Back to Journals » Journal of Inflammation Research » Volume 15
Risk Factors and the Role of the Albumin-to-Globulin Ratio in Predicting Recurrence Among Patients with Idiopathic Granulomatous Mastitis
Authors Ciftci AB , Bük F, Yemez K, Polat S, Yazıcıoğlu M
Received 9 June 2022
Accepted for publication 9 September 2022
Published 18 September 2022 Volume 2022:15 Pages 5401—5412
DOI https://doi.org/10.2147/JIR.S377804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ahmet Burak Ciftci,1 Ömer Faruk Bük,1 Kürşat Yemez,1 Süleyman Polat,1 İrem Melike Yazıcıoğlu2
1Department of General Surgery, Samsun University, Samsun Training and Research Hospital, Samsun, Turkey; 2Department of Pathology, Samsun University, Samsun Training and Research Hospital, Samsun, Turkey
Correspondence: Ahmet Burak Ciftci, Department of General Surgery, Samsun University, Samsun Training and Research Hospital, Barış Bulvarı, Kadıköy Mahallesi, No: 199 PK:55090 ilkadım, Samsun, Turkey, Tel +90 530 527 7302, Fax +90 362 277 8865, Email [email protected]
Background: Idiopathic granulomatous mastitis (IGM) is a rare inflammatory disease of the breast with a high recurrence rate. The serum albumin to globulin ratio (AGR) is a relatively novel biomarker in inflammatory diseases, and one whose role in the recurrence of IGM remains unknown. This study primarily investigated the potential risk factors for IGM recurrence and whether AGR can be used as a predictive factor.
Methods: Patients diagnosed with IGM from pathology reports between 2016 and 2021 were enrolled in the study, and their medical records were analyzed retrospectively. The patients were divided into two groups – recurrence and non-recurrence. Clinical, demographic characteristics, and laboratory parameters were compared.
Results: Eighty-five patients were included in the study, recurrence being detected in 16 (18.8%) of these, with a median follow-up time of 39.99± 18.93 months. No relationship was determined between childbearing, breastfeeding, disease severity, or therapeutic approaches and IGM recurrence. While AGR was significantly lower in the recurrence group (p < 0.001), neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) results were comparable in the two groups (p = 0.472 and p = 0.421, respectively). Multivariate analysis identified low AGR (odds ratio (OR): 50.7, 95% CI 5.93– 434.1 P < 0.001) and smoking (OR: 4.45, 95% CI 1.04– 18.9 P = 0.044) as independent risk factors for IGM recurrence.
Conclusion: The study findings indicated that AGR at a cut-off value of ≤ 1.179 at diagnosis and smoking exhibited a remarkable performance in predicting the recurrence of IGM. Developing new risk stratification systems for IGM recurrences and using AGR in these classifications may increase the success of treatment.
Trial Registration: This study was registered with ClinicalTrials.gov, NCT05409586.
Keywords: idiopathic granulomatous mastitis, recurrence, albumin-to-globulin ratio, risk factors
Introduction
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory disease of the breast that usually affects women of reproductive age.1 Although the etiopathogenesis is uncertain, the implicated factors include autoimmunity, some infectious agents (Corynebacterium species), local irritation, and oral contraceptive use.2–4 Clinically, IGM may present with findings such as pain, palpable mass, tenderness, redness, abscess, and fistulae.5,6 Definitive diagnosis is made pathologically, and other diseases that cause granulomatous reactions (tuberculosis, sarcoidosis, and granulomatous polyangiitis) must be excluded.2,7 Different therapeutic approaches are available, from simple antibiotics and follow-ups to large mastectomies. No universally accepted standard treatment protocol for IGM has yet been established.2,3,6,8 Reported recurrence rates in the literature are high, at up to 50%, despite the various therapeutic methods available.7,9
Recurrence after treatment and resistance to treatment are the most important difficulties in the management of this disease. Smoking, pregnancy, breastfeeding history, and breast infections are clinical parameters linked to the development of recurrence.2 However, studies evaluating the place of biomarkers in predicting IGM recurrence are growing in importance. One study evaluating various hematological indices reported significantly higher neutrophil-to-lymphocyte ratio (NLR) and fibrinogen-to-albumin ratio (FAR) values in recurrent cases.10 Another study reported that while there was a significant relationship between NLR levels and IGM recurrence, platelet-to-lymphocyte ratio (PLR) values did not affect the recurrence rate.11 However, there is still a need for new, more reliable, and robust markers with high accuracy capable of predicting the recurrence of IGM.
