Back to Journals » Infection and Drug Resistance » Volume 15
Risk Factors and Outcomes of Multidrug-Resistant Bacteria Infection in Infected Pancreatic Necrosis Patients
Authors Lu J, Ding Y, Qu Y, Mei W, Guo Y , Fang Z, Qu C, Gao C, Cao F, Li F , Feng Y
Received 24 August 2022
Accepted for publication 18 November 2022
Published 2 December 2022 Volume 2022:15 Pages 7095—7106
DOI https://doi.org/10.2147/IDR.S387384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jiongdi Lu,1,2 Yixuan Ding,1,2 Yuanxu Qu,1,2 Wentong Mei,1,2 Yulin Guo,1,2 Zhen Fang,1,2 Chang Qu,1,2 Chongchong Gao,1,2 Feng Cao,1,2,* Fei Li,1,2,* Yulu Feng3
1Clinical Center of Acute Pancreatitis, Capital Medical University, Beijing, People’s Republic of China; 2Department of General Surgery, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Pediatric, Chui Yang Liu Hospital Affiliated Tsinghua University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fei Li; Feng Cao, Clinical Center of Acute Pancreatitis, Capital Medical University, Department of General Surgery, Department of Surgery, Xuanwu Hospital, Capital Medical University, No. 45, Changchun Street, Xicheng District, Beijing, 100053, People’s Republic of China, Tel +86-10-83198731, Fax +86-10-83198868, Email [email protected]; [email protected]
Objective: The incidence of acute pancreatitis (AP) is increasing. Twenty percent of AP patients with developing necrotizing pancreatitis (NP), while ~40– 70% of NP patients develop potentially fatal infectious complications. When patients are suspected or confirmed infected pancreatic necrosis (IPN), antibiotics should be administered timeously to control the infection, but long-term use of antibiotics can lead to multidrug-resistant bacteria (MDRB) infection and eventually to increased mortality. Our study aimed to determine the incidence of MDRB infection and evaluate the risk factors for MDRB infection in IPN patients.
Methods: Clinical data of IPN patients admitted to the general surgery department of Xuanwu Hospital of Capital Medical University between January 1, 2014, and December 31, 2021, were retrospectively analyzed.
Results: IPN patients (n = 267) were assigned to MDRB infection (n = 124) and non-MDRB infection (n = 143) groups. On admission, patients in the MDRB group had a higher modified computer tomography severity index (CTSI) score (P < 0.05), pancreatic necrosis degree, and PCT level (P < 0.05) than those in the non-MDRB group, and the prognosis of patients in MDRB group was poor. The most common gram-negative bacteria were Acinetobacter baumannii (n = 117), the most common gram-positive bacteria were Enterococcus faecium (n = 98), and the most common fungal infection was Candida albicans (n = 47). Multivariable analysis showed that complications of EPI (OR: 4.116, 95% CI: 1.381– 12.271, P = 0.011), procalcitonin (PCT) level at admission (OR: 2.728, 95% CI: 1.502– 4.954, P = 0.001), and degree of pancreatic necrosis (OR: 2.741, 95% CI: 1.109– 6.775, P = 0.029) were independent risk factors for MDRB infection in IPN patients.
Conclusion: We identified common infectious strains and risk factors for MDRB infection in IPN patients.
Keywords: infected pancreatic necrosis, extra-pancreatic infection, multidrug-resistant bacteria, risk factor analysis
Introduction
The morbidity of acute pancreatitis (AP) is increasing annually, and its clinical symptoms are complicated and variable. Although 80% of AP cases are mild, self-limiting, and have a very low mortality rate, 20% of AP patients suffer increased mortality due to various complications such as organ failure, peritoneal septal syndrome, and infected pancreatic necrosis.1,2 In the early stages of the disease (≤14 days), persistent organ failure (POF) caused by systemic inflammatory response syndrome (SIRS) is the main cause of death in AP patients. In the late stages of the disease (>14 days), pancreatic parenchyma and/or peripancreatic fat tissue necrosis develop, and AP progresses to necrotizing pancreatitis (NP). More patients die due to infected pancreatic necrosis (IPN) caused by complications such as septic shock and death.1–5
The current guidelines and meta-analysis suggest that prophylactic application of antibiotics does not reduce the risk of infection in AP patients and recommends that antibiotics only for patients with suspected or confirmed infection.6,7 In addition, in the early stages of AP patients, because of similar clinical symptoms of SIRS and infection (eg, fever, elevated white blood cells, shortness of breath), about 2/3 of patients received at least one course of antibiotic therapy history during their admission.8,9 In recent years, the decline in production costs of antibiotics, lack of antibiotic management processes in the community and local hospitals, and non-standard use of antibiotics have promoted the emergence and development of multidrug-resistant bacteria (MDRB) infection.10,11 Some studies highlighted that MDRB infection is an important factor contributing to the death of IPN patients.12,13
Therefore, to help prevent and reduce the incidence of MDRB infection and improve the prognosis of IPN patients, it is necessary to determine the incidence of MDRB infection in IPN patients, the common strains of MDRB infection, and the risk factors that are associated with MDRB infection.
