Back to Journals » International Journal of General Medicine » Volume 13
Risk Factors and Clinical Characteristics of Severe Fever with Thrombocytopenia Syndrome
Authors Wang F , Wu Y, Jiao J, Wang J, Ge Z
Received 18 November 2020
Accepted for publication 10 December 2020
Published 30 December 2020 Volume 2020:13 Pages 1661—1667
DOI https://doi.org/10.2147/IJGM.S292735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fei Wang,1 Yunjuan Wu,2 Jie Jiao,3 Jun Wang,1 Zheng Ge1
1Department of Hematology (Key Department of Jiangsu Medicine), Zhongda Hospital, Medical School, Southeast University, Institute of Hematology Southeast University, Nanjing 210009, People’s Republic of China; 2Department of Rheumatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, People’s Republic of China; 3Department of Nuclear Medicine, Nanjing First Hospital, Nanjing Medical University, Nanjing 210006, People’s Republic of China
Correspondence: Zheng Ge
Department of Hematology (Key Department of Jiangsu Medicine), Zhongda Hospital, Medical School, Southeast University, Institute of Hematology Southeast University, Number 87, Dingjiaqiao Street, Nanjing 210009, People’s Republic of China
Tel +86-25-83262468
Email [email protected]
Purpose: This study was to investigate the clinical characteristics and laboratory parameters of severe fever with thrombocytopenia syndrome (SFTS).
Patients and Methods: A detailed retrospective analysis of clinical records for SFTS patients was conducted. Fifty-one cases confirmed SFTS virus infected were enrolled. The clinical characteristics and laboratory parameters between survivors and non-survivors were analyzed.
Results: All patients aged between 30 and 80 years were farmers or residing in wooded and hilly areas. All patients occurred between April and October. The major clinical manifestations were fever, fatigue, diarrhea, myalgia, nausea and vomiting. Conscious disturbance, lymph node enlargement and hemorrhage were common. Fatal outcome occurred in 31.4% (16/51) of patients. Compared with survivors group, in non-survivors group, the proportion of consciousness disturbance, age, the levels of AST, LDH, Bun, Cr, PT and APTT were significantly increased, and PLT was significantly decreased. The age, PLT, AST, LDH, Cr, PT and APTT were the risk factors for fatal outcomes. Moreover, the age (OR, 1.245; 95% CI, 1.052– 1.474) and APTT (OR, 1.095; 95% CI, 1.005– 1.192) were the independent risk factors for fatal outcomes. Heteromorphic lymphocyte and hemophagocytosis could be found in SFTS patients, especially the proportion of finding hemophagocytosis was significantly higher in non-survivors group compared with survivors group.
Conclusion: These results suggest SFTS is a systemic infection, the age and APTT can be used as potential predictors referring to severe SFTS cases.
Keywords: severe fever with thrombocytopenia syndrome; SFTS, hemophagocytosis, laboratory parameters, risk factors
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease firstly described in rural areas of China in 2010, and caused by SFTS virus (SFTSV), a novel tick-borne virus classified into Bandavirus genus, Phenuiviridae family, Bunyavirales order.1,2 The disease has been recognized in Japan and South Korea since 2013.3,4 Recent years, SFTS cases has also been reported in Taiwan, Vietnam and Myanmar, indicating a broader global distribution.5–7 The major clinical manifestations of SFTS include acute fever (≥38°C), thrombocytopenia, leucopenia, gastrointestinal symptoms, central nervous system symptoms, even disseminated intravascular coagulation and multiple organ dysfunctions. The average mortality rate of SFTS is 16.2%, with higher risk to elder people in China.8 And Nanjing, located on the border between Jiangsu and Anhui provinces, is a high incidence area of SFTS.9 Therefore, We retrospectively analyze the SFTS patients at Zhongda hospital in Nanjing, between January 2013 and January 2019, summarize the clinical characteristics and laboratory parameters, and identify useful markers to predict disease severity.
Patients and Methods
Study Patients
A total of 51 laboratory-confirmed cases were from Zhongda hospital, and identified by Jiangsu Provincial Center for Disease Control and Prevention. The complete medical records were obtained and reviewed to analyze clinical characteristics and laboratory parameters. We also divided the patients into survivors group and non-survivors group, comparing clinical and laboratory features. This study was approved by the research ethics committee of Zhongda Hospital, Southeast University (Reference Number: 2020ZDSYLL226-P01) and performed in accordance with the principles of the Declaration of Helsinki. The requirement to obtain written informed consent from each patient was waived because this was an observational retrospective study. The patients’ information were anonymous and non identifiable.
