Back to Journals » Infection and Drug Resistance » Volume 15
Risk Factors and Prognosis of Carbapenem-Resistant Organism Colonization and Infection in Acute Cholangitis
Authors Li K, Jiang S, Fu H, Hao Y, Tian S, Zhou F
Received 21 November 2022
Accepted for publication 14 December 2022
Published 28 December 2022 Volume 2022:15 Pages 7777—7787
DOI https://doi.org/10.2147/IDR.S398581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Kaili Li, Sanle Jiang, Hongxue Fu, Yingting Hao, Shijing Tian, Fachun Zhou
Department of Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Shijing Tian; Fachun Zhou, Emergency Department, the First Affiliated Hospital of Chongqing Medical University, 1 Youyi Road, Yuzhong Qu, 17th Floor, Building 1, Chongqing, People’s Republic of China, Tel +86 023-89011756, Email [email protected]; [email protected]; [email protected]
Background: To identify the risk factors and prognosis of carbapenem-resistant organisms (CRO) in patients with acute cholangitis.
Methods: This retrospective observational study was conducted to explore the risk factors and prognosis of CRO infection in 503 acute cholangitis patients diagnosed between July 2013 and January 2022 at the First Affiliated Hospital of Chongqing Medical University, who were divided into a CRO group and non-CRO group based on the presence or absence of CRO. Univariate, multivariate analyses, and the proportional hazards model were used to compare the risk factors and prognosis of CRO suffering in patients with acute cholangitis.
Results: We identified 35 patients colonized with CRO from 503 acute cholangitis patients. In the multivariate analysis, tumor (OR=7.09, 95% CI=1.11– 45.30, P=0.038) and chronic kidney disease (OR=8.70, 95% CI=2.11– 35.88, P=0.003) were ascertained as the risk factors of the occurrence on CRO infection under the background of acute cholangitis. CRO infection was identified as an independent risk factor for acute cholangitis patient death (HR=5.147, 95% CI=1.475– 17.595, P=0.01) by Cox proportional-hazards regression.
Conclusion: Tumor and chronic kidney disease may be risk factors for CRO infection. Patients diagnosed with acute cholangitis further infected with CRO had a poor prognosis and a more severe mortality. Active screening for CRO is expected to facilitate early prevention, diagnosis, and treatment of high-risk patients.
Keywords: CRO, acute cholangitis, risk factors, prognosis
Introduction
Acute cholangitis is still deemed to be a fatal disease of the biliary system caused by bile duct stone obstruction and tumor formation, which can be accompanied by multiple organ dysfunction, and is one of the clinical problems that needs to be solved urgently due to its higher mortality and poor prognosis.1,2 According to the Tokyo Guidelines 2018 (TG18), patients with mild, moderate, and severe acute cholangitis may need antibacterial treatment, especially for more severe cases with systemic inflammation, for which antibacterial treatment may be relatively effective, and more severe patients even need antibacterial treatment until the implementation of surgical treatment such as cholecystectomy.3
At present, the multiple drug resistance (MDR) and multiple organ dysfunction associated with acute cholangitis bring heavily dilemma in improving the prognosis of patients.4 In addition, antimicrobial selection associated with antimicrobial therapy for acute cholangitis, is often reported to be affected by the presence of carbapenemase-producing bacteria.5 Carbapenems once used to treat multidrug-resistant bacterial infections, are threatened by the widespread of carbapenems resistant gut bacteria.6 The increasing detection rate of CRO brings severe challenges to clinical infection of acute cholangitis.7
CRO is a class of bacteria characterized by different mechanisms of drug resistance, and the incidence of CRO is increasing worldwide.8 In recent years, more and more studies have focused on the analysis of risk factors for CRO infection in patients afflicted with different diseases, aiming to further explore the optimal utilization of antibiotics and the maximization of patients’ benefits.9–13 The influence of carbapenem-resistant nonfermenting gram-negative bacteria (such as Acinetobacter baumannii and Pseudomonas aeruginosa) which caused a global epidemic is increasingly being recognized.14 Some retrospective studies elaborated that mechanical ventilation, septic shock, and low platelet count represent three independent risk factors to the mortality of ASOT recipients with CR-GNB infection.5,15 Studies have indicated that patients admitted to the ICU with high Sequential Organ Failure Assessment (SOFA) and Nutric scores, prolonged ICU LOS, previous surgery, dialysis and mechanical ventilation, and prior aminoglycosides and carbapenems use may be at increased risk for CRE infection.16,17 A systematic review referring to 92 studies identified that the most common risk factors for CRO infection but further exploration of CRO infection in patients with acute cholangitis was omitted.13 Therefore, to explore the risk factors of CRO infection in acute cholangitis is of great significance to further guide the prevention and control of CRO infection and to explore the potential causes of CRO infection in patients with acute cholangitis is of significance, which may ensure continuous access to effective treatment.