Methods
Study Design and Setting
In this study, clinical data of patients with AP admitted to the general surgery department of Xuanwu Hospital of Capital Medical University between January 1, 2014, and December 31, 2021, were retrospectively analyzed. This study was reviewed and approved by the Ethical Review Committee of Xuanwu Hospital of the Capital Medical University (No.2020158). This study was designed in accordance with the principles of the Declaration of Helsinki (as revised in 2013). All patient data were anonymously analyzed using an electronic data acquisition system without informed consent. A detailed flowchart is illustrated in Figure 1.
Inclusion and Discharge Criteria
The inclusion criteria of patients were as follows: 1) AP patients with pancreatic and/or peripancreatic necrosis confirmed by imaging examination (enhanced CT/MRI, etc.) and pathogen culture positive, 2) Enhanced CT showed bubble sign in pancreatic necrotic tissue. The exclusion criteria: 1) Mild acute pancreatitis (MAP) or sterile pancreatic necrosis; 2) NP patients need emergency surgery; 3) Acute exacerbation of chronic pancreatitis or recurrent AP (RAP). The definitions of relevant clinical factors in this study are shown in Table 1.
 |
Table 1 Definitions of the Clinical Factors |
Pathogen Culture
Pathogenic culture and drug sensitivity tests were routinely performed in patients with suspected infection symptoms. Common culture samples included 1) necrotic pancreatic tissue obtained by puncture or surgery; 2) blood; 3) respiratory sputum; 4) urine; 5) abdominal puncture drainage fluid; 6) gallbladder puncture drainage fluid; 7) wound swab.
Patient Management
According to the current international guidelines,7 after admission, patients received routine administration of trypsin inhibitors, fluid resuscitation, analgesia, nutritional support, and other treatments, and antibiotics can only be used in patients with suspected or confirmed infection. Regular laboratory investigations (routine blood tests, blood biochemistry, inflammatory indicators, etc.) and imaging investigations (abdominal ultrasound or CT) were performed to monitor the patient’s disease development, and different therapeutic measures were taken according to the changes in the patient’s condition. If the patient’s condition improved, conservative treatment was continued. If the patient’s condition deteriorated (such as new-onset organ failure [NOF], IPN), a multidisciplinary team (MDT), including pancreatic surgeons, anesthetists, intensivists, and imaging specialists, collaborated and evaluated the patient. For patients with suspected or confirmed infection symptoms, third- and fourth-generation cephalosporins or carbapenem antibiotics were empirically administered, and the antibiotics were adjusted according to the results of drug sensitivity tests. Patients with suspected or confirmed NOF were provided with relevant organ support therapy (continuous pumping of vasoactive drugs, mechanical ventilation therapy [MVT], and continuous renal replacement therapy [CRRT]).
