors Han Y, Zhang R, Yang D , Li D, Han H, Qiao H, Chen S, Wang Y, Yu M, Hong Y, Wang Z , Zhao X, Liu G
Received 21 August 2021
Accepted for publication 16 November 2021
Published 12 January 2022 Volume 2022:18 Pages 61—70
DOI https://doi.org/10.2147/TCRM.S335401
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yongjun Han,1,* Runhua Zhang,2,3,* Dandan Yang,4 Dongye Li,5 Hualu Han,6 Huiyu Qiao,6 Shuo Chen,6 Yu Wang,2,3 Miaoxin Yu,2,3 Yin Hong,2,3 Zhiqun Wang,1 Xihai Zhao,6,* Gaifen Liu2,3,*
1Department of Radiology, Aerospace Center Hospital, Beijing, People’s Republic of China; 2Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Beijing, People’s Republic of China; 4Center for Brain Disorders Research, Capital Medical University and Beijing Institute of Brain Disorders, Beijing, People’s Republic of China; 5Department of Radiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 6Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University School of Medicine, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xihai Zhao
Center for Biomedical Imaging Research, School of Medicine, Tsinghua University, Haidian District, Beijing, 100084, People’s Republic of China
Tel +86-10-62792662
Fax +86-10-62796175
Email [email protected]
Gaifen Liu
Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Fengtai District, Beijing, 100070, People’s Republic of China
Tel +86-10-59976746
Email [email protected]
Background and Purpose: The association between risk factors and intracranial atherosclerosis disease (ICAD) determined by magnetic resonance (MR) vessel wall imaging in Chinese population has not been investigated. The aim of this study was to investigate the associations of conventional vascular risk factors with asymptomatic and symptomatic ICAD using MR vessel wall imaging in Chinese population.
Methods: The study population was recruited from two cohort studies of ICASMAP and CAMERA comprised 104 symptomatic ICAD subjects (57.1 ± 11.1 years; 35.6% females), 51 asymptomatic ICAD subjects (70.1 ± 8.4 years; 50.0% females) and 418 controls (58.0 ± 13.3 years; 61.0% females) defined as asymptomatic subjects without ICAD on MR vessel wall imaging. We compared the vascular risk factors between the three groups using a multivariate logistic regression analysis.
Results: Compared with controls, there was a significant positive association between age (OR: 1.07, 95% CI: 1.03– 1.10, p < 0.001) and hypertension (OR: 3.03, 95% CI: 1.45– 6.36, p = 0.003) and asymptomatic ICAD. There was a positive association of smoking (OR: 3.41, 95% CI: 1.57– 7.42, p = 0.001), hypertension (OR: 7.43, 95% CI: 3.81– 14.49, p < 0.001) and diabetes (OR: 3.54, 95% CI: 1.93– 6.49, p < 0.001) and an inverse association of high-density lipoprotein (HDL) (p < 0.017) with symptomatic ICAD. Compared to asymptomatic ICAD, there was a significant inverse association of age (OR: 0.86, 95% CI: 0.81– 0.92, p < 0.001) and HDL (p < 0.001) with symptomatic ICAD.
Conclusion: Old age and hypertension are associated with asymptomatic ICAD and smoking, hypertension, diabetes and lower HDL are associated with an increased risk of symptomatic ICAD in Chinese population.
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT03417063.
Keywords: intracranial artery, atherosclerosis, risk factors, magnetic resonance, vessel wall imaging
Introduction
Stroke is one of the leading causes of death worldwide.1–3 As one of the most common causes of ischemic stroke, intracranial atherosclerosis disease (ICAD) accounts for up to 50% of ischemic stroke or transient ischemic attacks (TIA) in Asia.4–6 A number of studies have shown that age, gender, hypertension, hyperlipidemia, diabetes, obesity, and smoking are important risk factors for ICAD.7 Unfortunately, the ICAD in previous studies was mainly assessed by transcranial Doppler ultrasound or magnetic resonance (MR) angiography, which could not directly provide the information of the atherosclerotic plaques in the vessel wall. These imaging modalities lead to underestimation and even misdiagnosis, especially for the ICAD with positive remodeling. MR vessel wall imaging can accurately evaluate the morphology and compositions of atherosclerotic plaques in intracranial arteries with high spatial resolution and excellent reproducibility.8–10 However, the relationship between risk factors and ICAD determined by MR vessel wall imaging in Chinese population has not been investigated. Better understanding the determinants of the risk of developing ICAD and cerebrovascular events is of importance for refining the management strategy of ICAD patients and prevention of future events.