Patients and Methods
Study Design
All 1,570 inpatients with acute cholangitis admitted to the First Affiliated Hospital of Chongqing Medical University from July 2013 to January 2022 were considered for inclusion. For inclusion and exclusion criteria refer to our previous studies.18 The inclusion criteria for acute cholangitis and the identification of cases are shown in Figure 1. In view of the following exclusion factors:) Bile culture and blood culture were not obtained, 2) Received treatment before admission, 3) Organ failure before onset, 4) Transfer to another hospital, and 5) Neither ERCP and surgical drainage were performed, 503 patients were finally included in this research. Under the implementation of patient’s blood or bile culture of CRO infection, a total of 35 cases were included in the CRO group, while 468 cases were arranged into the non-CRO group, as shown in Figure 1. This research was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
 |
Figure 1 Inclusion and exclusion procedures for 503 acute cholangitis patients and CRO grouping between 503 acute cholangitis patients. |
Basic Information
Information for all 503 patients was collected in the light of the form uniformly formulated by the team in the preliminary study, including patient’s gender, age, hospital stays, Acute Physiology And Chronic Health Evaluation II (APACHE II), SOFA, TG18 severity Grade (I, II, III), fever, abdominal pain, jaundice, mental status change, shock, biliary tract tumor, bile stones, relapse, 14 days antibiotic use, endoscopic retrograde cholangio-pancreatography (ERCP), biliary stent, biliary anastomosis, renal insufficiency (after the occurrence of CRO), baseline diseases (cardiovascular, chronic pulmonary, malignancies, diabetes mellitus, chronic kidney disease [(GFR <60 mL/(min·1.73 m2) over 3 months], chronic liver disease, neurological disease), laboratory tests for post-admission testing: platelet (PLT), international normalized ratio (INR), white blood cell (WBC), neutrophil (N), total bilirubin (TB), direct bilirubin (DB), alanine transaminase (ALT), aspartate transaminase (AST), albumin (ALB), blood urea nitrogen (BUN), creatinine (Cr), pathogen culture, MDR, extended-spectrum β-lactamases (ESBL), bile culture, and blood culture. In addition, organ functional injury in our study was determined according to the Organ injury criteria in the TG18: respiratory dysfunction (PaO2/FiO2 <300), hepatic dysfunction (INR >1.5, renal dysfunction (oliguria and serum creatinine >2.0 mg/dL), hematological dysfunction (platelet count <100,000/mm3), and neurological dysfunction (disturbance of consciousness).
The following factors prior to hospitalization were also taken into account: infection-related risk factors, whether CRE infection was detected before being hospitalized, pre-admission factors included hospitalization within 3 months (emergency admission, duration of emergency stay, use of carbapenems within 3 months prior to admission, and presence of infection before admission), whether or not a ventilator or artificial airway were used within a month prior to infection, whether the catheter was indwelled, whether the blood flow catheter was indwelled, catching infection previously, whether the patients were admitted to the intensive care unit (ICU), usage of antibacterial drugs, that is, combination of drugs, use of carbapenems, cephalosporins, tetracycline, fluoroquinolones, aminoglycosides, glycopeptides, sulfamides, and other antibacterial drugs, and their hours of use.
The separation and identification of bacteria were carried out in strict accordance with the national Clinical Laboratory Operating Procedures (4th edition) in 2016; the drug sensitivity test was conducted by automatic instrument method or K-B disk diffusion method,19 a modified Hodge test was used to confirm the phenotype of the carbapenemase-producing strain. All drug sensitivity results were interpreted based on the American Institute for Clinical Laboratory Standardization (CLSI) standards.