Albumin and globulin are the two most abundant proteins of human plasma and are indicators of nutritional status and inflammation. The albumin-to-globulin ratio (AGR) represents the calculated ratio between these two basic proteins and is thought to be a more specific indicator because it is not affected by body fluid balance.12,13 AGR is therefore increasingly used in determining the prognosis and severity of many cancers and inflammatory diseases. Previous studies have identified low AGR values as a poor prognostic factor in solid cancers such as those of the esophagus, stomach, and colon, and nasopharyngeal cancers.14–17 In addition, AGR is an inflammation-based index that has been shown to be capable of predicting the severity of inflammatory bowel diseases,18 predicting the risk of developing lupus nephritis in patients with systemic lupus erythematosus.19 However, the association between AGR and IGM recurrence has never been studied so far. The purpose of this study was to evaluate the association between the AGR and recurrence of IGM and to determine potential risk factors for the disease.
Materials and Methods
Study Participants and Ethics
This retrospective study was conducted at the Samsun Education and Research Hospital, a university-affiliated health center for medical research and practice, in Turkey. The design of the study was approved by the clinical trials ethics committee of Samsun Ondokuz Mayis University (OMUKAEK no: 2022/176, date: 19.04.2022). The medical records of patients with a pathologically confirmed diagnosis of granulomatous mastitis between January 2016 and April 2021 were reviewed. All patients were over 18 years of age, with no diagnosis of malignant disease. Individuals who were later diagnosed with tuberculous mastitis and Wegener’s granulomatosis were excluded from the study. Men, patients with insufficient follow-up information, and those with missing data were also excluded. The study flow diagram can be seen in Figure 1.
 |
Figure 1 The study flow diagram for selection of patients. |
Study Data
Pathological sampling methods for diagnosis included tru-cut biopsy, excisional-incisional biopsy, segmental mastectomy, and fine-needle aspiration cytology (FNAC). The patients’ clinical, demographic, and radiological characteristics, including age, body mass index (BMI), marital status, parity, breastfeeding, the time of the latest delivery, smoking status, lesion side and localization, disease severity, and treatment modalities were recorded. Laboratory findings such as serum albumin, total protein, sedimentation (ESR), C-reactive protein (CRP), and hematological parameters (neutrophil, lymphocyte, platelet, hemoglobin, and leukocyte levels) were also recorded. AGR, NLR, and PLR were calculated by dividing the relevant parameters by one another. Globulin was calculated as total protein-albumin. Disease severity was classified into three categories – mild, moderate, and severe, as suggested by Kaviani et al.20
Definition of Remission and Recurrence
Remission of IGM was defined as improvement in the principal complaints (such as breast pain, mass, or nipple discharge) and complete disappearance of inflammatory signs (skin ulcers and fistulas), although there is no definitively agreed definition of remission in the literature. Recurrence was defined as the reappearance of complaints such as pain, mass, abscess, and fistula after at least three months of remission with treatment. IGM developing in the contralateral breast after treatment was also regarded as a recurrent case. In addition to analyzing the hospital medical records of the patients, IGM recurrence was confirmed by making inquiries by telephone.