We hypothesized that patients with symptomatic ICAD have different clinical profiles compared to those with asymptomatic ICAD or the normal subjects. The aim of this study was to determine the associations of conventional vascular risk factors with asymptomatic and symptomatic ICAD in Chinese population using MR vessel wall imaging.
Materials and Methods
Study Sample
This is a retrospective study. The study population was recruited from two cohort studies of ICASMAP (Intracranial Artery Stenosis MR imaging: Aetiology and Progression, NCT03417063) and CAMERA (Cardio- and Cerebro-vascular Accident Monitoring, Epidemiology and Care Quality System). The ICASMAP study was an observational and prospective study which aimed to investigate the etiology of intracranial artery stenosis (ICAS) and the progression rate of ICAD in symptomatic population using MR imaging. The design and rational of the ICASMAP study have been published.11 The inclusion criteria were as follows: 1) 18 to 80 years old; 2) patients within 2 weeks after onset of ischemic stroke or TIA; 3) patients with ICAS with a range from 30% to 100% stenosis in at least one vascular bed determined by computed tomography angiography or MR angiography. The ICAS lesions can be located in intracranial internal carotid artery, basilar artery, M1 segment of middle cerebral artery, A1 segment of anterior cerebral artery, or P1 segment of posterior cerebral artery. The exclusion criteria included: 1) severe carotid artery atherosclerotic disease (stenosis ≥70%); 2) cardiogenic thrombosis; 3) heart failure or respiratory failure; 4) renal dysfunction (serum creatinine ˃133umol/L); 5) serious disturbance of consciousness; 6) cerebral neoplasms; 7) intracranial hemorrhage; 8) claustrophobia; 9) contraindications to MR; and 10) pregnant or plan to get pregnant within recent 2 years. The CAMERA study is a community-based prospective study which aimed to evaluate the cerebrovascular risk of asymptomatic population at Tsinghua community. The inclusion criteria included: 1) age ≥30 years old; and 2) no cerebrovascular symptoms within recent 6 months. Subjects with contraindications to MR examination were excluded.
Clinical Information Collection
For symptomatic patients, clinical information including age, gender, body mass index (BMI), overweight, smoking, alcohol use, hypertension, serum lipid levels (low-density lipoprotein [LDL], high-density lipoprotein [HDL], total cholesterol [TC], and triglycerides [TG]), statin use, diabetes mellitus and history of coronary heart disease (CHD), history of stroke and history of TIA were collected from the medical record. For subjects from CAMERA study, the above clinical data were obtained from the questionnaires. Overweight was defined as BMI ≥25 kg/m2. Current smoking was defined as at least one cigarette per day during the past one year.12 Current alcohol use was defined as at least one drink per day during the past one year.13 Hypertension was defined as history of elevated blood pressure (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) diagnosed by physician or treatment with antihypertensive drugs. Diabetes mellitus was defined as fasting blood glucose ≥7.0 mmol/L (126 mg/dL), a known past history of diabetes mellitus or use of hypoglycemic drugs therapy. Institutional review board approvals were obtained for the entire study and for each participating institution. All subjects provided written consent form before participation.
MR Imaging
All the enrolled subjects from two cohorts underwent MR vessel wall imaging for intracranial arteries which included the intracranial internal carotid artery, basilar artery, M1 segment of middle cerebral artery, A1 segment of anterior cerebral artery, and P1 segment of posterior cerebral artery. The detailed imaging protocol and parameters for ICASMAP study have been published.11 Briefly, the intracranial arterial MR vessel wall imaging at each participating center was performed on 3.0T Philips or Siemens MR scanners with 8-channel phase-array head coil or 16-channel neurovascular coil. A standardized imaging protocol includes T1 volumetric isotropic turbo spin echo acquisition (T1-VISTA) at Philips MR platform or T1 sampling perfection with application‐optimized contrast using different flip angle evolutions (T1-SPACE) sequence at Siemens MR platform. The T1-VISTA/SPACE imaging sequence was acquired using the following parameters: fast spin echo sequence, repetition time (TR) 800/900 ms, echo time (TE) 19/24 ms, field of view (FOV) 200 × 181 × 45/158 × 158 × 158 mm3, matrix 332 × 300 × 150/256 × 256 × 246, and scan time 7 min 1 sec/8 min 6 sec. The intracranial arterial MR vessel wall imaging of CAMERA study was performed on a 3T Philips MR scanner equipped with a 36-channel head coil. The imaging protocol was used to acquire the T1-VISTA sequence using the following parameters: turbo spin echo, transverse plane, TR/TE 800/21 ms, FOV 200 × 180 × 40 mm3, matrix 332 × 332 × 133, scan time 6 min 18 sec. An identical spatial resolution of 0.6 × 0.6 × 0.6 mm3 was used in both ICASMAP and CAMERA studies.