Statistical Analysis
Data were represented as medians and ranges. Continuous variables were performed using Mann–Whitney U-tests and categorical variables were compared using Chi-square test or Fisher’s exact test (theoretical frequency <5). All significance tests were two-tailed and P values<0.05 were considered statistically significant. Binary logistic regression analysis was performed to identify the risk factors for mortality of SFTS, variables with P value<0.05 were entered into the multivariate model by method of enter. All analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical, Demographic and Epidemiologic Condition of Patients
All 51 SFTS patients aged between 30 and 80 years were farmers or residing in wooded and hilly areas. And all cases occurred between April and October (Table 1). Though we found a tick from one patient (Figure 1), most cases did not realize that they had been bitten.
 |
Table 1 Information and Clinical Characteristics of SFTS |
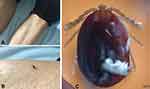 |
Figure 1 A patient bitten by a tick. The location of bite is under left knee (A and B); Tick morphology under microscope (×40) (C). |
Symptoms and Sign
As shown in Table 1, the major clinical manifestations of SFTS were fever (100%), fatigue (76.47%), diarrhea (49.02%), myalgia (47.06%), nausea (43.14%) and vomiting (31.37%). The symptoms of central nervous system characterized by conscious disturbance were found in 29 cases (56.86%). Lymph node enlargement and hemorrhage were common.
The proportion of consciousness disturbance was significantly higher in non-survivors group compared with survivors group, but other clinical manifestations showed no difference (Table 2).
 |
Table 2 Symptoms and Sign of SFTS Patients Between Survivors and Non-Survivors |
Laboratory Parameters
As shown in Table 3, the non-survivors group were found to have significantly increased age, and increased levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), prothrombin time (PT) and activated partial thromboplastin time (APTT), also have renal function depravation. Platelet count (PLT) was significantly decreased in non-survivors group.
 |
Table 3 Clinical Data of Survivors and Non-Survivors with SFTS Patients |
Risk Factors for Mortality in SFTS Patients
Univariate logistic regression analysis was performed to identify the risk factors for mortality of SFTS patients (Table 4). The age, PLT, AST, LDH, creatinine (Cr), PT and APTT were the risk factors for fatal outcomes. These variables were used for a multivariate logistic regression analysis, and the result indicated that the age (OR, 1.245; 95% CI, 1.052–1.474; P=0.011) and APTT (OR, 1.095; 95% CI, 1.005–1.192; P=0.038) were the independent risk factors for fatal outcomes.
 |
Table 4 Risk Factors of Mortality in SFTS Patients |
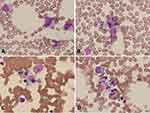
Morphological Changes of Bone Marrow in SFTS Patients
Bone marrow examinations were performed in 23 patients including 14 survivors and 9 non-survivors. Heteromorphic lymphocyte and hemophagocytosis could be found in SFTS patients (Figure 2.). And the proportion of heteromorphic lymphocyte and hemophagocytosis were more common in non-survivors group, especially the proportion of finding hemophagocytosis was significantly higher in non-survivors group compared with survivors group (Table 5.).
 |
Table 5 Heteromorphic Lymphocyte and Hemophagocytosis in Patients’ Bone Marrow |
Discussion
SFTSV has been detected in patients from China, South Korea, Japan, Vietnam and Myanmar in recent years. Generally, tick bite is a risk factor associated with SFTSV infection. However, many cases are unclear whether they had tick bite histories because of being unfamiliar with ticks or painless bites.10 Although most cases occur through tick bites, clusters of SFTS in family member or healthcare personnel also have been reported. Person-to-person transmission of SFTSV occurs rarely through contact with infected blood, bloody respiratory secretions, cadaveric blood11,12 and probable aerosol,13,14 which highlight the importance of adding universal precaution including airborne precaution and full personal protective equipment.