Statistical Analysis
All analyses were performed via the SPSS (Version 22, SPSS Inc., Chicago, USA) package program. The Student’s t-test was used for normally distributed data and the Mann–Whitney U-test for non-normally distributed data. Depending on the sample sizes, the Chi-square test or Fisher’s exact test were performed for categorical variables. ROC (Receiver Operating Characteristic) analysis was employed to determine whether serum proteins (albumin, total protein, globulin, and AGR) are prognostic indicators for IGM recurrence prediction. The Youden index was used to determine the best cut-off point in the ROC analysis. Univariate and Multivariate Binary Logistic Regression analysis was used to determine the risk factors that are effective in IGM recurrence. The statistical significance level was accepted as P < 0.05.
Results
One hundred nine patients were assessed for enrollment, of whom 85 were finally included in the study. Patients diagnosed with Wegener’s granulomatous mastitis (n = 2) and tuberculous mastitis (n = 1), men (n = 2), patients without regular follow-up controls (n = 11), and those with missing data (n = 8) were excluded from the study. The mean age (±standard deviation) of the patients included in the study was 36.47±9.63 years (range 21–73 years), and the mean follow-up (±standard deviation) time was 39.99±18.93 months (range 6–75 months). Recurrence was observed in 18.8% (n = 16) of the patients. Additionally, 89.4% (n = 76) of the patients were married, and all these had a history of at least one childbirth. The great majority of patients (81.2%, n = 69) had a history of breastfeeding. The median time (±interquartile range) between delivery and diagnosis was 6±6 years. In addition, 29.4% (n = 25) of the patients were active smokers, and the mean body mass index (BMI) of the study group was 28.1±4.72. The patients’ demographic and clinical characteristics and blood laboratory values are shown in Table 1. The table also shows a comparison of patients with and without IGM recurrence.
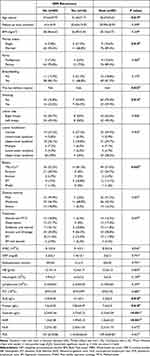 |
Table 1 Baseline Characteristics and Comparison of Variables Among IGM Recurrence Groups |
A comparison of the groups with and without recurrence revealed no statistically significant difference in terms of BMI, parity, breastfeeding, lesion side, lesion localization, disease severity, follow-up time, or treatment methods (p > 0.05, Table 1). However, mean age was significantly lower, while rates for smoking and being unmarried were significantly higher, in the recurrence group (p = 0.019, p = 0.014, and p = 0.01, respectively, Table 1). In terms of laboratory parameters, albumin, globulin, and AGR were significantly lower in the recurrence group, while protein was significantly higher (p < 0.05 for all). No significant difference was observed in terms of other laboratory parameters (WBC, CRP, ESR, HB, neutrophil, lymphocyte, PLT) and inflammatory markers (NLR and PLR) (p > 0.05 for all).
Table 2 shows the sensitivity, specificity, PPV, NPV, LR(+), and cut-off values for the parameters found to be significant in predicting IGM recurrence. Accordingly, AGR had the highest sensitivity (93.7%, 95% CI 67.7–99.6), specificity (76.8%, 95% CI 64.8–85.7), and LR(+) (4.04, 95% CI 2.58–6.32) values. The cut-off values determined for albumin, protein, globulin, and AGR were ≤4.15, ≥7.35, ≥3.35, and ≤1.179, respectively. According to these cut-off values, the number of patients with and without recurrence is shown in Table 3. The box plot showing the distribution of albumin, protein, globulin, and AGR values among the groups, and the ROC cut-off points, is shown in Figure 2. ROC curves for significant values in IGM recurrence prediction are presented in Figure 3.
