Back to Journals » Infection and Drug Resistance » Volume 14
Risk Factors for and Clinical Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Nosocomial Infections: A Retrospective Study in a Tertiary Hospital in Beijing, China
Authors Zhang H , Guo Z, Chai Y, Fang YP , Mu X, Xiao N , Guo J, Wang Z
Received 6 January 2021
Accepted for publication 18 March 2021
Published 13 April 2021 Volume 2021:14 Pages 1393—1401
DOI https://doi.org/10.2147/IDR.S298530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Huijuan Zhang,1,2 Zhe Guo,1,3 Yan Chai,1 Yi-Peng Fang,1 Xiangdong Mu,1,2 Nan Xiao,4 Jun Guo,1,2 Zhong Wang1,5
1School of Clinical Medicine, Tsinghua University, Beijing, 100084, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, 102218, People’s Republic of China; 3Department of Liver Intensive Care Unit, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, 102218, People’s Republic of China; 4Department of Laboratory Medicine, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, 102218, People’s Republic of China; 5Department of General Practice Medicine, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, 102218, People’s Republic of China
Correspondence: Jun Guo
Department of Pulmonary and Critical Care Medicine, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, No. 168 Litang Road, Changping District, Beijing, 102218, People’s Republic of China
Tel/Fax +86-10-56119539
Email [email protected]
Zhong Wang
Department of General Practice Medicine, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, No. 168 Litang Road, Changping District, Beijing, 102218, People’s Republic of China
Tel/Fax +86-10-56119084
Email [email protected]
Purpose: Carbapenem-resistant Klebsiella pneumoniae (CRKP) infections have been increasingly reported worldwide. We aimed to identify the risk factors for nosocomial CRKP infections and assess the clinical outcomes.
Patients and Methods: We conducted a case-control study with data collected from January 2016 to December 2018 in China. Controls were selected at a ratio of 1:1 from patients with nosocomial carbapenem-susceptible Klebsiella pneumonia (CSKP) infections. Risk factors for nosocomial CRKP infections and clinical outcomes were assessed with univariate and multivariate analyses.
Results: A total of one hundred forty-two patients with CRKP infections and one hundred forty-two patients with CSKP infections were enrolled in this study. Multivariate analysis showed that exposure to antibiotics within 3 months prior to admission (odds ratio OR, 2.585; 95% confidence interval [CI], 1.425– 4.691; P=0.002), exposure to carbapenems (OR, 2.532; 95% CI, 1.376– 4.660; P=0.003), exposure to fluoroquinolones (OR, 3.309; 95% CI, 1.326– 8.257; P=0.010), and the presence of a nasogastric tube (OR, 2.796; 95% CI, 1.369– 5.712; P=0.005) were independent risk factors for CRKP infections. The 30-day mortality rate in the CRKP group was 19.7%, while the in-hospital mortality rate was 28.9%. In the CRKP group, a higher creatinine level (OR, 1.009; 95% CI, 1.002– 1.016; P = 0.013), being in shock at the time of a positive culture (OR, 4.454; 95% CI, 1.374– 14.443; P = 0.013), and co-infection with other resistant bacteria (OR, 4.799; 95% CI, 1.229– 18.740; P = 0.024) were independent predictors of in-hospital mortality in patients with CRKP infections. Kaplan–Meier curves showed that the CRKP group had a shorter survival time than the CSKP group.
Conclusion: Nosocomial CRKP infection was associated with exposure to carbapenems and fluoroquinolones within 3 months prior to hospitalization and the presence of a nasogastric tube. Patients infected with CRKP had higher 30-day and in-hospital mortality rates. A higher creatinine level, shock and co-infection with other resistant bacteria were independent predictors of in-hospital mortality in patients with CRKP infections.
Keywords: carbapenem-resistant Klebsiella pneumoniae, risk factors, clinical outcomes, nosocomial infection
Introduction
Over the past two decades, the risk of antimicrobial resistance among gram-negative bacteria has grown to become a serious worldwide problem due to the potential for the rapid spread of resistance mechanisms and limited treatment options. In particular, the global increase in multidrug-resistant (MDR) strains, which are resistant to three or more classes of antimicrobials, or extensively drug-resistant (XDR) strains, which are resistant to all but one or two classes of antimicrobials, is a cause for major concern.1,2 Nosocomial infections caused by multidrug-resistant bacteria significantly increase in-hospital mortality rates and medical costs, and solutions are urgently needed.