Statistical Analysis
SPSS 20.0 (IBM, Armonk, NY) was used for statistical analysis. Descriptive statistics included the frequency (percentage) for categorical variables and mean ± standard deviation (x±s) and median (interquartile range, IQR) for continuous variables. Variables were compared between CRO group and non-CRO group through univariable analysis to assess any statistical significance using Mann-Whitney U-test, chi-square, or Fisher’s exact test, as appropriate. Subsequently, multivariate logistic regression was further used to verify single factors that were identified through univariate analysis or worth discussing according to clinical experience. Cox proportional-hazards regression model was used to assess the risk of death. P<0.05 indicated that the difference was statistically significant.
Results
Characteristics of All 503 Patients
According to drug sensitivity results, 35 patients with CRO infection were defined as the CRO group, and 468 patients without CRO infection were included in the non-CRO group. The baseline characteristics of 503 patient with acute cholangitis are summarized and described in Table 1. Tumor, bile stones, biliary stent, chronic pulmonary disease, chronic kidney disease, abdominal pain, mental status change, INR, INR>1.5, TB, DB, ALB, Grade I, Grade II, Grade III, APACHE II, SOFA, ICU admission, and Death, as shown in Table 1, could be associated with an increased incidence of CRO infection when compared with the non-CRE group (P<0.05).
 |
Table 1 Demographic Characteristics of Patients |
Microbiological Characteristics of Blood Culture and Bile Culture
As described in Table 2, 313 (62.2%) blood positive culture records and 365 (72.6%) bile positive culture records were verified among 503 enrolled acute cholangitis patients. Aeromonas hydrophila, Proteus vulgaris, Enterococcus avium, Enterobacter aerogenes, other Enterococcus spp and Enterococcus raffinosus were not detected in the blood culture, but were in the bile culture. In addition, for Gram-negative bacilli, Escherichia coli and Klebsiella pneumoniae occupy a higher proportion both in blood culture (57.3%, 20.7%) and bile culture (40.1%, 15.8%). For Gram-positive cocci, Enterococcus faecalis and Enterococcus faecium accounted for a higher proportion both in blood culture (7.3%, 7.3%) and bile culture (20.4%, 15.8%). Results of blood culture and bile culture in 35 CRO patients from 503 acute cholangitis are shown in Figure 2. K. pneumoniae accounts for the highest proportion both in blood culture (8/44.4%) and bile culture (10/37.0%) followed by E.coli, Pseudomonas aeruginosa, A.baumannii, Enterobacter cloacae, and E.aerogenes.
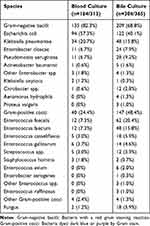 |
Table 2 Results of Blood and Bile Cultures in CRO Patients with Acute Cholangitis |
 |
Figure 2 Both blood culture and bile culture results of 35 CRO patients. |
The Cox Proportional-Hazards Regression Model and Organ Function Between CRO and Non-CRO
CRO infection identified as an independent risk factor for acute cholangitis patient death (HR=5.147, 95% CI=1.475–17.959, P=0.01) by Cox proportional-hazards regression (60 days of follow-up) when compared with the non-CRO group in Figure 3. Cox proportional-hazards regression model was adjusted for age, gender, fever, mental status change, shock, tumor, biliary stent, malignancies, chronic liver disease, chronic kidney disease, chronic pulmonary disease, cardiovascular, diabetes, relapse, surgery PLT <100×109, INR >1.5, WBC, TB, and CRE.
The results of the risk ratio of organ dysfunction expounded that the hepatic dysfunction was more severe in the CRO group than the non-CRO group [OR=3.63, 95% CI=1.49–8.81, P=0.004], while the respiratory dysfunction was worse in thr CRO group than non-CRO group [OR=6.77, 95% CI=2.93–15.64, P<0.001]. Correspondingly, there was no significant difference in ICU admission, ICU stay, Hospital stays, Grade, mental status change, renal insufficiency, shock, hospital stays, or cardiovascular sequelae between the CRO group and non-CRO group, as described in Table 3.