 |
Table 2 ROC Analysis Results and Sensitivity, Specificity, PPV, NPV, and Likelihood Ratio (+) Values of ALB, Protein, Globulin, and AGR Parameters in IGM Recurrence Prediction |
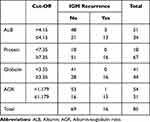 |
Table 3 The Success of Cut-off Values Determined by ROC Analysis in IGM Recurrence Prediction |
We determined the effective parameters in IGM recurrence with regression analysis and the results are presented in Table 4. Univariate analysis identified marital status (single or married), smoking (yes or no), age, date of the last delivery, and low AGR values (≤1.179) as risk factors in IGM recurrence. In the multivariate model, smoking (p = 0.044, OR: 4.45; 95% confidence interval (CI), 1.04–18.9) and AGR below the cut-off value of 1.179 (p < 0.001, OR: 50.7; 95% CI, 5.93–434.1) emerged as being independently associated with IGM recurrence.
 |
Table 4 The Results of Univariate and Multivariate Binary Logistic Regression Analysis Performed to Determine the Risk Factors That are Effective in IGM Recurrence |
Discussion
IGM, a form of non-lactational mastitis, was first described by Kessler and Wolloch in 1972.21 Since it cannot be distinguished from breast cancers and other benign diseases of the breast either clinically or radiologically, pathological confirmation is necessary for diagnosis.9 Although surgical approaches were previously recommended for treatment, medical pharmacological approaches and combined treatments are now more common.8,22,23 Although IGM has been recognized as a self-limiting and slowly healing benign inflammatory disease, the development of recurrence and persistent symptoms still pose a problem in the management of the disease. Recurrence is reported in up to 50% of patients, and long-term treatment and follow-up are required. The recurrence rate in the present study was 18.6%, with a mean follow-up period of 39.99 months, consistent with the previous literature.2,9,11,22,24,25 The classification of patients according to recurrence risk groups at the time of first diagnosis and determination of prognostic factors will increase the success of treatment by permitting personalized treatment strategies. Clinical factors that can be used in recurrence risk classification and safe biomarkers therefore need to be identified. The present study evaluated clinical and laboratory parameters that may affect the development of IGM recurrence, and AGR levels emerged as capable of use as safe biomarkers of the development of recurrence. Smoking was also identified as an independent risk factor for recurrence.
AGR has been evaluated in many inflammatory, autoimmune, and malignant diseases and its prognostic role determined in numerous previous studies.13–19,26 However, we encountered no studies evaluating the relationship between AGR and IGM. In the present study, we investigated the role of AGR in patients with IGM for the first time in the literature. AGR was significantly lower in the recurrence group, with a cut-off value of 1.179 at ROC analysis. Below this value, AGR emerged as an independent risk factor for recurrence at multivariate logistic regression analysis. Albumin is a negative acute phase reactant, the synthesis of which decreases in inflammatory conditions and malignant diseases.27,28 Conversely, globulin increases in cases of infection and chronic inflammation. AGR may therefore be expected to decrease in inflammatory conditions. This prediction suggested us that AGR can be used in IGM patients and in the prediction of IGM recurrence.
Laboratory parameters such as WBC, CRP, and ESR, and the inflammatory biomarkers NLR and PLR were also evaluated in this study, but no significant relationship was found between these and recurrence. In contrast to our findings, studies evaluating recurrent IGM have observed a significant relationship between NLR and IGM recurrence.10,11,29 However, the numbers of patients in these studies, 55, 41, and 59, respectively, were lower than in the present study. In addition, neutrophil and lymphocyte values are dynamic and may vary daily. There may also be differences in these values depending on whether blood samples are collected at the time of admission or before treatment after pathological diagnosis. In the present study, all laboratory parameters were calculated based on the values at the time of first admission. However, in the other studies mentioned, these parameters were based on values measured before surgical or medical treatment.