Carbapenems, which are beta-lactam antibiotics with the broadest spectrum against gram-negative organisms, used to be considered the last option for multidrug-resistant gram-negative bacterial infections, including Klebsiella pneumoniae. However, carbapenem-resistant Klebsiella pneumoniae (CRKP) with the ability to produce extended-spectrum beta-lactamases (ESBLs) was first reported in northeastern Scotland in 1997.3 In the past ten years, the spread of CRKP has posed a threat to public health worldwide. In CRKP, plasmids containing the gene for carbapenemase often carry additional genes that confer resistance to other antibiotics, leading to limited treatment options. Moreover, these plasmids easily spread among bacteria, resulting in outbreaks of nosocomial CRKP infections.4,5 According to data from the China Antimicrobial Surveillance Network (CHINET, www.chinets.com), the rates of resistance to meropenem and imipenem in Klebsiella pneumoniae rapidly increased from 2.9% and 3.0% in 2005 to 28% and 26.4% in 2020, respectively. Infections with CRKP are widespread in developed and developing countries,6–9 with a poor prognosis, high mortality rate and high associated economic costs.10,11
Only a few drugs, such as tigecycline and polymyxins, may be effective against CRKP. Although new sensitive antimicrobial agents for the treatment of CRKP infections, such as ceftazidime-avibactam, have emerged in recent years, they are still ineffective against New Delhi metallo-beta-lactamase (NDM)-producing CRKP.12 Therefore, it is important to identify the risk factors for nosocomial CRKP infections to support the development of effective prevention and treatment measures. In recent years, a number of risk factors for CRKP infections have been identified in retrospective clinical studies. However, the conclusions of these clinical studies have not been entirely consistent. Furthermore, a few studies have focused on the clinical outcomes and predictors of mortality in patients with CRKP infections in China.10,13 More systematic studies about nosocomial CRKP infections are needed to facilitate the development of appropriate methods of control. Here, we conducted a case-control study to identify the risk factors, mortality and predictors of mortality.
Patients and Methods
Ethics Statement
This study was approved by the Ethics Committee of Beijing Tsinghua Changguang Hospital with approval number 20357-0-01 and adhered to the principles of the Declaration of Helsinki. This was a retrospective study, and the privacy of the involved subjects was not affected. Therefore, the need to obtain written informed consent from the participants was waived.
Study Design and Patient Population
This study was conducted in Beijing Tsinghua Changguang Hospital, a 1000-bed tertiary-care hospital with approximately 35,000 hospital admissions per year. CRKP in this study was defined as either meropenem- or imipenem-resistant according to the minimum inhibitory concentration (MIC) cut-off values established by the Clinical and Laboratory Standards Institute (CLSI).14
A case–control study was conducted to assess the risk factors for CRKP infections and the clinical outcomes. Patients diagnosed with CRKP infections for the first time between January 2016 and December 2018 were identified from the database, and those from whom strains were isolated within the first 48 h after admission were excluded. For each patient with a CRKP infection, we selected a matched control patient from the pool of patients with CSKP infections. The CSKP group was selected from the source population admitted to the same ward during the same time period (within 30 days).
