Back to Journals » Vascular Health and Risk Management » Volume 18
Risk Factors for and Treatment of Chronic Venous Disease in Thai Patients
Authors Taengsakul N
Received 19 July 2022
Accepted for publication 29 August 2022
Published 30 August 2022 Volume 2022:18 Pages 667—676
DOI https://doi.org/10.2147/VHRM.S382726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Nawaphan Taengsakul
Surgical Unit, Chulabhorn Hospital, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, Bangkok, Thailand
Correspondence: Nawaphan Taengsakul, Surgical Unit, Chulabhorn Hospital, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, 906 Kamphaeng Phet 6 Road, Talat Bang Khen, Lak Si, Bangkok, 10210, Thailand, Tel/Fax +66-2-576-6791, Email [email protected]
Introduction: The prevalence of chronic venous disease (CVD), a common health care problem, is still underestimated. A few previous epidemiologic studies have report Asian patients with this condition in western countries, but not in Asian countries. The aim of this study was to determine risk factors for CVD and its treatment in Thai individuals.
Methods: In this cross-sectional study, we collected data of patients with CVD visiting Chulabhorn Hospital Vascular Clinic from 1 December 2018– 1 October 2021. We reviewed medical records for patient characteristics, comorbidities, Clinical, Etiology, Anatomy, Pathophysiology (CEAP) categories, Venous Clinical Severity Score (VCSS), ultrasound findings and treatment.
Results: The study cohort comprised 260 CVD patients with CVD of mean age 61.92 ± 12.82 years. Almost 80% of participants were female. A history of deep vein thrombosis (DVT) was the strongest risk factor for severe CVD. Other identified risk factors comprised body–mass index (BMI) > 30 kg/m2, and older age. The most common CEAP categories were C2 (39%) and C1 (33.8%). Superficial venous reflux was the most common location of venous reflux in this study, 67.32% of participants having great saphenous vein reflux and 16.99% small saphenous vein reflux. Only 4.76% of our cohort had both reflux and obstruction. Most of the participants had undergone compression therapy, approximately half of them complying well with wearing of stockings. Nineteen percent of our cohort had undergone sclerotherapy and 14% surgery, which comprised radiofrequency ablation in 97% of them.
Conclusion: The major risk factors for severe CVD identified in this study were deep vein thrombosis, body mass index> 30 kg/m2 and older age. The most common CEAP category was C2 (39%). GSV was the most commonly involved venous system. Involvement of numerous venous systems was a risk factor for severe CVD.
Keywords: chronic venous disease, chronic venous insufficiency, CEAP classification, risk factor, Thailand
Introduction
The prevalence of chronic venous disease (CVD), a common health care problem, remains underestimated because early evidence of CVD is frequently overlooked by general practitioners.1 Vuylsteke et al estimated the global prevalence of CVD as relatively high, ranging from 2–56% in men and 1–73% in women.1 Its prevalence has not been established in Asia, previous epidemiologic studies having only reported data for Asian individuals in western countries.2–5 The pathophysiology of CVD includes both morphological and functional abnormalities in the lower extremities, venous return being impaired by reflux, obstruction, or muscular pump failure.6 Both superficial and deep veins are responsible for venous return in the lower limbs, these veins being connected by perforator veins. Effective venous drainage is achieved through unidirectional blood flow. Valvular dysfunction causes increasing venous hypertension, which manifests clinically as CVD.7,8 Risk factors known to be associated with development and progression of CVD include obesity, smoking, prolonged orthostasis, age, and family history of CVD.2 The clinical presentation of CVD is highly variable, ranging from mild telangiectasia, reticular veins, varicosity, leg edema, and hyperpigmented skin to ulceration. The severity of CVD can be categorized according to the CEAP classification. The aim of this study was to determine risk factors for advanced CVD and treatment of CVD in a Thai cohort in Thailand.
Methods
This was a cross-sectional of data of patients with CVD who had attended Chulabhorn Hospital Vascular Clinic, Bangkok, Thailand. from 1 December 2018 to 1 October 2021 being collected. The inclusion criteria were CVD diagnosed by a vascular surgeon and close follow up at Chulabhorn Hospital Vascular Clinic. The exclusion criteria included history of peripheral arterial bypass, combined arterial-venous disease, congenital venous malformations (such as Klippel-Trenaunay syndrome and Parkes-Weber syndrome) and leg edema from others causes, particularly heart disease, kidney disease and liver disease. Others exclusion criteria comprised incomplete medical records, loss to follow up and non-Thai ethnicity. We reviewed the medical records for patient characteristics, comorbidities, Clinical, Etiology, Anatomy, Pathophysiology (CEAP) categories, Venous Clinical Severity Score (VCSS), ultrasound findings and treatment. We assessed the CEAP category and VCSS in the higher scoring leg in patients with bilateral CVD. To determine the risk factors that influence advanced stage CVD, we divided the participants into two groups: mild to moderate CVD, defined as patients with clinical classification 1–3 (C1-C3); and severe CVD, defined as patients with clinical classification 4–6 (C4-C6) for determined the risk factors that influence advanced stage of CVD.