The mean age at diagnosis of the patients in this study was 36.47±9.63 years. This finding was consistent with the previous literature, showing that most patients are of reproductive age.2,6,24,25,30–33 Age was significantly associated with recurrence at univariate analyses (p = 0.023), and recurrence was found to occur more frequently at younger ages. However, this finding was not significant at multivariate analysis. Additionally, the mean age of both groups lay in the third decade and can be considered clinically insignificant. In terms of marital status, the great majority of patients (89.4%) in this study were married, similarly to many other studies in the literature.11,34 Interestingly, however, the proportion of singletons was statistically significantly higher in the recurrence group than in the non-recurrence group (31.3% vs 5.8%, p = 0.01). Although we cannot fully explain this difference, marriage rates increase with age. In parallel with the younger age of the patients in the recurrence group, the proportion of single individuals was also higher in this group.
In agreement with previous studies, most of the patients in our study had given birth at least once (89.4%) and had a history of breastfeeding (81.2%).2,3,35 Also consistent with many previous studies, these factors had no effect on recurrence.9,31,33 However, there are also studies in the literature reporting a statistically significant relationship between pregnancy and breastfeeding and recurrence.2,33,35 In contrast to the findings in the previous literature, the median time elapsed between the time of the most recent delivery and diagnosis was six years in this study. Patients diagnosed with IGM are within the first few years after delivery.6,9 We surmised that the difference between this study and other studies in terms of the most recent delivery might be due to some patients in the early lactation period being misdiagnosed with lactational mastitis in our study, or to the patients in this study presenting to the hospital later because of regional differences. Although the time to the most recent delivery was statistically significantly shorter in the recurrence group, this was not considered clinically significant since it lay outside the lactation period by approximately five years or more in both groups.
The relationship between smoking and IGM is uncertain. Although Al-Khafaff et al suggested that smoking is not associated with IGM,36 other studies have described smoking as an important risk factor for IGM recurrence.2,37,38 The proportion of smokers among IGM patients in previous studies ranged from 16.7% to 77.8%.4,36,39 The equivalent figure in the present was 29.4% and was significantly higher in the recurrence group. In addition, logistic regression multivariate analyses identified that smoking as an independent risk factor for recurrence. However, other studies have observed no significant relationship between smoking and IGM recurrence.25,34
Bilaterality is rarely reported in the literature, and IGM is usually seen unilaterally.6,20,30 All the patients in this study presented with unilateral symptoms at the time of initial diagnosis. Only two cases were regarded as bilateral because of new lesions that developed in the contralateral breast during follow-up after treatment. However, lesions were detected almost equally in the right and left breasts in the present study (50.6% vs 49.4%), and the side involved had no effect on recurrence. A similar finding was reported by Emre et al.34 Consistent with the literature in terms of lesion localization, the upper quadrants were those with the highest incidence of the disease, at 52.9%, in the present study.7,34 However, there was no significant relationship between recurrence and lesion localization.
Ultrasound-guided tru-cut biopsy was the most frequently employed diagnostic method in this study, at 57.6%. This method is noteworthy for its ability to provide a highly accurate diagnosis, as well as being less invasive and involving less scarring than surgical biopsies (excisional-incisional). Although the use of FNAC in the initial diagnosis of breast lesions is increasing, it is still controversial because it requires an experienced cytopathologist and the exact cytological diagnostic criteria have not been fully defined.40 In addition, the diagnostic power of FNAC is reported to be low. For these reasons, FNAC was the least used diagnostic approach in this study.