The indications for minimally invasive intervention in patients are as follows: 1) After active conservative treatment, the patient’s condition has no significant improvement or has continued deterioration (new organ failure or increased temperature and inflammatory indicators, etc.); 2) The presence of infected pancreatic necrosis is confirmed; 3) The range of necrosis in patients is enlarged, resulting in compression symptoms of surrounding organs (such as digestive tract or biliary tract obstruction). Our pancreatic surgeon has rich experience in laparoscopic necrotic tissue debridement. Pancreatic surgeons adopted different minimally invasive intervention methods according to the nature, site, and integrity of the necrotic material in patients, and the specific surgical methods have been discussed in our previous studies.19,20
Statistical Analysis
In this study, Excel 2018 (Microsoft, Redmond, CA, USA) was used to record the clinical data of patients, SPSS 23.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, United States) were used for statistical analysis. The Shapiro–Wilk test was used to evaluate whether the study data fit the normal distribution. Data with normal distribution were expressed as mean ± standard deviation (mean ± SD), and the differences between groups were analyzed using the independent sample t-test. Data of skewed distribution were presented in the form of the median (range), and between-group differences were analyzed using the rank sum test. Quantitative data were presented as rates, and differences between groups were analyzed using the chi-square test or Fisher’s exact probability method. Logistic regression was used to analyze the risk factors for MDR infection, and the odds ratio (OR) and 95% confidence interval (CI) were calculated; ROC curve analysis was employed to investigate the diagnostic ability of risk factors and to find the ideal cut-off value. P value <0.05 was considered statistically significant.
Results
A total of 267 IPN patients were included in this study, including 176 men and 92 women (average age: 50.72 ± 14.94 years). Among them, 144 patients had biliary pancreatitis, 78 had hyperlipidemia, and 45 other patients (10 patients with alcohol, 21 patients with unknown etiology, and 14 patients with postoperative endoscopic retrograde cholangiography [ERCP]). Depending on the results of pathogen culture in IPN patients, patients were divided into the MDRB infection group (n = 124) and the non-MDRB infection group (n = 143).
Baseline Data
There was no statistical difference between the two groups in sex, age, and etiology. In the MDRB group, the transfer time (30 [1–120] days vs 25 [1–150] days, P < 0.05), modified computer tomography severity index (CTSI) score (8 [4–10] vs 6 [2–10], P < 0.05), pancreatic necrosis degree, and PCT level (1.65 ± 1.39 ng/mL vs 0.82 ± 0.79 ng/mL, P < 0.05) were higher than those in the non-MDRB group. In the MDRB group, hemoglobin level (94.49 ± 32.05 g/L vs 112.36 ± 28.34 g/L, P < 0.05), hematocrit level (29.39 ± 6.78% vs 32.64 ± 8.31%, P < 0.05), and albumin level (27.76 ± 5.39 g/L vs 30.27 ± 6.55 g/L, P < 0.05) were lower than those in the non-MDRB group (Table 2).
 |
Table 2 Comparison of Baseline Data on Admission Between MDRB and Non-MDRB Groups |
Clinical Outcomes
In the clinical management of the MDRB group, the number of patients requiring combined nutritional support (80.65% vs 48.95%, P < 0.05), the parenteral nutrition (PN) support time (32.07 ± 26.92 days vs 20.71 ± 18.61 days, P < 0.05), the enteral nutrition (EN) support time (30.25 ± 27.44 days vs 20.07 ± 19.08 days, P < 0.05) days, the number of patients needing antibiotics treatment (88.71% vs 73.43%, P < 0.05), the number of patients requiring surgical intervention (88.71% vs 65.03%, P < 0.05), and the number of surgical interventions (2 [1–10] vs 2 [1–7] times, P < 0.05) were all higher than those in the non-MDRB group, and the differences were statistically significant (Table 3).
 |
Table 3 Clinical Management and Prognosis Comparison by Group |
In terms of clinical outcome, the in-hospital mortality (24.19% vs 13.99%; P < 0.05), the incidence of postoperative complications (23.39% vs 11.89%, P < 0.05), the incidence of postoperative new-onset organ failure (NOF) (11.29% vs 1.4%, P < 0.05), length of intensive care unit (ICU) stay (30.13 ± 28.67 days vs 17.94 ± 13.72 days, P < 0.05), and total length of hospital stay (49.98 ± 31.08 days vs 28.99 ± 21.45 days, P < 0.05) in the MDRB group were significantly higher than those in the non-MDRB group (P < 0.05) (Table 3).