 |
Table 3 Risk Ratio of Organ Dysfunction in Different Culture Results by Logistic-Regression Model |
Risk Factors for CRO Infection
According to the results of univariate analysis in Table 4, tumor may be a risk factor for CRO infection (OR=3.52, 95% CI=1.24–10.00, P=0.018). Furthermore, biliary stent placement (OR=3.76, 95% CI=1.59–8.90, P=0.003), and chronic kidney disease (OR=9.87, 95% CI=2.55–36.82, P=0.001) were also ascertained as risk factors of the occurrence of CRO infection by univariate analysis.
 |
Table 4 Univariate and Multivariable Analysis of Risk Factors for the Occurrence of CRO in Acute Cholangitis |
On the grounds of univariate analysis, age, tumor, biliary stent placement, malignancies, and chronic kidney disease were included in the multivariable analysis, as described in Table 4. The results suggested that tumor (OR=7.09, 95% CI=1.11–45.30, P=0.038) and chronic kidney disease (OR=8.70, 95% CI=2.11–35.88, P=0.003) were regarded as the risk factors of the occurrence on CRO infection by further multivariable analysis.
Discussion
Carbapenem-resistant organisms (CRO) represent a growing threat to human health globally, which usually requires a longer treatment time and higher treatment costs.7 The reduction in mortality from CRO infection requires a combination of appropriate antibiotic use, infection severity, and CRO strain characteristics.12 However, optimal antimicrobial treatments available against CRO are limited, with only a few active antimicrobials left for use. Subsequently, the burden and source of carbapenem resistance is still an urgent problem to be solved,20 and understanding the risk factors for CRO infection is also becoming critical.
Acute cholangitis, a potentially life-threatening clinical syndrome with a mortality rate of more than 50%, occurs in most cases with biliary obstruction, followed by bacterial transplantation and infection.21 The main treatment methods for acute cholangitis are fluid resuscitation, antibiotic therapy, and biliary drainage.22,23 Antibiotic use and invasive biliary drainage including endoscopic retrograde cholangiopancreatography (ERCP) have been widely reported as possible risk factors for CRO infection.24,25 Therefore, we hypothesized that the analysis of risk factors for CRO infection in patients with acute cholangitis may play a suggestive role in clinical diagnosis and treatment and guide the improvement of prognosis.
At present, precision medicine is increasingly being recommended for personalized management of patients with CRO infection because it is a multicomponent approach, but the mortality associated with CRO infection remains high.26 Reyes and Nicolau27 mentioned that important prerequisites for precision treatment of CRO include in-depth understanding of the clinical characteristics of patients and analysis of risk factors related to CRO infection. Comprehension toward the characteristics of patients with different diseases and the risk factors for CRO infection is of great help for precise treatment and personalized medicine of CRO suffering.
Our results suggested that tumor (OR=7.09, 95% CI=1.11–45.30, P=0.038) and chronic kidney disease (OR=8.70, 95% CI=2.11–35.88, P=0.003) may be risk factors for CRO infection in patients with acute cholangitis. Consistent with our studies, some studies confirm that the risk of CRO infection was higher in patients suffering from tumor.28–30 Tumor was also found to be an independent risk factor for CRO infection by several retrospective systematic analyses.31,32 In Hong Kong, a study involving 1,408 residents from 28 northern Hemisphere regions identified an increased risk of CRO infection in cancer patients.33 A study of 551 residents of Italy confirmed a possible six-fold increase in the risk of CRO infection among cancer patients.34 In addition, cancer and metastasis were seen as the independent risk factors for 28-day mortality for CRO infection.35 The presence of underlying diseases such as cancer and diabetes have been identified as possible causes for CRO, based on multivariate analysis.30,36
Biliary stent placement, one of the invasive interventions, may increase the risk of bloodstream infections caused by Enterobacter carbapenem-resistant bacteria. Several studies have indicated that the association of health care exposures and devices with invasive infections in CRO patients has become somewhat of a health consensus in clinical practice (22–24). Previous studies among hospitalized patients have also explored risk factors for progression from colonization with CRO to invasive infection, and have identified invasive procedures, central line placement, ICU admission, receipt of antibiotics, and underlying diagnoses of diabetes and cancer as risk factors (25–27). In our study, biliary stent placement was found to be a significant risk factor for CRO infection in acute cholangitis by univariate analysis, but not in the process of multivariate analysis due to some limitations.