Ultrasound examinations were performed with the patients upright and supporting their weight on the contralateral leg. Venous reflux was elicited in two ways: using the Valsalva maneuver for the saphenofemoral junction and common femoral vein, followed by manual compression and release at distal to the point of examination for reflux from other veins. Reflux time of more than 500 milliseconds was defined as superficial venous reflux and perforator vein reflux, whereas a reflux time of more than 1000 milliseconds was defined as deep venous reflux. Iliocaval venous obstruction (ICVO) was evaluated by assessing loss of respiratory variation and reversed flow in the superior epigastric vein in all included patients. Individuals with suspected ICVO was evaluated by computed tomography venogram. This study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee for Human Research of Chulabhorn Research Institution approved the study (Number 146/2564). Moreover, full written informed consent for publication of this article was obtained from the patients. Confidentiality of data was secured using codes for each record.
The statistical analysis was essentially descriptive, findings being expressed as mean ± standard deviation for quantitative variables and counts and percentages for categorical variables All univariate analyses were performed by logistic regression. The χ2 test was used to assess associations with outcome variables. Where data were sparse, Fisher’s exact test was used. Exploratory bivariate analyses were also performed using the Wilcoxon signed-rank test for quantitative variables. Analysis was performed using STATA version 16.0 (Stata, College Station, TX, USA), P<0.050 for two-sided tests being considered to denote statistical significance.
Results
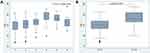
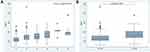
The study cohort comprised 260 CVD patients of mean age 61.92 ± 12.82 years. Relevant patient characteristics are presented in Table 1. Almost 80% of participants were female and most were postmenopausal. The mean BMI was 24.96 ± 5.44; Thus, our cohort were overweight, especially those with severe CVD (C4–C6), the mean BMI of this subgroup being 28.21 ± 5.80. Most of our cohort were non-smokers. The rate of smoking did not differ significantly between those with mild–moderate versus severe CVD. Two-thirds of our cohort had no relevant comorbidities. The commonest comorbidities were hypertension (HT; 21.54%), diabetes mellitus (DM; 6.9%), malignancy (3.85%) and thyroid disease (2.31%), as shown in Table 1. Only one patient had a history of CVD treatment in another hospital. Eight percent of participants had a family history of chronic venous disease and 1.5% a personal history of deep vein thrombosis. Both legs were involved in 85% of participants, only 14% having unilateral involvement. The initial overall VCSS was 3.37 ± 3.47, whereas it was 2.28 ± 1.86 in patients with mild to moderate CVD and 8.30 ± 4.66 in those with severe CVD, as shown in Table 1. We identified older age, male sex, high BMI, DM, HT and history of DVT as risk factors for severe CVD, as shown in Table 1. Univariate analysis (Table 2A) showed that a history of DVT was the strongest risk factor for severe CVD (odds ratio 14.45 [1.47, 142.22] P= 0.022). Other factors significantly associated with a higher risk of severe CVD were BMI>30, DM, HT, male sex, and BMI 25–29, respectively. Multivariate analysis (Table 2B) confirmed that a history of DVT was the strongest risk factor for severe CVD. As determined by univariate analysis, multivariate analysis confirmed that BMI>30 and age were significantly associated with severe CVD; however, DM, male sex, HT, and BMI 25–29 were not significantly associated with risk of severe CVD. No comorbidity was identified as protective by either univariate or multivariate analysis. However, DM, male sex, HT tended to be associated with an increased risk of severe CVD; none of these associations was statistically significant. In our cohort, 97% of patients (253 cases) had primary disease of CVD, only seven (3%) having secondary CVD. The commonest CEAP category was C2 39%, followed by C1 33.8%, C4 12%, C3 9.23%, C6 3.46% and C5 2.34% in that order. C2s was the commonest CEAP category in both sexes in our study cohort, as shown in Table 3. Age and BMI tended to increase in parallel with CEAP category. As shown in Table 3, the mean VCSS increased with increasing CEAP category. More than 80% of our cohort had mild to moderate CVD (C1–C3). Figures 1–4 shows the correlations between risk factors and CEAP category: Patients with severe CVD were significantly older age and had higher BMIs than patients with mild to moderated CVD. Furthermore, patients with severe CVD tended to be smokers and to have a family history of CVD. Smoking and family history of CVD were more prevalent in the C6 category. The commonest site of reflux was superficial veins (71.9% of patients: greater saphenous vein [GSV] in 67.32%, small saphenous vein [SSV] in 16.99%), followed by perforator veins (45.10%), and deep veins (12.42%) (femoral vein 12.42%, popliteal vein 1.96%), as shown in Table 4. Approximately half of our study cohort had involvement of only one venous system was involved in approximately half of our study cohort. We found a significant association between number of involved venous systems and higher CEAP categories. Only 4.76% of our participants had both reflux and obstruction. As shown in Table 5, more than 75% of our cohort had undergone compression therapy, approximately half of them complying well with wearing of compression stockings. Sclerotherapy was performed on 19% of our participants, comprising foam sclerotherapy in 29/50 (average 1.2 sessions, range 1–5 sessions) and liquid sclerotherapy in 23/50 (average 1.17 sessions, range 1–2 sessions). The most commonly prescribed medication was micronized purified flavonoid fraction (17.76%), followed by Aescin (12.02%). Surgery was performed on 14% of our cohort, this mostly comprising venous radiofrequency ablation (97%). Overall, VCSS scores improved by 3.62 ± 2.25, decreasing by 2.96 ± 1.60 in those with mild to moderate CVD group and by 5.57 ± 2.74 in those with severe CVD.