MR Image Analysis
The vessel wall images of intracranial arteries were interpreted by two experienced radiologists with consensus blinded to all clinical information. Both radiologists had >3 years’ experience in cardiovascular plaque imaging. The image quality was rated per artery on a 4-point scale (1, poor; 2, marginal; 3, good; and 4, excellent) according to the clearness and signal-to-noise-ratio of vessel wall boundaries and images with a quality rating <2 were excluded from this analysis.14 The MR images with image quality ≥2 were interpreted. The ICAD was defined as presence of atherosclerotic plaque characterized by eccentric arterial wall thickening in any intracranial vascular bed for each subject.15 The identification of ICAD was determined by experienced neuroradiologists (>5 years’ experience) according to the characteristics of imaging in ICASMAP study.16 Intracranial atherosclerotic plaque was characterized by eccentric wall thickening on MR vessel wall images. Presence or absence of atherosclerotic plaque at each vascular bed of intracranial arteries was determined. For each subject, if there was an atherosclerotic plaque in any vascular bed, this subject was identified to have atherosclerotic plaque.
Statistical Analysis
The study population was divided into three groups: symptomatic ICAD group: the subjects of this group were from ICASMAP study who had intracranial atherosclerotic plaques and symptoms of ischemic stroke or TIA; asymptomatic ICAD group: the subjects of this group were from CAMERA study who had intracranial atherosclerotic plaques but were asymptomatic; and controls group: the subjects of this group were from CEMERA study who were asymptomatic and did not have intracranial atherosclerotic plaques. Continuous variables were summarized as mean ± standard deviation and categorical variables were presented as percentage. Clinical characteristics were compared with One-way ANOVA and Tukey’s studentized range testing for continuous variables and Chi-square analysis or Fisher’s exact test for categorical variables among three groups, as appropriate. We compared the association between outcome variable (3 groups) and each risk factor including age, gender, overweight, smoking, alcohol use, hypertension, HDL, LDL, TC, TG, diabetes mellitus and history of CHD by using univariate logistic regression analysis. In the multivariate logistic regression models, we included all the covariates and used stepwise method to select the covariate. This statistical analysis may partially minimize the selective bias of study population from different cohort studies. Two-tailed p-values <0.05 were considered statistically significant. During above logistic regression, the level of significance was adjusted to p < 0.017 after correcting multiple comparisons (0.05/3 = 0.017). Statistical analysis was performed using the software of SPSS 16.0 (IBM, Chicago, IL) and SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
The derivation of the study groups is shown in Figure 1. Of 663 included subjects, 90 were excluded due to the following reasons: 1) other etiologies of ICAS (n = 28) including 4 moyamoya disease, 2 artery dissection and 22 others; 2) insufficient clinical information (n = 16) and poor image quality (n = 10); and 3) asymptomatic subjects with history of stroke (n = 20) and history of TIA in CAMERA study (n = 16). The remaining 573 subjects were included in final statistical analysis.
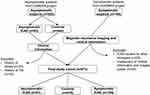 |
Figure 1 The derivation of the study groups. Abbreviations: ICAD, intracranial atherosclerosis disease; ICAS, intracranial artery stenosis; TIA, transient ischemic attacks. |
Comparison of Clinical Characteristics Among Three Groups
The clinical characteristics of the study subjects are shown in Table 1. Asymptomatic ICAD subjects were significantly older (70.1 ± 8.4 years vs 57.1 ± 11.1 years and 58.0 ± 13.3 years, p < 0.001) and had higher prevalence of history of CHD (17.6% vs 6.7% and 5.0%, p = 0.002) than symptomatic ICAD and controls. Symptomatic and asymptomatic ICAD subjects showed more current alcohol use (15.4% and 21.6% vs 8.9%, p = 0.007) and greater prevalence of hypertension (86.5% and 74.5% vs 40.2%, p < 0.001) than controls. Symptomatic ICAD subjects are less likely to female (35.6% vs 50.0% and 61.0%, p < 0.001) and had greater BMI (25.3 ± 3.4kg/m2 vs 24.6 ± 3.2kg/m2 and 24.3 ± 3.3kg/m2, p = 0.018), higher prevalence of overweight (69.2% vs 60.8% and 51.4%, p = 0.003), current smoking (26.9% vs 7.8% and 5.0%, p < 0.001), diabetes (44.2% vs 25.5% and 12.4%, p < 0.001) and less statin use (9.6% vs 37.3% and 20.1%, p < 0.001) than asymptomatic ICAD and controls. In contrast, the levels of HDL (1.11 ± 0.24 mmol/L vs.1.53 ± 0.44 mmol/L and 1.49 ± 0.37 mmol/L, p < 0.001) and TC (4.40 ± 1.49 mmol/L vs 4.78 ± 0.74 mmol/L and 4.85 ± 0.92 mmol/L, p < 0.001) of symptomatic ICAD subjects were significantly lower than asymptomatic ICAD and controls. There were no significant differences in LDL and TG among the 3 groups (all p > 0.05).