Microbiologic Distribution
In terms of infection, patients in both groups had been diagnosed with IPN in other hospitals (35.48% vs 39.16%; P > 0.05), and the incidence of EPI (63.71% vs 34.27%, P < 0.05) and fungal infection (35.48% vs 19.58%, P < 0.05) in the MDRB group was significantly higher than that in the non-MDRB group. A total of 995 positive strains were cultured. Of these, 402 strains of pathogenic bacteria were cultured in the MDRB group, including 307 strains of MDRB bacteria and 95 strains of fungi. In the non-MDRB group, 593 strains of pathogenic bacteria were cultured, including 35 strains of fungi and 558 strains of bacteria. The most common gram-negative bacteria were Acinetobacter baumannii (n = 117), Pseudomonas aeruginosa (n = 118), Klebsiella pneumonia (n = 118), and Escherichia coli (n = 88). The incidence of MDRB bacteria was 72.65%, 32.20%, 66.95%, and 36.36%, respectively. The most common gram-positive bacteria were Enterococcus faecium (n = 98), Staphylococcus epidermidis (n = 57), and Staphylococcus hominis (n = 38), and the incidence of MDRB bacteria was 16.67%, 15.09%, and 11.43%, respectively. The most common fungal infection was Candida albicans (n = 47) (Table 4).
 |
Table 4 Microbiological Profile of Organisms in IPN Patients |
Risk Factor Analysis for MDRB Infection
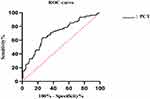
Table 5 shows the univariable and multivariable analyses of risk factors for MDR infection in the IPN patients. In univariable analysis, patients with long transfer time (OR: 1.011, 95% CI: 1.000–1.021, P = 0.044), high PCT level on admission (OR: 2.141, 95% CI: 1.557–2.994, P = 0.001), low hemoglobin (OR: 0.979, 95% CI: 0.965–0.994, P = 0.006), hematocrit (OR: 0.943, 95% CI: 0.908–0.980, P = 0.003) and albumin level (OR: 0.930, 95% CI: 0.868–0.996, P = 0.038), combined EPI (OR: 3.947, 95% CI: 2.240–6.957, P = 0.001), need antibiotic therapy (OR: 2.911, 95% CI: 1.425–5.946, P = 0.003) or surgical intervention (OR: 4.364, 95% CI: 2.080–9.156, P = 0.001) are more likely to develop MDR infection. Multivariable analysis identified combined EPI (OR: 4.116, 95% CI: 1.381–12.271, P = 0.011), the level of PCT at admission (OR: 2.728, 95% CI: 1.502–4.954, P = 0.001), and the degree of pancreatic necrosis (OR: 2.741, 95% CI: 1.109–6.775, P = 0.029) as the independent risk factors for MDRB infection in IPN patients (Table 5). The ROC analysis of the PCT level at admission found that when the PCT value was 1.055 ng/mL, the sensitivity was 0.636, the specificity was 0.741, and the ROC curve area was 0.705 (Figure 2).
 |
Table 5 Multivariable Analysis of Risk Factors for MDRB Infection in IPN Patients |
Discussion
In this study, we found that compared with the non-MDRB group, the prognosis of patients in the MDRB group was poor, which reconfirmed the view of previous studies.12,13 In addition, we identified the risk factors of MDRB infection in IPN patients and provided the references for clinicians to prevent and treat MDRB infection.
There were no between-group differences regarding age, sex, etiology, BMI, and other baseline characteristics of patients. The most common condition in the two groups was biliary pancreatitis, which is consistent with the results of our previous study.18−21, However, the incidence of hyperlipidemic pancreatitis showed an increasing trend, which may be related to improvements in living standards. Changes in dietary habits are also associated with a lack of exercise.23,24
The incidence of MDRB infection in this study was 46.44% (124/267), which was much higher than that in other studies (38.28% [80/209] and 29.50% [164/556]),12,13 and similar to Li et al (45.10% [23/51]),24 it may be caused by the different severity of the included patients (eg, AP, SAP, IPN) and clinical treatment measures (eg, early intensive care, minimally invasive interventions, application of antibiotics, etc.). Possible reasons are as follows: 1) Our hospital has a special acute pancreatitis diagnosis and treatment center, 91.76% of the patients (n = 245) are referred from other hospitals, local hospitals may lack experience in monitoring and treating local complications of severe AP patients, which more likely to have IPN and MDRB infections,25 the results of the present study also further support this view. 2) The high resistance of Acinetobacter baumannii in the environment and its ability to develop resistance to antibiotics have resulted in it becoming the main pathogen in nosocomial infections.26,27 3) More patients in the MDRB group required surgical intervention to control the disease than patients in the non-MDRB group. Treatment measures such as the placement of long-term indwelling drainage tubes after surgery, regular tube replacement, and tube lavage may also increase the incidence of MDRB infection.28 Therefore, clinicians should perform multiple and multi-site pathogen cultures to avoid prolonged empiric antibiotic administration. After identifying the pathogenic strains and sensitive antibiotics, timely antibiotic adjustment is necessary to reduce the time of empiric antibiotic administration to reduce the risk of MDR pathogen infection.