Like other studies,15 except for abnormal blood routine, our data also have shown alanine aminotransferase (ALT), AST, LDH, creatine phosphokinase (CK), D-Dimer, APTT and serum ferritin are elevated. They are all useful laboratory markers though lack specificity. SFTS is easily misdiagnosed as hemorrhagic fever with renal syndrome because of similar clinical features characterized by fever, diarrhea, myalgia, thrombocytopenia and abnormal coagulation.16 However, SFTS has its own characteristics. In our data, all SFTS cases occurred from April to October, about a half patients had consciousness disturbance. Significantly, SFTS rarely induced severe kidney injury, only 13 of 51 patients suffered from renal dysfunction, most of them were in non-survivor group, complicated with multiple organ dysfunction syndrome. Besides, owing to leukopenia, thrombocytopenia and abnormal coagulation function, some SFTS patients can be suspected of leukemia, especially hypoproliferative acute promyelocytic leukemia. However, acute leukemia patients usually does not have extremely elevated ALT, AST and CK, which are markers indicating liver and muscle injury. And according to the result of bone marrow aspirate, these two diseases can be finally identified.
Ding et al reported the incidence rate of SFTS was significantly higher in patients over 40 years old and fatal cases only occurred in patients over 50-years-old.17 This is consistent with our data. Moreover, APTT, as another independent risk factors for fatal outcomes, indicated the coagulation disorder caused by SFTS virus might be related to disease severity.
SFTSV can inhibit the host immune response, leading to rapid virus replication, affect multiple organs, causing conscious disturbance, liver dysfunction, coagulation dysfunction and rhabdomyolysis. The mechanism remains unclear, potential reason may include direct organ invasion by virus and immune mediated inflammatory process.18,19 SFTSV can target microvascular endothelium, resulted in hyperpermeability owning to the disruption of intercellular junction, causing cytokine storm.20 SFTSV-infected endothelium also can capture circulating platelets, adhere to white blood cells and transmigrate them into interstitial space, contributing to leukopenia and thrombocytopenia.21
Some studies suggest cytokine storm might be associated with disease severity.22,23 For instance, many cytokines are significantly higher in SFTS patients than in healthy controls.23 SFTSV infection-induced cytokine storm indirectly triggers consciousness disturbance.24 More than half of patients suffered consciousness disturbance in our data. In clinical settings, steroid pulse therapy and plasma exchange are widely used to suppress excessive cytokine production, showing some visible effects.24,25
Meanwhile, cytokine storm can contribute to hemophagocytic lymphohistiocytosis (HLH).4,26 Takahashi et al reported that five SFTS patients who underwent bone marrow aspirate, all exhibited hemophagocytosis which was also observed in lymph nodes and spleen.4 Jung et al suggested SFTS cases complicated by HLH have a worse prognosis.27 In our data, bone marrow aspirate was performed in 23 patients. Heteromorphic lymphocyte can be found in more than half of surviving patients. More hemophagocytosis occurs in death group. Therefore, HLH might be a critical pathogenesis in fatal cases of SFTS.28 Recent study showed SFTSV could adhere to platelets and facilitate the phagocytosis of platelet by mouse primary macrophages.29 Furthermore, SFTSV RNA could be detected in cytoplasm of phagocytosing macrophages in bone marrow, liver, and spleen during the autopsy process, indicating that SFTSV infected macrophages may induce hemophagocytosis and cause a cytokine storm, which leads to viral hemorrhagic fever, even death.28 Therefore, bone marrow examination should be done in patients to identify hemophagocytosis.
This study had some limitations. First, the sample size was small, which limited the accuracy of the observation and conclusion. Second, we had not the data of viral load, so that we could not analyze the impact of viral load on survival. Third, the hospital collecting cases was the local central hospital, many cases were transferred from county hospitals and were in serious condition, so the mortality (31.4%) was higher than other reports (16.2%).8
Conclusions
SFTS is characterized by disturbance of consciousness, myalgia, abnormal coagulation function and rare severe renal injury. The age and APTT are useful markers for fatal cases. As a prevalent endemic disease in East Asia accompanied by high mortality, the identified clinical and laboratory parameters may predict fatal outcome, and new treatment strategies such as effective vaccine or anti-inflammatory therapy are needed.