 |
Table 1 The Clinical Characteristics of the Study Subjects |
Asymptomatic ICAD Subjects versus Controls
Compared with controls, asymptomatic ICAD subjects were more likely to be older (OR: 1.08, 95% CI: 1.05–1.11, p < 0.001) and have hypertension (OR: 4.63, 95% CI: 2.27–9.44, p < 0.001) and history of CHD (OR: 3.34, 95% CI: 1.33–8.38, p = 0.010) (Table 2). After adjusted for all other risk factors (Model 1 with covariates of age and hypertension), the differences in age (OR: 1.07, 95% CI: 1.03–1.10, p < 0.001) and hypertension (OR: 3.03, 95% CI: 1.45–6.36, p = 0.003) between asymptomatic ICAD subjects and controls remained statistically significant (Table 3).
 |
Table 2 Comparison of Vascular Risk Factor Prevalence Between 3 Groups by Univariate Logistic Regression Analysis |
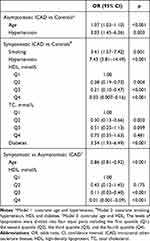 |
Table 3 Comparison of Vascular Risk Factor Prevalence Between 3 Groups by Multivariate Logistic Regression Analysis |
Symptomatic ICAD Subjects versus Controls
Compared with controls, symptomatic ICAD subjects were less likely to be female (OR: 0.35, 95% CI: 0.22–0.55, p < 0.001) and more likely to have overweight (OR: 2.11, 95% CI: 1.33–3.35, p = 0.001), current smoking (OR: 6.50, 95% CI: 3.51–12.06, p < 0.001), hypertension (OR: 9.93, 95% CI: 5.46–18.07, p < 0.001), diabetes (OR: 5.55, 95% CI: 3.40–9.05, p < 0.001), lower HDL (the second quantile (Q2)/the third quantile (Q3)/the fourth quantile (Q4) vs the first quantile (Q1), all p < 0.001), lower LDL (Q3 vs Q1, p = 0.010) and lower TC (Q2/Q3/Q4 vs Q1, all p < 0.001) (Table 2). After adjusted for all other risk factors (Model 2 with covariates of smoking, hypertension, HDL, TC and diabetes), the differences in smoking (OR: 3.41, 95% CI: 1.57–7.42, p = 0.001), hypertension (OR: 7.43, 95% CI: 3.81–14.49, p < 0.001), HDL (Q2/Q3/Q4 vs Q1, all p < 0.017), TC (Q2 vs Q1, p = 0.003) and diabetes (OR: 3.54, 95% CI: 1.93–6.49, p < 0.001) between asymptomatic ICAD subjects and controls remained statistically significant (Table 3).
Asymptomatic ICAD Subjects versus Symptomatic ICAD Subjects
Compared with asymptomatic ICAD subjects, symptomatic ICAD subjects were more likely to be younger (OR: 0.87, 95% CI: 0.83–0.92, p < 0.001) and have current smoking (OR: 5.03, 95% CI: 1.44–17.57, p = 0.011), lower HDL (Q3/Q4 vs Q1, p < 0.001) and lower TC (Q2 vs Q1, p = 0.004; Q3 vs Q1, p = 0.015) (Table 2). After adjusted for all other risk factors (Model 3 with covariates of age and HDL), the differences in age (OR: 0.86, 95% CI: 0.81–0.92, p < 0.001) and HDL (Q3/Q4 vs Q1, p < 0.001) between asymptomatic and symptomatic ICAD subjects remained statistically significant (Table 3).