Our results confirm that chronic kidney disease was also identified as a possible independent risk factor for CRO infection. In another study, the status of transplanted kidney and the degree of immunosuppression have been confirmed to be risk factors for carbapenem resistance in renal transplantation patients.37 Coagulation time, which may be affected by renal dysfunction, may also be an independent risk factor for CRO infection in patients with acute cholangitis.38,39
The results of bacterial culturing in 503 acute cholangitis patients suggested that the overall bacterial distribution in bile culture and blood culture showed similar trends. The results of bacterial culture indicated that the proportions of E.coli, E.pneumoniae, E.f aecalis, and E. faecium were all high in blood culture and bile culture, which may play a certain guiding role in the selection of antimicrobial agents in clinical practice. The bacterial profiles of 35 CRO patients also provided a basis for further clinical exploration of the bacterial distribution characteristics of CRO. Correlation analysis of colonizing bacteria may also contribute to early antibiotic intervention and multidrug resistance reduction guidance.
The results of cumulative hazard rates indicated that patients suffering CRO infection have more severe mortality and worse prognosis. Meanwhile, patients with chronic liver injury and chronic kidney damage in CRO infections were more likely than non-CRO infections which is worth exploring further.
Age was not a risk factor in our study. In most studies, age was analyzed as a categorical variable, it was found that those aged ≥90 were around nine times more likely to be infected in the adjusted analysis.30 An Italian study reported OR=4.63 (95% CI=1.12–19.1; P=0.034) in residents with age ≥86 years to be the risk factor toward CRO infection.40 Therefore, the inclusion of age as a categorical variable may be more scientifically analyzed than the relationship between age and CRO infection in patients with acute cholangitis. In addition, cholecystectomy, surgery, and ERCP were not found to be risk factors for CRO infection in patients with acute cholangitis, which is worthy of further data enrichment and discussion.
Conclusion
In conclusion, our results suggested that tumor and chronic kidney disease may be risk factors for CRO infection and further CRO infection in patients with acute cholangitis has poor prognosis and higher mortality.
There are some limitations in our study. The study excluded hospital-acquired CRO infection, and only focused on community-acquired CRO infection. Due to the low incidence of CRO appearance during the community, only 35 patients were included in the study, which brings some disadvantages for further study. Despite these limitations, we still hope that this study will help identify risk factors for CRO infection and provide guidance for lower mortality and improved prognosis in patients with acute cholangitis.
Abbreviations
CRO, Carbapenem-resistant organism; TG18, Tokyo Guidelines 2018; MDR, Multiple drug resistance; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; ERCP, Endoscopic Retrograde Cholangio-Pancreatography; PLT, Platelet; INR, International Normalized Ratio; WBC, White Blood Cell; N, Neutrophil; TB, Total Bilirubin; DB, Direct Bilirubin; ALT, Alanine Transaminase; AST, Aspartate transaminase; ALB, Albumin; BUN, Blood urea nitrogen; Cr, Creatinine; ESBL, Extended-spectrum β-lactamases; ICU, Intensive care unit; CLSI, Clinical Laboratory Standardization.
Data Sharing Statement
The data that support the findings of this study are available from the Department of Medical Records, the First Affiliated Hospital of Chongqing Medical University, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Ethics Approval and Consent to Participate
Our study was conducted in accordance with the Helsinki Declaration and all methods were carried out in accordance with relevant guidelines and regulations of the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The experimental protocols and reference number (2022-249) of our research was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Informed consent of our research was obtained from all subjects or their legal guardian.