Our study findings suggest that in IPN patients, the presence of EPI and the high level of degree of pancreatic necrosis are risk factors for MDRB infection. The reasons may be as follows: 1) 63.71% of MDRB cases were complicated by EPI, and the time of EPI development patient with AP was less than that of patients with IPN.15,16,22,29,30 EPI pathogens were also slightly different from IPN pathogens.15,22,29 Antibiotics that are sensitive to pancreatic pathogens may not be sensitive to extra-pancreatic pathogens, the duration of antibiotic use increases, and multiple antibiotics are needed, which increases the risk of MDRB infection. 2) Studies have confirmed that the risk of pancreatic infection is positively correlated with the degree of pancreatic necrosis.31 Patients need multiple surgical interventions, which are more prone to postoperative complications, resulting in prolonged hospital stay and increased mortality.32,33 3) With the expansion of pancreatic necrosis, it is suggested that patients have more pancreatic acinar cell damage, and a large amount of inflammatory factors into the blood, causing SIRS, clinicians have the possibility of prophylactic application of antibiotics due to the suspicion of patient infection, which prone to develop MDRB infection.8,32,34 Therefore, for patients with suspected infection, clinicians should base their treatment strategy on the time of symptom onset, the latest imaging findings, changes in clinical symptoms (cough, sudden or persistent high fever, etc.), and whether different treatment modalities have been performed (placement of deep venous catheters or indwelling urinary catheters, etc.) to exclude EPI.
PCT has both hormonal and cytokine characteristics, and when patients have bacterial infection, PCT has the role of maintaining vascular endothelial tension. The level of PCT increases rapidly under the proinflammatory stimulation of bacterial origin and decreases after the successful anti-inflammatory treatment.35 In our previous study, we identified the PCT level as an independent risk factor for infection recurrence in patients with IPN after surgical intervention,36 and He et al’s study indicated that PCT could be used to predict the risk of critically severe AP (CAP) and IPN within 48 hours after hospital admission.37 Siriwardena et al showed that a procalcitonin-based algorithm (whether PCT levels ≥1.0ug/L) to guide antibiotic use in patients with acute pancreatitis (PROCAP) reduced antibiotic use and did not increase patient’s infection.8 This study found that patients with PCT levels higher than 1.055 ng/mL on admission had an increased risk of MDRB infection. Clinicians can perform PCT testing regularly to reduce the risk of infection.
However, there were some limitations of this study: 1) Our hospital is a large pancreatitis diagnosis and treatment center in northern China, and the patients admitted to our hospital are mainly those with moderate and severe acute pancreatitis, which may drive MDRB infection; 2) Most patients in this study (n = 245) were referral patients who had different treatments before admission (such as the types of empiric antibiotics used, nutritional support, surgical treatment, etc.), and these factors may have introduced bias into the statistical analysis; 3) In this study, only one patient received the endoscopic intervention, and the difference in prognosis and incidence of MDRB infection between endoscopic intervention and surgical intervention was not compared.
Conclusion
In conclusion, MDRB infections are increasing in IPN patients, with gram-negative bacteria being the most common causative pathogen. Clinicians can assess the risk of MDRB infection in patients based on whether IPN patients have EPI, the degree of pancreatic necrosis, and the level of PCT at admission.