Discussion
This study compared clinical characteristics that were known as conventional vascular risk factors among asymptomatic ICAD, symptomatic ICAD and controls. In our study, there were differences in the distribution of conventional vascular risk factors in three groups. Symptomatic ICAD subjects were significantly younger, more likely male, higher prevalence of overweight, current smoking, current alcohol use, hypertension, diabetes, less statin use, lower HDL, TC and history of CHD than asymptomatic ICAD and/or controls. Compared with controls, asymptomatic ICAD subjects were more likely older and to have hypertension and symptomatic ICAD subjects were more likely to have current smoking, hypertension and diabetes. We also found that symptomatic ICAD subjects were younger and more likely to have lower HDL than those with asymptomatic ICAD. Our findings suggest that smoking cessation and modification of the level of blood pressure and blood glucose may be helpful for reducing the risk of developing intracranial atherosclerosis and cardiovascular events.
In our study, we found that old age and hypertension were associated with an increased risk of asymptomatic ICAD. The finding of higher prevalence of asymptomatic ICAD in older individuals is in line with previous reports.17–19 The probable explanation may be the fact that vascular aging and cellular senescence are associated with increased expression of proinflammatory cytokines and adhesion molecules further promoting inflammation which accelerate the formation of atherosclerotic plaques.20 Although hypertension is usually considered to be one of the major risk factors for ICAD,7 its role in development of asymptomatic ICAD is controversial. Several transcranial Doppler studies documented that hypertension had a positive association with asymptomatic ICAD with odds ratios ranging from 1.43 to 2.23 (all p <0.05).17,21,22 An MR angiography study by Park et al reported an increased prevalence of hypertension (OR: 2.40, 95% CI: 1.01–5.69, p <0.05) in asymptomatic ICAS subjects compared with those without stenosis.23 However, another MR angiography study showed no association between hypertension and asymptomatic ICAD subjects (OR: 0.98, 95% CI: 0.89–1.08, p = 0.76).24 Despite the literature reports on the relationship between hypertension and ICAD are inconsistent, most of investigators believed that elevated blood pressure is a risk factor for the development of atherosclerosis due to damage in the endothelium and vascular wall by elevated blood pressure through both mechanical and humoral factors.25 Another explanation of the association of hypertension with ICAD is that the elevated blood pressure induces oxidative stress on the arterial wall companion with other atherogenic stimuli such as hyperlipidemia.26 The role of blood pressure in development of asymptomatic ICAD needs to be further investigated in future studies.
In the present study, univariate logistic regression analysis showed that subjects with symptomatic ICAD were less female and more overweight than controls. Our results are in line with previous studies. Multiple studies have shown that high BMI among men who were overweight or obese was strongly associated with increased stroke mortality.27,28 Bos et al found that more than 80% of elderly Caucasians have ICAD and the volume of atherosclerosis is larger in males than that in females.29 The potential mechanisms for the subjects with symptomatic ICAD being less female and more overweight are complex. Estrogen, such as estradiol, which prevents fatty streak deposition and progression of atherosclerotic plaque, plays an important role in vascular protection and anti-atherosclerosis.30,31 In addition, Obesity may activate and promote endothelial cell migration, smooth muscle cell proliferation and vascular calcification due to the increase secretion of leptin, thus promotes the formation of atherosclerosis.32,33 We also found that subjects with symptomatic ICAD were found to be more likely to have current smoking, hypertension, and diabetes. Our results are consistent with previous studies to some extent. A study of ICAD in Korean population showed that cigarette smoking is associated with multiple ICAS.34 A Chinese acute ischemic stroke population study indicated that high diastolic blood pressure is a risk factor for ICAS (OR: 1.075, 95% CI: 1.016–1.138, p = 0.013).35 In addition, a Warfarin Aspirin Symptomatic Intracranial Disease study showed that systolic blood pressure ≥140 mm Hg (HR: 1.79, p = 0.0009, 95% CI: 1.27–2.52) was associated with an increased risk of stroke, myocardial infarction, or vascular death.36 Furthermore, a transcranial Doppler study reported that hypertension was associated with a more than 2‐fold higher likelihood of symptomatic ICAD (OR: 2.41, 95% CI: 1.02‐5.67, P = 0.045), while diabetes mellitus was the strongest predictive variable of symptomatic ICAD (OR: 4.25, 95% CI: 2.18‐8.26, P < 0.001).37 Symptomatic ICAD is usually characterized by the following vulnerable plaque features: intraplaque hemorrhage, larger lipid-rich necrotic core, and fibrous cap rupture. Cigarette smoking promotes the development of vulnerable plaques and plaque rupture by enhancing inflammation and activating matrix metalloproteinases.38 Recent studies proved that blood pressure, particularly pulse blood pressure or diastolic blood pressure,39–41 is associated with occurrence of intraplaque hemorrhage in carotid artery, which is presumed to be caused by erythrocyte leakage from dysfunctional intraplaque micro-vessels.42 Moreno et al found a larger content of high-risk plaque features such as a lipid core in coronary specimens of diabetics than in nondiabetics.43 It has been hypothesized that the alterations in vascular homeostasis due to endothelial and smooth muscle cell dysfunction are the main features of diabetic vasculopathy favouring a pro-inflammatory/thrombotic state which ultimately leads to atherosclerosis.44 The symptomatic ICAD may not be the outcome of only one risk factor, but the outcome of combination of multiple factors such as hypertension, high level of LDL and diabetes. In addition, TC was inversely associated with symptomatic ICAD which may be due to the higher proportion of statin use in control subjects than in symptomatic ICAD subjects.