Acknowledgments
Author disclosure: all authors have read and approved the manuscript. This article has not been published or accepted for publication. It is not under consideration by another journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Science and Technology Commission of Chongqing (project number X1-2703).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Takada T, Strasberg SM, Solomkin JS, et al. TG13: updated Tokyo guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20(1):1–7. doi:10.1007/s00534-012-0566-y
2. Yokoe M, Hata J, Takada T. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute. J Hepatobiliary Pancreat Sci. 2018;25(1):41–54. doi:10.1002/jhbp515
3. Mayumi T, Okamoto K, Takada T, et al. Tokyo guidelines 2018: management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25(1):96–100. doi:10.1002/jhbp.519
4. Shah RA, Kowdley KV. Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol Hepatol. 2020;5(3):306–315. doi:10.1016/S2468-1253(19)30343-7
5. Sung YK, Lee JK, Lee KH, Lee KT, Kang CI. The clinical epidemiology and outcomes of bacteremic biliary tract infections caused by antimicrobial-resistant pathogens. Am J Gastroenterol. 2012;107(3):473–483. doi:10.1038/ajg.2011.387
6. Tanaka A, Leung PS, Gershwin ME. Pathogen infections and primary biliary cholangitis. Clin Exp Immunol. 2019;195(1):25–34. doi:10.1111/cei.13198
7. Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur J Clin Microbiol Infect Dis. 2021;40(10):2053–2068. doi:10.1007/s10096-021-04296-1
8. Chamieh A, El-Hajj G, Zmerli O, Afif C, Azar E. Carbapenem resistant organisms: a 9-year surveillance and trends at Saint George University Medical Center. J Infect Public Health. 2020;13(12):2101–2106. doi:10.1016/j.jiph.2019.02.019
9. Sexton ME, Bower C, Jacob JT. Risk factors for isolation of carbapenem-resistant Enterobacterales from normally sterile sites and urine. Am J Infect Control. 2022;50(8):929–933. doi:10.1016/j.ajic.2021.12.007
10. O’Hara LM, Nguyen MH, Calfee DP, et al.; CDC Prevention Epicenters Program. Risk factors for transmission of carbapenem-resistant Enterobacterales to healthcare personnel gloves and gowns in the USA. J Hosp Infect. 2021;109:58–64. doi:10.1016/j.jhin.2020.12.012
11. Moghnieh R, Abdallah D, Jadayel M, et al. Epidemiology, risk factors, and prediction score of carbapenem resistance among inpatients colonized or infected with 3rd generation cephalosporin resistant Enterobacterales. Sci Rep. 2021;11(1):1–4. doi:10.1038/s41598-021-94295-1
12. Zhou C, Jin L, Wang Q, et al. Bloodstream infections caused by Carbapenem-resistant Enterobacterales: risk factors for mortality, antimicrobial therapy and treatment outcomes from a prospective multicenter study. Infect Drug Resist. 2021;14:731. doi:10.2147/IDR.S294282
13. Routsi C, Pratikaki M, Platsouka E, et al. Risk factors for carbapenem-resistant Gram-negative bacteremia in intensive care unit patients. Intensive Care Med. 2013;39(7):1253–1261. doi:10.1007/s00134-013-2914-z
14. Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Supplement_7):S521–8. doi:10.1093/cid/ciz824
15. Wu D, Chen C, Liu T, Jia Y, Wan Q, Peng J. Epidemiology, susceptibility, and risk factors associated with mortality in carbapenem-resistant gram-negative bacterial infections among abdominal solid organ transplant recipients: a retrospective cohort study. Infect Dis Ther. 2021;10(1):559–573. doi:10.1007/s40121-021-00411-z
16. Teo JQ, Chang CW, Leck H, et al. Risk factors and outcomes associated with the isolation of polymyxin B and carbapenem-resistant Enterobacteriaceae spp.: a case-control study. Int J Antimicrob Agents. 2019;53(5):657–662. doi:10.1016/j.ijantimicag.2019.03.011
17. Aleidan FAS, Alkhelaifi H, Alsenaid A, et al. Incidence and risk factors of carbapenem-resistant Enterobacteriaceae infection in intensive care units: a matched case-control study. Expert Rev Anti Infect Ther. 2021;19(3):393–398. doi:10.1080/14787210.2020.1822736
18. Tian S, Li K, Tang H, et al. Clinical characteristics of gram-negative and gram-positive bacterial infection in acute cholangitis: a retrospective observational study. BMC Infect Dis. 2022;22(1):1. doi:10.1186/s12879-021-06964-1
19. Morris CP, Bergman Y, Tekle T, Fissel JA, Tamma PD, Simner PJ. Cefiderocol antimicrobial susceptibility testing against multidrug-resistant Gram-negative bacilli: a comparison of disk diffusion to broth microdilution. J Clin Microbiol. 2020;59(1):e01649–20. doi:10.1128/JCM.01649-20
20. Olalekan A, Onwugamba F, Iwalokun B, Mellmann A, Becker K, Schaumburg F. High proportion of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae among extended-spectrum β-lactamase-producers in Nigerian hospitals. J Glob Antimicrob Resist. 2020;21:8–12. doi:10.1016/j.jgar.2019.09.007
21. Sulzer JK, Ocuin LM. Cholangitis: causes, diagnosis, and management. Surg Clin. 2019;99(2):175–184. doi:10.1016/j.suc.2018.11.002
22. Aboelsoud M, Siddique O, Morales A, Seol Y, Al-Qadi M. Early biliary drainage is associated with favourable outcomes in critically-ill patients with acute cholangitis. Gastroenterol Rev. 2018;13(1):16–21. doi:10.5114/pg.2018.74557
23. Carbone M, Milani C, Gerussi A, Ronca V, Cristoferi L, Invernizzi P. Primary biliary cholangitis: a multifaceted pathogenesis with potential therapeutic targets. J Hepatol. 2020;73(4):965–966. doi:10.1016/j.jhep.2020.05.041
24. Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6(9):533–541. doi:10.1038/nrgastro.2009.126