Abbreviations
AP, acute pancreatitis; NP necrotizing pancreatitis; IPN infected pancreatic necrosis; EPI, extra-pancreatic infection; MDR multidrug-resistant; PCT procalcitonin; POF, persistent organ failure; SIRS systemic inflammatory response syndrome; NP, necrotizing pancreatitis; CT computed tomography; MAP mild acute pancreatitis; OF, organ failure; MDT, multidisciplinary team; MVT, mechanical ventilation therapy; CRRT, continuous renal replacement therapy; OR, odds ratio; CI, confidence interval; ROC, receiving operating characteristic; CTSI, computer tomography severity index; ERCP, endoscopic retrograde cholangiography; EN, enteral nutrition; PN, parenteral nutrition; NOF, new-onset organ failure; ICU, intensive care unit.
Ethics Approval and Consent to Participate
The clinical data of patients in this study were collected from the database of Xuanwu Hospital, Capital Medical University, and have been reviewed and approved by the Review Committee of Xuanwu Hospital, Capital Medical University (No. 2020-158). Because this was a retrospective study that only analyzed existing clinical and follow-up data, the need to obtain informed patient consent was waived.
Acknowledgments
We would like to thank Professor Chun-jing Bian and Professor Ang Li for their guidance in this manuscript.
Funding
The research was supported by 1. Beijing Municipal Science & Technology Commission (grant number: Z171100001017077); 2. Beijing Municipal Science and Technology Commission Clinical Diagnosis and Treatment Technology Research and Demonstration Application Project (grant number: Z191100006619038); 3. Capital Medical Development and Research Special Project (grant number: 2020-1-2012); 4. Construction Project of Clinical Advanced subjects of Capital Medical University (grant number: 1192070312); 5. Capital Medical Development and Research Special Project (grant number: Z201100005520090); 6. Beijing Medical Authority Cultivation Program (grant number: PX2022035).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382–390. doi:10.1001/jama.2020.20317
2. van Dijk SM, Hallensleben NDL, van Santvoort HC, et al. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66(11):2024–2032. doi:10.1136/gutjnl-2016-313595
3. Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi:10.1136/bmj.l6227
4. Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–734. doi:10.1016/S0140-6736(20)31310-6
5. Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology. 2019;156(7):1994–2007 e1993. doi:10.1053/j.gastro.2019.01.269
6. Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;2010(5):CD002941.
7. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
8. Siriwardena AK, Jegatheeswaran S, Mason JM; PROCAP Investigators. A procalcitonin-based algorithm to guide antibiotic use in patients with acute pancreatitis (PROCAP): a single-centre, patient-blinded, randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7(10):913–921. doi:10.1016/S2468-1253(22)00212-6
9. Andersson B, Andrén-Sandberg A, Nilsson J, Andersson R. Survey of the management of acute pancreatitis in surgical departments in Sweden. Scand J Gastroenterol. 2012;47(8–9):1064–1070. doi:10.3109/00365521.2012.685752
10. Lim C, Takahashi E, Hongsuwan M, et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. Elife. 2016;5. doi:10.7554/eLife.18082
11. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Supplement_2):S82–S89. doi:10.1086/499406
12. Jain S, Mahapatra SJ, Gupta S, Shalimar GPK, Garg PK. Infected pancreatic necrosis due to multidrug-resistant organisms and persistent organ failure predict mortality in acute pancreatitis. Clin Transl Gastroenterol. 2018;9(10):190. doi:10.1038/s41424-018-0056-x
13. Moka P, Goswami P, Kapil A, Xess I, Sreenivas V, Saraya A. Impact of antibiotic-resistant bacterial and fungal infections in outcome of acute pancreatitis. Pancreas. 2018;47(4):489–494. doi:10.1097/MPA.0000000000001019
14. Balthazar EJ, Ranson J, Naidich D, Megibow A, Caccavale R, Cooper M. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156(3):767–772. doi:10.1148/radiology.156.3.4023241
15. Pando E, Alberti P, Hidalgo J, et al. The role of extra-pancreatic infections in the prediction of severity and local complications in acute pancreatitis. Pancreatology. 2018;18(5):486–493. doi:10.1016/j.pan.2018.05.481