Patients in this study were classified into three disease severity levels based on clinical and radiological findings and using the classification suggested by Kaviani et al.20 Accordingly, most cases were evaluated as mild or moderate (85.6%, n = 71), and no significant relationship was found between disease severity and recurrence. Similarly, in Huang et al’s retrospective study involving 41 IGM patients, although 21 patients (51.2%) were in the severe group, no significant relationship was also observed between the degree of disease and recurrence.31
Half a century after IGM was first described, there is still no clear consensus on its treatment.3,8,34,41,42 The good response to steroid and immunosuppressant treatments reported in studies shows that the disease is an immunological condition rather than a surgical one, and pharmacological treatments predominate.22,23 Six different treatment options were determined in this study, and surgical methods (SM and SM + steroid) were applied in only 15.3% of cases, while 38.8% of patients were treated with antibiotics after abscess drainage and 17.6% were treated by observation only. Methotrexate has been given in recurrent cases, those resistant to steroid therapy, or in cases where the steroid dosage needs to be reduced. On the other hand, no statistically significant relationship was found between treatment options and IGM recurrence in the present study. Similarly, other studies investigating the factors affecting IGM recurrence have found that the treatment methods applied had no effect on recurrence, although the treatment classifications were different.2,24,31
There are some limitations to our study to be considered. First, due to the retrospective nature of the study, some patient data could not be accessed or had to be excluded, resulting in a relative decrease in the number of patients included. Second, the treatment protocols applied are not dependent on a specific algorithm, due to the lack of consensus in the literature, and were based instead on the preference of the physician. Finally, the fact that the study was conducted in a single-center makes it difficult to generalize our findings. For these reasons, further prospective, multicenter studies with larger sample sizes and a standard treatment algorithm are therefore needed.
Conclusions
The findings of this study show that IGM patients with an AGR value lower than 1.179 at first admission and smokers are more prone to recurrence. These patients should be informed about the risk of recurrence and should be followed-up more closely with personalized treatment.
Abbreviations
IGM, Idiopathic granulomatous mastitis; AGR, Albumin-to-globulin ratio; NLR, Neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; FAR, Fibrinogen-albumin ratio; GM, granulomatous mastitis; SM, Segmental mastectomy; FNAC, Fine-needle aspiration cytology; BMI, Body mass index; ESR, Sedimentation; CRP, C-reactive protein; Hb, Hemoglobin; PLT, Platelets.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
Our study obtained approval from the ethics committee of Samsun Ondokuz Mayis University (OMUKAEK no: 2022/176, date: 19.04.2022) and accorded with the Helsinki Declaration. Considering the retrospective nature of the study, the patients’ consents were waived by the ethics committee. All data about the patients was anonymized or maintained confidentially.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Shojaee L, Rahmani N, Moradi S, Motamedi A, Godazandeh G. Idiopathic granulomatous mastitis: challenges of treatment in Iranian women. BMC Surg. 2021;21(1):206. doi:10.1186/s12893-021-01210-6
2. Uysal E, Soran A, Sezgin E; Granulomatous Mastitis Study Group. Factors related to recurrence of idiopathic granulomatous mastitis: what do we learn from a multicentre study? ANZ J Surg. 2018;88(6):635–639. doi:10.1111/ans.14115