Data Collection
We reviewed the medical records and collected the case information. A standard case report form (CRF) was used to collect the epidemiologic and clinical data, including the department, patient demographics (sex and age), intensive care unit (ICU) stay within 3 months prior to a positive culture, exposure to antibiotics within 3 months prior to admission, underlying diseases (pulmonary disease, cardiovascular disease, liver disease, neurologic disease, malignancy, diabetes mellitus, autoimmune disease, use of corticosteroids or immunosuppressors within 6 months), emergency surgery within 1 month prior to a positive culture, blood transfusion within 1 month prior to a positive culture, biochemical indicators (albumin, prealbumin, creatinine) within 1 week prior to a positive culture, Acute Physiology And Chronic Health Evaluation II (APACHE II) score (only for patients in the ICU), presence of drainage tube for ≥48 hours (thoracic drainage tube, abdominal drainage tube, ventricular drainage tube, central venous catheters, arterial catheters, tracheal cannula and mechanical ventilation, Foley catheter, nasogastric tube, intravenous nutrition) within 1 month prior to a positive culture, and types of antibiotic used (≥48 hours) within 1 month prior to a positive culture. We also collected information from the period after a positive culture, including the procalcitonin (PCT) level, presence of shock, co-infection with other resistant bacteria and appropriate definitive treatment. Most of the variables were recorded as binary results (yes/no), while the specific values were recorded for the biochemical indicators. Patients with a positive culture from blood or any other sterile source were defined as having an infection. Patients with positive cultures from respiratory, urine and surgical wounds were defined as having infections according to the Centers for Disease Control and Prevention (CDC) and National Healthcare Safety Network (NHSN) criteria.15 Co-infections with other drug-resistant bacteria were defined by the isolation of drug-resistant pathogens other than CRKP from the same infection site. Appropriate definitive treatment was defined as at least one active drug treatment given for more than 48 hours based on the antimicrobial susceptibility testing reports.
The clinical outcomes were defined as follows: 30-day mortality (within the 30 days after the first positive culture), in-hospital mortality (death during hospitalization after the first positive culture), and total hospital length of stay (duration from the first positive culture to hospital discharge).
Microbiologic Methods
The identification of CRKP and other pathogens was performed using routine microbiological methods. Susceptibility testing was performed in the clinical microbiology laboratory using the Vitek 2 automated system (bioMérieux, Marcy l’Etoile, France). Carbapenem (meropenem and/or imipenem) resistance was confirmed by the Kirby-Bauer (K-B) method, according to the manufacturer’s instructions (Thermo Fisher Scientific, United Kingdom).
Statistical Analysis
In this study, continuous variables were compared with Student’s t-test (for normally distributed variables) or the Mann–Whitney U-test (for non-normally distributed variables) and are presented as the means ± standard deviations (SDs) or medians (interquartile ranges, IQRs). The infection types and clinical outcomes in the CRKP group were compared to those in the CSKP group using χ2 tests. Logistic regression models were used to analyse the risk factors for and predictors of in-hospital mortality due to nosocomial CRKP infection. All variables with a P value <0.05 in univariate analysis were included in the multivariate analysis. Cox proportional hazards regression analysis was performed to estimate the survival rates in the CRKP and CSKP groups. All variables with a P value <0.05 in univariate analysis were considered probable predictors in the multivariable Cox proportional hazards regression analysis. The number of days from the first positive culture to death are displayed in Kaplan-Meier curves. A Log rank test was used to compare the results between the different groups. All tests were two-tailed, with the significance level set at 0.05. SPSS 19 (SPSS Inc., Chicago, IL, USA) was used for the data analysis.
Results
Department Distribution and Types of Infection
Among the one hundred forty-two patients with CRKP infections identified during the study period, the ICU treated the most patients, followed by the gastrointestinal surgery department. The department distribution is shown in Table 1. The most common type of infection was respiratory tract infection, followed by urinary tract infection and intra-abdominal infection. One hundred forty-two patients with CSKP infections were included. The infection types in the CRKP group and the CSKP group are listed in Table 2. There was no significant difference in infection type between the two groups.
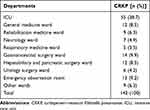 |
Table 1 Distribution of Nosocomial CRKP Infections Among Departments |
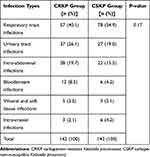 |
Table 2 Infection Types in the CRKP and CSKP Groups |
Risk Factors
The characteristics of the study population are shown in Table 3. There was no difference in demographic features between the two groups. In the CRKP group, there were greater proportions of patients with ICU stays within 3 months prior to a positive culture (OR, 1.812 95% CI, 1.119–2.935; P=0.016), exposure to antibiotics within 3 months prior to admission (OR, 2.217; 95% CI, 1.340–3.668; P=0.002), blood transfusions (OR, 2.145; 95% CI, 1.251–3.678; P=0.006), the presence of arterial catheter (OR,1.640; 95% CI, 1.019–2.637; P=0.041), the presence of a Foley catheter (OR, 2.1; 95% CI, 1.181–3.734; P=0.011), the presence of a nasogastric tube (OR, 3.397; 95% CI, 1.991–5.794; P=0.001), and the use of intravenous nutrition (OR, 2.229; 95% CI, 1.359–3.656; P=0.001). The CRKP group had a lower albumin level than the CSKP group (OR, 0.939; 95% CI, 0.894–0.986; P=0.01). In addition, the proportions of patients exposed to antibiotics, including carbapenems (OR, 3.437; 95% CI, 2.025–5.833; P=0.001), β-lactam/β-lactamase inhibitor combinations (OR, 2.119; 95% CI, 1.315–3.413; P=0.002), fluoroquinolones (OR, 2.709; 95% CI, 1.201–6.114; P=0.016), and glycopeptides (OR, 3.711; 95% CI, 1.918–7.184; P=0.001) were higher in the CRKP group than in the CSKP group.