We found that symptomatic ICAD subjects were more likely to be younger and have lower HDL compared with asymptomatic ICAD subjects. An association between symptom and age was less evidenced previously. Similar findings about the effect of HDL on symptoms in patients with ICAD have been reported in previous studies. A study by Kim et al demonstrated that a high HDL level strongly predicted a favorable course for symptomatic ICAS (p = 0.005).45 It has been assumed that high level of HDL may prevent the development of the cerebrovascular symptoms. The explanation for the decreased frequency of symptoms in ICAD subjects is probably due to the reduction of plaque growth and inflammatory and favor positive vascular remodeling by high level of HDL.46–48
The major strengths of our study are as follows: (1) the use of MR vessel wall imaging guaranteed the accurate detection of atherosclerotic plaques in intracranial arteries which effectively avoided underestimation or misdiagnosis. (2) to investigate the conventional vascular risk factors of three groups may be provide targets for ICAD therapy and clues for prevention the occurrence of ICAD symptoms. Several limitations in this study need to be acknowledged. Firstly, the inclusion criteria, the sample size and age distribution of each subject group of the present study are heterogeneous. For example, the sample size of the asymptomatic ICAD subjects (n = 55) was small and the age of them (70.6 ± 8.4 years) were old. This study population may not represent the general population due to narrow range of the age. The power analysis showed that under the current sample size, the power to detect OR <0.50 was greater than 0.92. Secondly, MR scanners from multiple vendors were applied to this study which may introduce inter-platform bias due to the inconsistent imaging parameters. However, we believe this influence should be minor because we only determined presence or absence of atherosclerotic plaque and no quantitative measurements were performed. Finally, in our study, we only cross-sectionally evaluated the conventional vascular risk factors of symptomatic and asymptomatic intracranial atherosclerosis. Long-term follow-up studies are suggested to determine the effect of vascular risk factors on the time course of intracranial atherosclerosis in a single study population.
In conclusion, old age and hypertension are associated with asymptomatic ICAD and smoking, hypertension, diabetes and lower HDL are associated with an increased risk of symptomatic ICAD in Chinese population. Our findings suggest that management of the modifiable risk factors including smoking, hypertension, diabetes and HDL may reduce the risk of developing intracranial atherosclerotic plaques and cerebrovascular events.
Abbreviations
BMI, body mass index; CHD, coronary heart disease; CAMERA, Cardio- and Cerebro-vascular Accident Monitoring, Epidemiology and Care Quality System; FOV, field of view; HDL, high-density lipoprotein; ICAD, intracranial atherosclerosis disease; ICAS, intracranial artery stenosis; ICASMAP, Intracranial Artery Stenosis MR imaging: Aetiology and Progression; LDL, low-density lipoprotein; MR, magnetic resonance; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attacks; TC, total cholesterol; TG, triglycerides; T1-VISTA, T1 volumetric isotropic turbo spin echo acquisition; T1-SPACE, T1 sampling perfection with application‐optimized contrast using different flip angle evolutions; TR, repetition time; TE, echo time.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author [X.Z. and G.L.]. The data are not publicly available due to them containing information that could compromise research participant privacy/consent.
Ethics Approval and Consent to Participate
The study protocol was approved by institutional review board of Tsinghua University School of Medicine and each participating institution and the written consent forms were obtained from all the subjects prior to the initiation of this study.
Author Contributions
Y.H. carried out the study, analyzed and interpreted data, and drafted the manuscript. R.Z. performed statistical analysis. D.Y., D.L. and H.H. participated in acquisition of data. H.Q. and S.C. carried out the study. Y.W. participated in acquisition of data and interpreted the data. M.Y. and Y.H. participated in acquisition of data. Z.W. made critical revision of the manuscript. X.Z. and G.L. carried out the conception, study design, revised the manuscript, and made critical revision of the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is supported by grants of Beijing Municipal Science and Technology Project (D171100003017003, D131100002313002), Ministry of Science and Technology of China (2017YFC1307904, 2017YFC1307702), and National Natural Science Foundation of China (81771825).