 |
Table 1 Patient Characteristics According to Severity of Chronic Venous Disease |
 |
Table 2 Risk Factors for Severe CVD in Our Study Cohort |
 |
Table 3 Risk Factors for CVD and VCSS According to CEAP Category |
 |
Table 4 Ultrasound Findings According to Severity of CVD |
 |
Table 5 Treatment According to Severity of CVD |
Discussion
The study cohort comprised 260 patients with diagnoses of CVD. The commonest age group of participants was >60 years and the participants were predominantly female. Older age was significantly associated with severe CVD (P<0.001). The San Diego study reported an odds ratio of up to 4.85 for older age; this association was statistically significant.2 In the Bonn Vein Study, the most important risk factor for CVD was older age, especially ages 70–79 in whom the risk was 15.9-fold that of younger patients.9 Over 75% of our cohort were female but that male sex tended to be associated with severe CVD. Most previous researchers have reported that CVD is more prevalent in female than in male patients, as was the case in our study. One previous study reported that female patients had significantly more severe CVD (higher CEAP category) than did male patients.1 The Edinburgh Vein Study reported the greater prevalence of severe CVD in male than in female patients.10 This is in agreement with the present findings. Although more than 30% of our study patients were of normal weight (BMI <25 kg/m2), the mean BMI met the criteria for overweight. Additionally, high BMI (BMI>30 kg/m2) was significantly associated with severe CVD (CEAP category C4–C6) (P =0.014). A previous study found an association between BMI >30 kg/m2 and increased risk of CVD, the odds ratios for men and women being 6.5 and 3.1, respectively.11 Furthermore, DM and HT tended to be associated with a higher risk of severe CVD. Patients with no comorbidities were at lower risk of severe CVD than were those with relevant comorbidities (P=0.026). A previous study found that patients with CVD had more comorbidities than the general population.12 Oral contraception was not a risk factor for CVD in the present study, contrary to the finding of a previous study.9 A history of DVT was a risk factor for severe CVD. Although we identified neither smoking nor a family history of CVD as risk factors for severe CVD in the present study, patients in the C6 category were more likely to be smokers and to have a family history of CVD, as shown in Figure 1. A previous study showed a positive correlation between smoking and severity of CVD only in men aged 35–65 years; however, these researchers did not provide data concerning the relationship between smoking and the severity of CVD in women.1 Although, we did not identify a significant relationship between family history of CVD and severe CVD in our study, several studies have found a correlation between these variables.1,9 A review of published reports revealed considerable evidence for a strong association between family history of CVD and the presence of varicose veins. According one study, more than 70% of patients with varicose veins have a family history of CVD.13 In a study of 4033 nuclear families, heritability of CVD was identified in 17.3%, suggesting a genetic component.14 A twins’ study found a higher degree of concordance between monozygotic twins (75%) than between dizygotic twins (52%).15 A case-control study has shown that offspring of parents who both have CVD have a 90% risk of developing varicose veins, whereas the risks 25% for male and 62% for female offspring when one parent is affected.16 The result of analysis nuclear families is compatible with autosomal dominant inheritance for 70%-92%, some pedigrees being compatible with autosomal recessive inheritance, whereas 37% of cases are sporadic.16 There is evidence that varicose veins disease are linked to mutation of FOXC2 gene (associated with lymphedema-distichiasis syndrome), Notch 3 gene (associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), angiodysplasia (Ehlers-Danlos syndrome, Klippel-Trenaunay syndrome) and the Norries disease gene (associated with ocular anomaly and sensory neural deafness).17 Furthermore, many inherited metabolic disorders are associated with CVD, particularly hyperhomocysteinemia, and genetic influences on wall remodelling.17 There are had limited data concerning a possible correlation between family history and chronic venous ulcer.17 In the West London Study, in which patients with leg ulcers were studied over one-year period, the prevalence of such ulcers was higher in white than in a South Asian individuals (odds ratio=4.43).18 However, history of DVT, age, arterial hypertension and prolonged standing are the main factors in the development of ulcers.17 However, whether there is a correlation between family history and venous ulcer is subject to ongoing debated.17 Apart from an established associated between occurrence of venous ulcers and familial abnormality in iron mechanism such as hemochromatosis, estrogen receptor beta gene and hereditary thrombophilia.17 In our patients, those in the C6 category were more likely to have a family history of CVD.