 |
Table 3 Risk Factors for Nosocomial CRKP Infections |
Multivariate analysis showed that exposure to antibiotics within 3 months prior to admission (OR, 2.585; 95% CI, 1.425–4.691; P=0.002), the presence of a nasogastric tube (OR, 2.796; 95% CI, 1.369–5.712; P=0.005), exposure to carbapenems (OR, 2.532; 95% CI, 1.376–4.660; P=0.003), and exposure to fluoroquinolones (OR, 3.309; 95% CI, 1.326–8.257; P=0.010) were independent risk factors for CRKP infections.
Outcomes
In our study, 28 patients (19.7%) died within 30 days of a positive culture of CRKP, and 13 patients (9.2%) in the CSKP group died. The 30-day mortality rate was higher in the CRKP group than in the CSKP group (P=0.022). A significantly longer hospitalization duration was observed in the CRKP group than in the CSKP group (P=0.001). The clinical outcomes in the two groups are shown in Table 4. Variables associated with 30-day in-hospital mortality in the Cox proportional hazard model were CRKP infection (HR, 2.825; 95% CI, 1.157–6.896; P = 0.023), higher creatinine level (HR, 1.005; 95% CI, 1.002–1.008; P = 0.004), and shock (HR, 11.667; 95% CI, 4.145–32.835; P<0.001). Kaplan-Meier survival analysis was performed, and the results are shown in Figure 1.
 |
Table 4 Clinical Outcomes in the Two Groups |
 |
Figure 1 Kaplan–Meier curves showing 30-day mortality in the CRKP group versus the CSKP group (P = 0.044). |
Forty-one patients (28.9%) in the CRKP group died during hospitalization, while 20 patients (14.1%) in the CSKP group died. Univariable predictors of in-hospital mortality in the CRKP group are shown in Table 5. Age (P = 0.027), a history of an emergency operation within 1 month (P = 0.038), lower prealbumin levels (P = 0.024), higher creatinine levels (P = 0.007), the presence of arterial catheters (P = 0.02), the presence of a tracheal cannula (P = 0.009), shock at the time of a positive culture (P = 0.001), and co-infection with other resistant bacteria (P = 0.003) were predictors of in-hospital mortality in the CRKP group. Multivariable analysis showed that higher creatinine level (OR, 1.009; 95% CI, 1.002–1.016; P = 0.013), shock at the time of a positive culture (OR, 4.454; 95% CI, 1.374–14.443; P = 0.013), and co-infection with other resistant bacteria (OR, 4.799; 95% CI, 1.229–18.740; P = 0.024) were independent predictors of in-hospital mortality in the CRKP group.
 |
Table 5 Univariable and Multivariable Analyses of In-Hospital Mortality in the Nosocomial CRKP Infection Group |
Discussion
In this study, we found that nosocomial CRKP infections were concentrated in the ICU, gastrointestinal surgery ward, hepatobiliary and pancreatic surgery ward, general medicine ward and emergency observation room in our hospital. We know that the proportion of drug-resistant bacteria, including CRKP, is significantly higher in ICU patients than in patients in other departments. Patients in the ICU are in critical condition and require treatment with a variety of broad-spectrum antibiotics and life-support systems. Patients in hepatobiliary, pancreatic, and gastrointestinal surgery wards often have complicated conditions, need to have various drainage tubes for a long time and use broad-spectrum antibiotics (especially carbapenems) due to repeated infections. In China, the emergency observation room is actually a transitional ward, and patients with various infectious diseases are often treated there. The general medicine ward treats many emergency patients with various complicated infections. Therefore, the situation in the general medicine ward with regard to CRKP infections is similar to that in the emergency observation room.