Disclosure
The authors declare that they have no competing interests.
References
1. Mortality GBD; Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171.
2. Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42(12):3651–3654. doi:10.1161/STROKEAHA.111.635755
3. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480687 adults. Circulation. 2017;135(8):759–771. doi:10.1161/CIRCULATIONAHA.116.025250
4. Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet. 2014;383(9921):984–998. doi:10.1016/S0140-6736(13)61088-0
5. López-Cancio E, Galán A, Dorado L, et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-Asia (asymptomatic intracranial atherosclerosis) study. Stroke. 2012;43(10):2712–2719. doi:10.1161/STROKEAHA.112.661702
6. Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke. 2014;45(2):645–651. doi:10.1161/STROKEAHA.113.002491
7. Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res. 2017;120(3):502–513. doi:10.1161/CIRCRESAHA.116.308441
8. Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke. 2016;47(2):434–440. doi:10.1161/STROKEAHA.115.009955
9. Qiao Y, Guallar E, Suri FK, et al. MR imaging measures of intracranial atherosclerosis in a population-based study. Radiology. 2016;280(3):860–868. doi:10.1148/radiol.2016151124
10. Zhang N, Zhang F, Deng Z, et al. 3D whole-brain vessel wall cardiovascular magnetic resonance imaging: a study on the reliability in the quantification of intracranial vessel dimensions. J Cardiovasc Magn Reson. 2018;20(1):39. doi:10.1186/s12968-018-0453-z
11. Han Y, Qiao H, Chen S, et al. Intracranial artery stenosis magnetic resonance imaging aetiology and progression study: rationale and design. Brain Behav. 2018;8(12):e01154. doi:10.1002/brb3.1154
12. Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40(4):1195–1203. doi:10.1161/STROKEAHA.108.529883
13. Griswold MG, Fullman N, Hawley C; GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. doi:10.1016/S0140-6736(18)31310-2
14. Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104(17):2051–2056. doi:10.1161/hc4201.097839
15. Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010;212(2):507–511. doi:10.1016/j.atherosclerosis.2010.06.035
16. Choi YJ, Jung SC, Lee DH. Vessel wall imaging of the intracranial and cervical carotid arteries. J Stroke. 2015;17(3):238–255. doi:10.5853/jos.2015.17.3.238
17. Bae HJ, Lee J, Park JM, et al. Risk factors of intracranial cerebral atherosclerosis among asymptomatics. Cerebrovasc Dis. 2007;24(4):355–360. doi:10.1159/000106982
18. Huang HW, Guo MH, Lin RJ, et al. Prevalence and risk factors of middle cerebral artery stenosis in asymptomatic residents in Rongqi county, Guangdong. Cerebrovasc Dis. 2007;24(1):111–115. doi:10.1159/000103125
19. Uehara T, Tabuchi M, Mori E. Risk factors for occlusive lesions of intracranial arteries in stroke-free Japanese. Eur J Neurol. 2005;12(3):218–222. doi:10.1111/j.1468-1331.2004.00959.x
20. Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111(2):245–259. doi:10.1161/CIRCRESAHA.111.261388
21. López-Cancio E, Dorado L, Millán M, et al. The Barcelona-asymptomatic intracranial atherosclerosis (Asia) study: prevalence and risk factors. Atherosclerosis. 2012;221(1):221–225. doi:10.1016/j.atherosclerosis.2011.12.020
22. Zhang S, Zhou Y, Zhang Y, et al. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur J Neurol. 2013;20(11):1479–1485. doi:10.1111/ene.12210
23. Park KY, Chung CS, Lee KH, Kim GM, Kim YB, Oh K. Prevalence and risk factors of intracranial atherosclerosis in an asymptomatic Korean population. J Clin Neurol. 2006;2(1):29–33. doi:10.3988/jcn.2006.2.1.29
24. Kamal AK, Majeed F, Pasha O, et al. Clinical, lifestyle, socioeconomic determinants and rate of asymptomatic intracranial atherosclerosis in stroke free Pakistanis. BMC Neurol. 2014;14(1):155. doi:10.1186/s12883-014-0155-6