16. Brown LA, Hore TA, Phillips AR, Windsor JA, Petrov MS. A systematic review of the extra-pancreatic infectious complications in acute pancreatitis. Pancreatology. 2014;14(6):436–443. doi:10.1016/j.pan.2014.09.010
17. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
18. Lee HS, Lee SK, Park DH, et al. Emergence of multidrug resistant infection in patients with severe acute pancreatitis. Pancreatology. 2014;14(6):450–453. doi:10.1016/j.pan.2014.10.003
19. Li A, Cao F, Li J, et al. Step-up mini-invasive surgery for infected pancreatic necrosis: results from prospective cohort study. Pancreatology. 2016;16(4):508–514. doi:10.1016/j.pan.2016.03.014
20. Cao F, Duan N, Gao C, Li A, Li F. One-step verse step-up laparoscopic-assisted necrosectomy for infected pancreatic necrosis. Dig Surg. 2020;37(3):211–219. doi:10.1159/000501076
21. Zheng Y, Zhou Z, Li H, et al. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas. 2015;44(3):409–414. doi:10.1097/MPA.0000000000000273
22. Lu JD, Cao F, Ding YX, Wu YD, Guo YL, Li F. Timing, distribution, and microbiology of infectious complications after necrotizing pancreatitis. World J Gastroenterol. 2019;25(34):5162–5173. doi:10.3748/wjg.v25.i34.5162
23. Zhu Y, Pan X, Zeng H, et al. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised Atlanta classification in Jiangxi, China over an 8-year period. Pancreas. 2017;46(4):504–509. doi:10.1097/MPA.0000000000000776
24. Li X, Li L, Liu L, et al. Risk factors of multidrug resistant pathogens induced infection in severe acute pancreatitis. Shock. 2020;53(3):293–298. doi:10.1097/SHK.0000000000001371
25. Mallick B, Dhaka N, Sharma V, et al. Impact of timing of presentation of acute pancreatitis to a tertiary care centre on the outcome. Pancreatology. 2019;19(1):143–148. doi:10.1016/j.pan.2018.10.005
26. Cisneros JM, Rodríguez-Baño JJ. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Infection. 2002;8(11):687–693.
27. Wroblewska MM, Towner KJ, Marcher H, Luczak M. Emergence and spread of carbapenem-resistant strains of Acinetobacter baumannii in a tertiary-care hospital in Poland. Clin Microbiol Infect. 2007;13(5):490–496. doi:10.1111/j.1469-0691.2007.01694.x
28. van Grinsven J, van Santvoort HC, Boermeester MA, et al. Timing of catheter drainage in infected necrotizing pancreatitis. Nat Rev Gastroenterol Hepatol. 2016;13(5):306–312. doi:10.1038/nrgastro.2016.23
29. Zhou H-Y, Yuan Z, Du Y-P. Prior use of four invasive procedures increases the risk of Acinetobacter baumannii nosocomial bacteremia among patients in intensive care units: a systematic review and meta-analysis. Int J Infect Dis. 2014;22:25–30. doi:10.1016/j.ijid.2014.01.018
30. Besselink MG, van Santvoort HC, Boermeester MA, et al. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96(3):267–273. doi:10.1002/bjs.6447
31. Perez A, Whang EE, Brooks DC, et al. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25(3):229–233. doi:10.1097/00006676-200210000-00003
32. Sanden L, Paul M, Leibovici L, Andreassen S. Quantifying the associations between antibiotic exposure and resistance-a step towards personalised antibiograms. Eur J Clin Microbiol Infect Dis. 2016;35(12):1989–1996. doi:10.1007/s10096-016-2751-4
33. Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;1:47. doi:10.1186/2110-5820-1-47
34. Tan C, Yang L, Shi F, et al. Early systemic inflammatory response syndrome duration predicts infected pancreatic necrosis. J Gastrointest Surg. 2020;24(3):590–597. doi:10.1007/s11605-019-04149-5
35. Schuetz P, Mueller B. Procalcitonin-guided antibiotic stewardship from newborns to centennials. Lancet. 2017;390(10097):826–829. doi:10.1016/S0140-6736(17)31628-8
36. Gao -C-C, Li J, Cao F, et al. Infection recurrence following minimally invasive treatment in patients with infectious pancreatic necrosis. World J Gastroenterol. 2020;26(22):3087–3097. doi:10.3748/wjg.v26.i22.3087
37. He WH, Zhu Y, Zhu Y, et al. Comparison of multifactor scoring systems and single serum markers for the early prediction of the severity of acute pancreatitis. J Gastroenterol Hepatol. 2017;32(11):1895–1901. doi:10.1111/jgh.13803
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.