Although most recent studies have suggested that CRKP infections are related to carbapenem exposure, the conclusions have not been consistent in the literature.16–18 Our study showed that the prior use of carbapenems was an independent risk factor for a CRKP infection, which is consistent with most previous clinical studies.7,10,11,19–21 The acquisition of the ability to produce carbapenemase is the main cause of carbapenem resistance, which can easily result in nosocomial spread.9 Han et al demonstrated that in China, the most prevalent carbapenemase gene in CRKP was KPC-2, followed by genes for other carbapenemases, including NDM, and OXA-48.4 Carbapenem exposure may induce the emergence of these resistance-conferring genes. We did not find an association of 3rd- or 4th-generation cephalosporins or β-lactam/β-lactamase inhibitors with CRKP infections, unlike in other studies identifying risk factors.10,17,20 Exposure to another very commonly used type of antibiotic, fluoroquinolones, was an independent risk factor for CRKP infections in our study, and this association has been reported in a few previous studies.13,22,23 However, other studies did not find an association between fluoroquinolone exposure and CRKP infection.24 A previous study suggested that in the presence of fluoroquinolones, the frequency of mutations conferring carbapenem resistance in Pseudomonas aeruginosa strains was enhanced. Loss of OprD activity or increased mexAB-oprM expression may be the main mechanisms;25 these changes can also occur in Klebsiella pneumoniae. The mechanism underlying fluoroquinolone-induced carbapenem resistance needs further study. The use of antibiotics within 3 months prior to admission means that the patient may have had repeated infections and antibiotic exposure, thereby increasing the risk of the development of antibiotic resistance. This conclusion is also consistent with the findings in other studies.26,27
Similar to a previous study, we found that indwelling gastric tubes are an independent risk factor for CRKP infection.28 Meta-analyses have shown that the use of medical devices (including gastric tubes) had the highest pooled estimate.19,29 Many studies have shown that CRKP can colonize the body, especially the gastrointestinal tract. Patients with CRKP colonization in the gastrointestinal tract are more likely to develop CRKP infections.20,30 The majority of patients in the clinic with gastric tubes have gastrointestinal dysfunction and poor nutritional status and have a higher risk of intestinal translocation of bacteria, including CRKP.
Mortality rates in patients with CRKP infections have ranged from 24% to 65% in various studies.11,16,22,24,31,32 In the studies by Schwaber et al and Wang et al, CRKP infection was found to be an independent risk factor for mortality.10,22 Our study had similar findings: patients with CRKP infections had a relatively higher mortality rate and a longer hospital stay.
Patients with CRKP infections who develop shock status are considered critically ill. The mortality rate was significantly higher in the patients who developed shock than in those who did not. High creatinine levels were an independent predictor of mortality in patients with nosocomial CRKP infections. This finding was also reported in other studies.16,17 It has been shown that factors such as underlying illnesses and comorbidities significantly affect mortality in patients infected with multidrug-resistant gram-negative bacteria.33 In our study, the mean creatinine level in the CRKP group was higher than that in the CSKP group. Treatment for infections with CRKP often involves the use of many different antibiotics. However, a higher creatinine level limits the use of antibiotics, and the antibiotic doses need to be reduced due to impaired renal function, resulting in inadequate treatment. At the same time, patients with poor renal function have reduced bacterial toxin clearance, leading to a more critical condition in these patients. Therefore, patients with renal insufficiency have a relatively higher mortality rate. Concomitant infections with other drug-resistant bacteria constitute another important predictor of mortality. In the past, few studies focused on patients co-infected with CRKP and other drug-resistant bacteria. Mixed infections with two or more drug-resistant bacteria are much more difficult to control. We did not find an association between the APACHE II score and mortality because of the limited sample size.