3. Yin Y, Liu X, Meng Q, Han X, Zhang H, Lv Y. Idiopathic granulomatous mastitis: etiology, clinical manifestation, diagnosis and treatment. J Invest Surg. 2022;35(3):709–720. doi:10.1080/08941939.2021.1894516
4. Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;2(12):852–858. doi:10.12998/wjcc.v2.i12.852
5. Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg. 2007;31(8):1677–1681. doi:10.1007/s00268-007-9116-1
6. Barreto DS, Sedgwick EL, Nagi CS, Benveniste AP. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat. 2018;171(3):527–534. doi:10.1007/s10549-018-4870-3
7. Chirappapha P, Thaweepworadej P, Supsamutchai C, Biadul N, Lertsithichai P. Idiopathic granulomatous mastitis: a retrospective cohort study between 44 patients with different treatment modalities. Ann Med Surg. 2018;36:162–167. doi:10.1016/j.amsu.2018.11.001
8. Zhou F, Liu L, Liu L, et al. Comparison of conservative versus surgical treatment protocols in treating idiopathic granulomatous mastitis: a meta-analysis. Breast Care. 2020;15(4):415–420. doi:10.1159/000503602
9. Azizi A, Prasath V, Canner J, et al. Idiopathic granulomatous mastitis: management and predictors of recurrence in 474 patients. Breast J. 2020;26(7):1358–1362. doi:10.1111/tbj.13822
10. Velidedeoglu M, Kundaktepe BP, Aksan H, Uzun H. Preoperative fibrinogen and hematological indexes in the differential diagnosis of idiopathic granulomatous mastitis and breast cancer. Medicina. 2021;57(7):698. doi:10.3390/medicina57070698
11. Cetinkaya OA, Celik SU, Terzioglu SG, Eroglu A. The predictive value of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in patients with recurrent idiopathic granulomatous mastitis. Eur J Breast Health. 2020;16(1):61–65. doi:10.5152/ejbh.2019.5187
12. Xuan Q, Yang Y, Ji H, et al. Combination of the preoperative albumin to globulin ratio and neutrophil to lymphocyte ratio as a novel prognostic factor in patients with triple negative breast cancer. Cancer Manag Res. 2019;11:5125–5131. doi:10.2147/CMAR.S195324
13. He J, Pan H, Liang W, et al. Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: a systematic review and meta-analysis. J Cancer. 2017;8(19):4002–4010. doi:10.7150/jca.21141
14. Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis. 2013;28(12):1629–1636. doi:10.1007/s00384-013-1748-z
15. Du XJ, Tang LL, Mao YP, et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS One. 2014;9(4):e94473. doi:10.1371/journal.pone.0094473
16. Mao MJ, Wei XL, Sheng H, et al. Clinical significance of preoperative albumin and globulin ratio in patients with gastric cancer undergoing treatment. Biomed Res Int. 2017;2017:3083267. doi:10.1155/2017/3083267
17. Oki S, Toiyama Y, Okugawa Y, et al. Clinical burden of preoperative albumin-globulin ratio in esophageal cancer patients. Am J Surg. 2017;214(5):891–898. doi:10.1016/j.amjsurg.2017.04.007
18. Wang Y, Li C, Wang W, et al. Serum albumin to globulin ratio is associated with the presence and severity of inflammatory bowel disease. J Inflamm Res. 2022;15:1907–1920. doi:10.2147/JIR.S347161
19. Liu XR, Qi YY, Zhao YF, Cui Y, Wang XY, Zhao ZZ. Albumin-to-globulin ratio (AGR) as a potential marker of predicting lupus nephritis in Chinese patients with systemic lupus erythematosus. Lupus. 2021;30(3):412–420. doi:10.1177/0961203320981139
20. Kaviani A, Vasigh M, Omranipour R, et al. Idiopathic granulomatous mastitis: looking for the most effective therapy with the least side effects according to the severity of the disease in 374 patients in Iran. Breast J. 2019;25(4):672–677. doi:10.1111/tbj.13300
21. Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58:642–646. doi:10.1093/ajcp/58.6.642
22. Basim P, Argun D, Argun F. Risk factors for idiopathic granulomatous mastitis recurrence after patient-tailored treatment: do we need an escalating treatment algorithm? Breast Care. 2022;17(2):172–179. doi:10.1159/000517399
23. Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38(2):330–356. doi:10.1148/rg.2018170095
24. Tan QT, Tay SP, Gudi MA, Nadkarni NV, Lim SH, Chuwa EWL. Granulomatous mastitis and factors associated with recurrence: an 11-year single-centre study of 113 patients in Singapore. World J Surg. 2019;43(7):1737–1745. doi:10.1007/s00268-019-05014-x
25. Velidedeoglu M, Umman V, Kilic F, et al. Idiopathic granulomatous mastitis: introducing a diagnostic algorithm based on 5 years of follow-up of 152 cases from Turkey and a review of the literature. Surg Today. 2022;52(4):668–680. doi:10.1007/s00595-021-02367-6
26. Zeng M, Liu Y, Liu F, Peng Y, Sun L, Xiao L. Association between albumin-to-globulin ratio and long-term mortality in patients with chronic kidney disease: a cohort study. Int Urol Nephrol. 2020;52(6):1103–1115. doi:10.1007/s11255-020-02453-7
27. Gabay C, Kushner I, Epstein FH. Acute-phase proteins and other systemic responses to inflammation. N Eng J Med. 1999;340(6):448–454. doi:10.1056/NEJM199902113400607
28. Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–255. doi:10.2147/IJGM.S102819
29. Kargin S, Turan E, Esen HH, Çetin Kargin N. Role of pre-treatment neutrophil-lymphocyte ratio in the prediction of recurrences after granulomatous mastitis treatment. Turkiye Klinikleri J Med Sci. 2020;40(1):46–51. doi:10.5336/medsci.2019-66835
30. Cetin K, Sikar HE, Goret NE, et al. Comparison of topical, systemic, and combined therapy with steroids on idiopathic granulomatous mastitis: a prospective randomized study. World J Surg. 2019;43(11):2865–2873. doi:10.1007/s00268-019-05084-x
31. Huang YM, Lo C, Cheng CF, Lu CH, Hsieh SC, Li KJ. Serum C-reactive protein and interleukin-6 levels as biomarkers for disease severity and clinical outcomes in patients with idiopathic granulomatous mastitis. J Clin Med. 2021;10:10. doi:10.3390/jcm10102077
32. Kaviani A, Noveiry BB, Jamei K, Rabbani A. How to manage idiopathic granulomatous mastitis: suggestion of an algorithm. Breast J. 2014;20(1):110–112. doi:10.1111/tbj.12216
33. Yilmaz TU, Gurel B, Guler SA, et al. Scoring idiopathic granulomatous mastitis: an effective system for predicting recurrence? Eur J Breast Health. 2018;14(2):112–116. doi:10.5152/ejbh.2018.3709
34. Emre A, Akbulut S, Sertkaya M, et al. Idiopathic granulomatous mastitis: overcoming this important clinical challenge. Int Surg. 2017;103(5–6):228–237. doi:10.9738/INTSURG-D-16-00225.1
35. Omranipour R, Mohammadi SF, Samimi P. Idiopathic granulomatous lobular mastitis - report of 43 cases from Iran; introducing a preliminary clinical practice guideline. Breast Care. 2013;8(6):439–443. doi:10.1159/000357320
36. Al-Khaffaf B, Knox F, Bundred NJ. Idiopathic granulomatous mastitis: a 25-year experience. J Am Coll Surg. 2008;206(2):269–273. doi:10.1016/j.jamcollsurg.2007.07.041
37. Co M, Cheng VCC, Wei J, et al. Idiopathic granulomatous mastitis: a 10-year study from a multicentre clinical database. Pathology. 2018;50(7):742–747. doi:10.1016/j.pathol.2018.08.010
38. Tang ELS, Ho CSB, Chan PMY, Chen JJC, Goh MH, Tan EY. The therapeutic dilemma of idiopathic granulomatous mastitis. Ann Acad Med Singap. 2021;50(8):598–605. doi:10.47102/annals-acadmedsg.2020645
39. Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11(2):108–114. doi:10.1111/j.1075-122X.2005.21576.x
40. Tse GMK, Poon CSP, Law BKB, Pang LM, Chu WCW, Ma TKF. Fine needle aspiration cytology of granulomatous mastitis. J Clin Pathol. 2003;56:519–521. doi:10.1136/jcp.56.7.519
41. Li J. Diagnosis and treatment of 75 patients with idiopathic lobular granulomatous mastitis. J Invest Surg. 2019;32(5):414–420. doi:10.1080/08941939.2018.1424270
42. Wolfrum A, Kummel S, Theuerkauf I, Pelz E, Reinisch M. Granulomatous mastitis: a therapeutic and diagnostic challenge. Breast Care. 2018;13(6):413–418. doi:10.1159/000495146
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