25. Sasamura H, Itoh H. Hypertension and arteriosclerosis. Nihon Rinsho. 2011;69(1):125–130.
26. Alexander RW; Theodore cooper memorial lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25(2):155–161. doi:10.1161/01.HYP.25.2.155
27. Zhou M, Offer A, Yang G, et al. Body mass index, blood pressure, and mortality from stroke: a nationally representative prospective study of 212,000 Chinese men. Stroke. 2008;39(3):753–759. doi:10.1161/STROKEAHA.107.495374
28. Kurth T, Gaziano JM, Berger K, et al. Body mass index and the risk of stroke in men. Arch Intern Med. 2002;162(22):2557–2562. doi:10.1001/archinte.162.22.2557
29. Bos D, van der Rijk MJ, Geeraedts TE, et al. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke. 2012;43(7):1878–1884. doi:10.1161/STROKEAHA.111.648667
30. Hsu SP, Lee WS. Effects of female sex hormones on the development of atherosclerosis. Chin J Physiol. 2020;63(6):256–262. doi:10.4103/CJP.CJP_69_20
31. Clarkson TB, Appt SE. Controversies about HRT–lessons from monkey models. Maturitas. 2005;51(1):64–74. doi:10.1016/j.maturitas.2005.02.016
32. Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88(9):954–960. doi:10.1161/hh0901.090975
33. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276(27):25096–25100. doi:10.1074/jbc.M007383200
34. Kim JS, Nah HW, Park SM, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43(12):3313–3318. doi:10.1161/STROKEAHA.112.658500
35. Li Y, Cai Y, Zhao M, Sun J. Risk factors between intracranial-extracranial atherosclerosis and anterior-posterior circulation stroke in ischaemic stroke. Neurol Res. 2017;39(1):30–35. doi:10.1080/01616412.2016.1250856
36. Chaturvedi S, Turan TN, Lynn MJ, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69(22):2063–2068. doi:10.1212/01.wnl.0000279338.18776.26
37. Tsivgoulis G, Vadikolias K, Heliopoulos I, et al. Prevalence of symptomatic intracranial atherosclerosis in caucasians: a prospective, multicenter, transcranial Doppler study. J Neuroimaging. 2014;24(1):11–17. doi:10.1111/j.1552-6569.2012.00707.x
38. Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10(4):219–230. doi:10.1038/nrcardio.2013.8
39. Sun J, Canton G, Balu N, et al. Blood pressure is a major modifiable risk factor implicated in pathogenesis of intraplaque hemorrhage: an in vivo magnetic resonance imaging study. Arterioscler Thromb Vasc Biol. 2016;36(4):743–749. doi:10.1161/ATVBAHA.115.307043
40. Selwaness M, van den Bouwhuijsen QJ, Verwoert GC, et al. Blood pressure parameters and carotid intraplaque hemorrhage as measured by magnetic resonance imaging: the Rotterdam study. Hypertension. 2013;61(1):76–81. doi:10.1161/HYPERTENSIONAHA.112.198267
41. Fassaert LMM, Timmerman N, van Koeverden ID, Pasterkamp G, de Kleijn DPV, de Borst GJ. Preoperative hypertension is associated with atherosclerotic intraplaque hemorrhage in patients undergoing carotid endarterectomy. Atherosclerosis. 2019;290:214–221. doi:10.1016/j.atherosclerosis.2019.09.008
42. van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC. Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam study. Eur Heart J. 2012;33(2):221–229. doi:10.1093/eurheartj/ehr227
43. Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102(18):2180–2184. doi:10.1161/01.CIR.102.18.2180
44. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34(31):2436–2443. doi:10.1093/eurheartj/eht149
45. Kim BJ, Hong KS, Cho YJ, et al. Predictors of symptomatic and asymptomatic intracranial atherosclerosis: what is different and why? J Atheroscler Thromb. 2014;21(6):605–617.
46. Johnsen SH, Mathiesen EB, Fosse E, et al. Elevated high-density lipoprotein cholesterol levels are protective against plaque progression: a follow-up study of 1952 persons with carotid atherosclerosis the tromsø study. Circulation. 2005;112(4):498–504. doi:10.1161/CIRCULATIONAHA.104.522706
47. Taylor AJ, Burke AP, Farb A, et al. Arterial remodeling in the left coronary system: the role of high-density lipoprotein cholesterol. J Am Coll Cardiol. 1999;34(3):760–767. doi:10.1016/S0735-1097(99)00275-2
48. Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P. Antiatherogenic functionality of high density lipoprotein: how much versus how good. J Atheroscler Thromb. 2008;15(2):52–62. doi:10.5551/jat.E571
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