The purpose of studies on nosocomial CRKP infections is to provide a basis for the development of effective control measures. In our hospital, CRKP infections are concentrated in the specific departments listed above. In these departments, CRKP transmission can be avoided by implementing measures such as appropriate hand hygiene protocols and environmental disinfection measures. Meanwhile, pre-emptive contact isolation should be implemented for patients who have used carbapenems and fluoroquinolones for a long time, were exposed to antibiotics within 3 months prior to admission and have indwelling nasogastric tubes. It is important to develop hospital-based policies to minimize CRKP infections. Our data also provide an impetus to redouble our efforts to ensure the rational usage of antibiotics and nasogastric tubes.
In recent years, an increasing number of studies have focused on active screening for CRKP. Through active screening in higher-risk departments or higher-risk patients, we can achieve early detection, isolation and intervention. The US CDC also suggested that the decision to implement active screening for CRKP should be based on local epidemiological conditions. In our hospital, nosocomial CRKP infections tend to occur in specific departments and patients with risk factors. This suggests that those departments should actively screen for CRKP. In addition, given the growing problem of antibiotic resistance and the identification of nosocomial infections with carbapenemase-resistant strains of Klebsiella pneumoniae, studies have increasingly focused on the possibility of using natural substances to combat multidrug-resistant Klebsiella pneumoniae.34–37
In summary, this retrospective study was performed to evaluate the risk factors for and clinical outcomes of nosocomial CRKP infections in China. Exposure to carbapenems and fluoroquinolones within 3 months and the presence of a nasogastric tube were independent risk factors for CRKP infections. Patients infected with CRKP had higher 30-day and in-hospital mortality rates. Higher creatinine levels, shock state and co-infection with other resistant bacteria were independent predictors of in-hospital mortality in patients with CRKP infections. However, we should note several limitations of our study. First, it was a single-centre study with a relatively small and non-stratified sample. Prospective, multicentre and large-sample clinical trials are needed. Second, a molecular epidemiological investigation of CRKP was not carried out, although different drug resistance mechanisms may influence the clinical outcomes. Therefore, we will perform molecular epidemiological investigations in future studies.
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med. 2013;80(4):225–233. doi:10.3949/ccjm.80a.12182
2. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
3. MacKenzie FM, Forbes KJ, Dorai-John T, Amyes SGB, Gould IM. Emergence of a carbapenem-resistant Klebsiella pneumoniae. Lancet. 1997;350(9080):783. doi:10.1016/s0140-6736(05)62567-6
4. Han R, Shi Q, Wu S, et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi:10.3389/fcimb.2020.00314
5. Baran I, Aksu N. Phenotypic and genotypic characteristics of carbapenem-resistant Enterobacteriaceae in a tertiary-level reference hospital in Turkey. Ann Clin Microbiol Antimicrob. 2016;15(1):20. doi:10.1186/s12941-016-0136-2
6. Jamal AJ, Garcia-Jeldes F, Baqi M, et al. Infection prevention and control practices related to carbapenemase-producing Enterobacteriaceae (CPE) in acute-care hospitals in Ontario, Canada. Infect Control Hosp Epidemiol. 2019;40(9):1006–1012. doi:10.1017/ice.2019.173
7. Tran DM, Larsson M, Olson L, et al. High prevalence of colonisation with carbapenem-resistant Enterobacteriaceae among patients admitted to Vietnamese hospitals: risk factors and burden of disease. J Infect. 2019;79(2):115–122. doi:10.1016/j.jinf.2019.05.013
8. Perovic O, Ismail H, Quan V, et al. Carbapenem-resistant Enterobacteriaceae in patients with bacteraemia at tertiary hospitals in South Africa, 2015 to 2018. Eur J Clin Microbiol Infect Dis. 2020;39(7):1287–1294. doi:10.1007/s10096-020-03845-4
9. David S, Reuter S, Harris SR, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4(11):1919–1929. doi:10.1038/s41564-019-0492-8
10. Wang Q, Zhang Y, Yao X, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microb Infect Dis. 2016;35(10):1679–1689. doi:10.1007/s10096-016-2710-0