In this study, the superficial venous system was the commonest site of venous reflux in this study (GSV, 67.32%; SSV, 16.99%). Reflux was also common in perforator veins (45.10%) and deep veins (12.42%). SSV and perforator reflux were associated with increased risk of severe CVD. Additionally, the more numerous the venous systems involved, the greater the risk of severe CVD; this association was significant. In this study, only one venous system was involved in 48% of participants; however, almost half of the patients with severe CVD had involvement of two venous systems. A previous study showed a high prevalence (more than 80%) of superficial vein reflux in Thai patients.19 Other Asian studies have reported similar findings, including studies from Hong Kong (93%)20 and Japan (90%)21. Of note, the prevalence of deep vein reflux in the present study was relatively low (12.42%) in present study compared with previous studies from Thailand (63.3%).19 Hong Kong (73.3%)20 and Japan (51.7%).21
Compression therapy was the main form of treatment administered to the participants; however, only 40% of patients complied well with it. Sclerotherapy, mostly foam sclerotherapy, was used in 20% of our patients. Foam sclerotherapy was used for truncal veins in both the mild to moderate and severe CVD groups; however, patients with severe CVD underwent more sessions than did those with mild to moderate CVD. Liquid sclerotherapy was only used in the mild to moderate CVD group, most patients undergoing one session. Medications were mainly used in the severe CVD group, the most common being micronized purified flavonoid fraction (17.76%), Aescin (12.02%) and sulodexide (1.16%). More than half of patients who received medical treatment (54%) received combined drug therapy. Only 14% of patients underwent surgery, the main procedure being endovenous radiofrequency ablation. We considered surgical management in symptomatic patients with clinical classifications of C2 or more. Perforator reflux in patients with C5-C6 disease was treated by thermal ablation or ultrasound guided foam sclerotherapy. Planning of operative procedures depended on location of venous reflux, severity of CVD and patients’ preferences. Adjunct stab avulsion was performed on 40% of patients, most of whom had mild to moderate CVD. After these interventions, significant improvements in VCSS categories occurred only in the severe CVD group.
The present study had some limitations. First, the prevalence of ICVO may have been underestimated because this diagnosis was based only on ultrasound findings (loss of respiratory variation; PPV100%, NPV 81.3%)22 and reverse flow in the superior epigastric vein (PPV 100%, NPV 45.3%).22 These variables are not the gold standard for diagnosis and depend on the operator. Second, there may have been selection bias as a result of clinician preferences. Third, this was a cross-sectional study, meaning that data were extracted from medical records and patients were not randomized. Thus, unrecognized confounding factors could have introduced bias, and weakening our conclusions. Last, data on symptom improvement after treatment interventions were not available. Moreover, only one participating hospital had data on non-Thai individuals. Further large studies are needed to clarify the epidemiology of CVD in Thai individuals.
Conclusions
The major risk factors for severe CVD identified in this study were history of DVT, BMI>30 and older age. The most common CEAP category was C2 (39%). GSV was the most commonly involved venous system. Involvement of numerous venous systems was a risk factor for severe CVD.