25. Lleo A, Wang GQ, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet. 2020;396(10266):1915–1926.
26. Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in. Expert Rev Anti Infect Ther. 2016;14(1):95–108. doi:10.1586/14787210.2016.1106940
27. Reyes S, Nicolau DP. Precision medicine for the diagnosis and treatment of carbapenem-resistant. Expert Rev Anti Infect Ther. 2020;18(8):721–740. doi:10.1080/14787210.2020.1760844
28. Kohler P, Fulchini R, Albrich WC, et al. Antibiotic resistance in Swiss nursing homes: analysis of national surveillance. Antimicrob Resist Infect Control. 2018;7(1):1–9.
29. Nucleo E, Caltagirone M, Marchetti VM. Colonization of long-term care facility residents in three Italian provinces by. Antimicrob Resist Infect Control. 2018;7:33. doi:10.1186/s13756-018-0326-0
30. Warren DK, Guth RM, Coopersmith CM, Merz LR, Zack JE, Fraser VJ. Prevalence of methicillin-resistant Staphylococcus aureus colonization in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2006;27(10):1032–1040.
31. Amanati A, Sajedianfard S, Khajeh S. Bloodstream infections in adult patients with malignancy, epidemiology. BMC Infect Dis. 2021;21(1):636. doi:10.1186/s12879-021-06243-z
32. Pouch SM, Satlin MJ. Carbapenem-resistant Enterobacteriaceae in special populations: solid organ. Virulence. 2017;8(4):391–402. doi:10.1080/2150559420161213472
33. Cheng VC, Chen JH, Ng WC, et al. Emergence of carbapenem-resistant Acinetobacter baumannii in nursing homes with. Infect Control Hosp Epidemiol. 2016;37(8):983–986. doi:10.1017/ice201684
34. Brugnaro P, Fedeli U, Pellizzer G, et al. Clustering and risk factors of methicillin-resistant Staphylococcus aureus. Infection. 2009;37(3):216–221.
35. Oka K, Matsumoto A, Tetsuka N. Clinical characteristics and treatment outcomes of carbapenem-resistant. J Glob Antimicrob Resist. 2022;29:247–252. doi:10.1016/j.jgar.2022.04.004
36. Mody L, Gibson KE, Horcher A, et al. Prevalence of and risk factors for multidrug-resistant Acinetobacter baumannii. Infect Control Hosp Epidemiol. 2015;36(10):1155–1162. doi:10.1017/ice.2015.143
37. Freire MP, de Oliveira Garcia D, Lima SG, et al. Performance of two methods of carbapenem-resistant Enterobacterales surveillance. Infection. 2022;9:1–9. doi:10.1007/s15010-022-01839-2
38. Wang ZQ, Guo ZL, Feng H, et al. Treatment of donor-derived Carbapenem-resistant Klebsiella pneumoniae infection. Curr Med Sci. 2021;41(4):770–776.
39. Wang Z, Qian Y, Bai H, Yang J, Li X. Allograft hemorrhage as a manifestation of carbapenem-resistant Klebsiella pneumonia infection in kidney transplant recipients: case series. Medicine. 2020;99(13):1.
40. March A, Aschbacher R, Dhanji H. Colonization of residents and staff of a long-term-care facility and adjacent. Clin Microbiol Infect. 2010;16(7):934–944. doi:10.1111/j.1469-0691.2009.03024.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