11. Liu J, Wang H, Huang Z, et al. Risk factors and outcomes for carbapenem-resistant Klebsiella pneumoniae bacteremia in onco-hematological patients. J Infect Dev Ctries. 2019;13(5):357–364. doi:10.3855/jidc.11189
12. Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2). doi:10.1128/cmr.00079-17
13. Wang Z, Qin RR, Huang L, Sun LY. Risk factors for carbapenem-resistant klebsiella pneumoniae infection and mortality of klebsiella pneumoniae infection. Chin Med J. 2018;131(001):56. doi:10.4103/0366-6999.221267
14. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
15. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi:10.1016/j.ajic.2008.03.002
16. Kofteridis DP, Valachis A, Dimopoulou D, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: a case-case-control study. J Infect Chemother. 2014;20(5):293–297. doi:10.1016/j.jiac.2013.11.007
17. Jiao Y, Qin Y, Liu J, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health. 2015;109(2):68–74. doi:10.1179/2047773215Y.0000000004
18. Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E. Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. J Infect. 2015;70(6):592–599. doi:10.1016/j.jinf.2014.11.003
19. In’t Holt Voor AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(1). doi:10.1128/aac.01730-17
20. Qin X, Wu S, Hao M, et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis. 2020;221(Supplement_2):S206–S214. doi:10.1093/infdis/jiz622
21. Xiao T, Zhu Y, Zhang S, et al. A retrospective analysis of risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteremia in nontransplant patients. J Infect Dis. 2020;221(Supplement_2):S174–S183. doi:10.1093/infdis/jiz559
22. Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52(3):1028–1033. doi:10.1128/AAC.01020-07
23. Ahn JY, Song JE, Kim MH, et al. Risk factors for the acquisition of carbapenem-resistant Escherichia coli at a tertiary care center in South Korea: a matched case-control study. Am J Infect Control. 2014;42(6):621–625. doi:10.1016/j.ajic.2014.02.024
24. Gómez Rueda V, Zuleta Tobón JJ. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae: a case-case-control study. Colomb Med. 2014;45(2):54–60. doi:10.25100/cm.v45i2.1417
25. Tanimoto K, Tomita H, Fujimoto S, Okuzumi K, Ike Y. Fluoroquinolone enhances the mutation frequency for meropenem-selected carbapenem resistance in Pseudomonas aeruginosa, but use of the high-potency drug doripenem inhibits mutant formation. Antimicrob Agents Chemother. 2008;52(10):3795–3800. doi:10.1128/AAC.00464-08
26. Kaye KS, Pogue JM. Infections caused by resistant gram-negative bacteria: epidemiology and management. Pharmacotherapy. 2015;35(10):949–962. doi:10.1002/phar.1636
27. Salomao MC, Guimaraes T, Duailibi DF, et al. Carbapenem-resistant Enterobacteriaceae in patients admitted to the emergency department: prevalence, risk factors, and acquisition rate. J Hosp Infect. 2017;97(3):241–246. doi:10.1016/j.jhin.2017.08.012
28. Akgul F, Bozkurt I, Sunbul M, Esen S, Leblebicioglu H. Risk factors and mortality in the carbapenem-resistant Klebsiella pneumoniae infection: case control study. Pathog Glob Health. 2016;110(7–8):321–325. doi:10.1080/20477724.2016.1254976
29. Li JH, Li YY, Song N, Chen Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. J Glob Antimicrob Resist. 2020;21:306–313. doi:10.1016/j.jgar.2019.09.006
30. Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control. 2016;44(5):539–543. doi:10.1016/j.ajic.2015.12.005
31. Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–1803. doi:10.1111/j.1469-0691.2011.03514.x
32. Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
33. Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66(5):401–414. doi:10.1016/j.jinf.2012.10.028
34. Trong Le N, Viet HD, Quoc Doan T, et al. In vitro antimicrobial activity of essential oil extracted from leaves of Leoheo domatiophorus Chaowasku, D.T. Ngo and H.T. Le in Vietnam. Plants. 2020;9(4):453. doi:10.3390/plants9040453
35. Le NT, Donadu MG, Ho DV, et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J Infect Dev Ctries. 2020;14(9):1054–1064. doi:10.3855/jidc.12469
36. Donadu MG, Trong Le N, Viet Ho D, et al. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of Hornstedtia bella Skornick. Antibiotics. 2020;9(6):334. doi:10.3390/antibiotics9060334
37. Mohamed SH, Mohamed MSM, Khalil MS, Azmy M, Mabrouk MI. Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumoniae. J Appl Microbiol. 2018;125(1):84–95. doi:10.1111/jam.13755
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