Acknowledgments
The author thank all patients and staff of Chulabhorn Hospital for their assistance and support towards the success and completion of this study. The author also thank Dr Trish Reynolds, MBBS, FRACP, from Edanz for editing a draft of this manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Vuylsteke ME, Thomis S, Guillaume G, Modliszewski ML, Weides N, Staelens I. Epidemiological study on chronic venous disease in Belgium and Luxembourg: prevalence, risk factors, and symptomatology. Eur J Vasc Endovasc Surg. 2015;49(4):432–439. doi:10.1016/j.ejvs.2014.12.031
2. Criqui MH, Jamosmos M, Fronek A, et al. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003;158:448–456. doi:10.1093/aje/kwg166
3. Sam RC, Hobbs SD, Darvall KA, et al. Chronic venous disease in a cohort of healthy UK Asian men. Eur J Vasc Endovasc Surg. 2007;34:92–96. doi:10.1016/j.ejvs.2006.12.034
4. Subramanian A, Patel V, Jacobs J, Myles F, Derodra J. Superficial venous pathology in the Asian population of South West London-a prospective study. Eur J Vasc Endovasc Surg. 2007;33:747–750. doi:10.1016/j.ejvs.2006.12.007
5. Hobbs SD, Sam R, Rehman A, Marshall T, Wilmink AB, Bradbury AW. The utilisation of superficial venous surgery for chronic venous insufficiency by the UK Asian population. Eur J Vasc Endovasc Surg. 2003;26:322–324. doi:10.1053/ejvs.2002.2001
6. Jonathan DB, Peter AG. Vascular and Endovascular Surgery.
7. Sameer AM, Srinivas P. Varicose veins: a Clinical Study. Int Surg J. 2016;4(2):529–533.
8. Robert BR. Vascular Surgery.
9. Rabe E, Pannier-Fisher F, Bromen K. Bonner Venenstudie der Deutschen Gesellschaft für Phlebologie e epidemiologische Untersuchung zur Frage der Häufigkeit und Ausprägung von chronischen Venenkrankheiten in der städtischen und länd- lichen Wohnbevölkerung. Phlebologie. 2003;32:1–14. in German. doi:10.1055/s-0037-1617353
10. Evan CG, Fowkes FGR, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53:149–153. doi:10.1136/jech.53.3.149
11. Rabe E. Vein Bonn Study. Phlebologie. 2006;2006:179–186. in German.
12. Rabe E, Regnier C, Goron F, Salmat G, Pannier F. The prevalence, disease characteristics and treatment of chronic venous disease: an international web-based survey. J Comp Eff Res. 2020;9(17):1205–1218. doi:10.2217/cer-2020-0158
13. Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175–184. doi:10.1016/j.annepidem.2004.05.015
14. Fiebig A, Krusche P, Wolf A, et al. Heritability of chronic venous disease. Hum Genet. 2010;127:669–674. doi:10.1007/s00439-010-0812-9
15. Niermann H. Zwillingsdermatologie. Vol. 1. Berlin: Springer-Verlag; 1964:32–33.
16. Cornu-Thenard A, Boivin P, Baud JM, De Vincenzi I, Carpentier PH. Importance of the familial factor in varicose disease. Clinical study of 134 families. J Dermatol Surg Oncol. 1994;20:318–326. doi:10.1111/j.1524-4725.1994.tb01631.x
17. Bolsseau MR. Chronic venous disease and the genetic influence. Phlebolymphology. 2014;21(2):100–111.
18. Franks PJ, Morton N, Campbell A, Moffatt CJ. Leg ulceration and ethnicity: a study in west London. Public Health. 1997;111:327–329.
19. Kanchanabat B, Stapanavatr W. Venous ultrasonography findings and clinical correlation in 104 Thai patients with chronic venous insufficiency of the legs. Singapore Med J. 2018;59(3):155–158. doi:10.11622/smedj.2017043
20. Ting ACW, Cheng SWK, Ho P, Llh W, Cheung GCY. Clinical outcomes and changes in venous hemodynamics after subfascial endoscopic perforating vein surgery. Surg Endosc. 2003;17:1314–1318. doi:10.1007/s00464-002-8764-3
21. Yamaki T, Nozaki M, Fujiwara O, Yoshida E. Comparative evaluation of duplex-derived parameters in patients with chronic venous insufficiency: correlation with clinical manifestations. J Am Coll Surg. 2002;195:822–830. doi:10.1016/S1072-7515(02)01670-8
22. Sermsathanasawadi N, Pruekprasert K, Pitaksantayothin W, et al. Prevalence, risk factors, and evaluation of iliocaval obstruction in advanced chronic venous insufficiency. J Vasc Surg. 2019;7(3):1–7.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.