Acknowledgments
This work was supported by Jiangsu Provincial Medical Youth Talent (QNRC2016812), Natural Science Foundation of Jiangsu Province for Youth (BK20180372) and Key Medical of Jiangsu Province (ZDXKB2016020).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–1532. doi:10.1056/NEJMoa1010095
2. Walker PJ, Siddell SG, Lefkowitz EJ, et al. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020). Arch Virol. 2020;165(11):2737–2748. doi:10.1007/s00705-020-04752-x
3. Kim KH, Yi J, Kim G, et al. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19(11):1892–1894. doi:10.3201/eid1911.130792
4. Takahashi T, Maeda K, Suzuki T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014;209(6):816–827. doi:10.1093/infdis/jit603
5. Tran XC, Yun Y, Van An L, et al. Endemic Severe Fever with Thrombocytopenia Syndrome, Vietnam. Emerg Infect Dis. 2019;25(5):1029–1031. doi:10.3201/eid2505.181463
6. Lin TL, Ou SC, Maeda K, et al. The first discovery of severe fever with thrombocytopenia syndrome virus in Taiwan. Em Microbes Infect. 2020;9(1):148–151. doi:10.1080/22221751.2019.1710436
7. Win AM, Nguyen YTH, Kim Y, et al. Genotypic Heterogeneity of Orientia tsutsugamushi in Scrub Typhus Patients and Thrombocytopenia Syndrome Co-infection, Myanmar. Emerg Infect Dis. 2020;26(8):1878–1881. doi:10.3201/eid2608.200135
8. Li H, Lu QB, Xing B, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: a prospective observational study. Lancet Infect Dis. 2018;18(10):1127–1137. doi:10.1016/s1473-3099(18)30293-7
9. Sun J, Lu L, Wu H, Yang J, Ren J, Liu Q. The changing epidemiological characteristics of severe fever with thrombocytopenia syndrome in China, 2011–2016. Sci Rep. 2017;7(1):9236. doi:10.1038/s41598-017-08042-6
10. Sun J, Gong Z, Ling F, et al. Factors associated with Severe Fever with Thrombocytopenia Syndrome infection and fatal outcome. Sci Rep. 2016;6:33175. doi:10.1038/srep33175
11. Jung IY, Choi W, Kim J, et al. Nosocomial person-to-person transmission of severe fever with thrombocytopenia syndrome. Clin Microbiology Infection. 2019;25(5):
12. Chen H, Hu K, Zou J, Xiao J. A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome bunyavirus. Int j Infect Dis. 2013;17(3):e206. doi:10.1016/j.ijid.2012.11.006
13. Moon J, Lee H, Jeon JH, et al. Aerosol transmission of severe fever with thrombocytopenia syndrome virus during resuscitation. Infect Control Hospital Epidemiology. 2018;19:1–4. doi:10.1017/ice.2018.330
14. Gong Z, Gu S, Zhang Y, et al. Probable aerosol transmission of severe fever with thrombocytopenia syndrome virus in southeastern China. Clin Microbiology Infection. 2015;21(12):1115–1120. doi:10.1016/j.cmi.2015.07.024
15. Kim UJ, Oh TH, Kim B, et al. Hyperferritinemia as a Diagnostic Marker for Severe Fever with Thrombocytopenia Syndrome. Dis Markers. 2017:6727184. doi:10.1155/2017/6727184
16. Qi R, Qin XR, Wang L, et al. Severe fever with thrombocytopenia syndrome can masquerade as hemorrhagic fever with renal syndrome. PLoS Negl Trop Dis. 2019;13(3):e0007308. doi:10.1371/journal.pntd.0007308
17. Ding S, Niu G, Xu X, et al. Age is a critical risk factor for severe fever with thrombocytopenia syndrome. PLoS One. 2014;9(11):e111736. doi:10.1371/journal.pone.0111736
18. Kim MG, Jung J, Hong SB, et al. Severe Fever with Thrombocytopenia Syndrome Presenting with Rhabdomyolysis. Infect Chem. 2017;49(1):68–71. doi:10.3947/ic.2017.49.1.68
19. Liu J, Wang L, Feng Z, Geng D, Sun Y, Yuan G. Dynamic changes of laboratory parameters and peripheral blood lymphocyte subsets in severe fever with thrombocytopenia syndrome patients. Int j Infect Dis. 2017;58:45–51. doi:10.1016/j.ijid.2017.02.017
20. Li XK, Zhang SF, Xu W, et al. Vascular endothelial injury in severe fever with thrombocytopenia syndrome caused by the novel bunyavirus. Virology. 2018;520:11–20. doi:10.1016/j.virol.2018.05.001
21. Li XK, Yang ZD, Du J, et al. Endothelial activation and dysfunction in severe fever with thrombocytopenia syndrome. PLoS Negl Trop Dis. 2017;11(8):e0005746. doi:10.1371/journal.pntd.0005746
22. Sun Y, Jin C, Zhan F, et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis. 2012;206(7):1085–1094. doi:10.1093/infdis/jis452
23. Liu MM, Lei XY, Yu H, Zhang JZ, Yu XJ. Correlation of cytokine level with the severity of severe fever with thrombocytopenia syndrome. Virol J. 2017;14(1):6. doi:10.1186/s12985-016-0677-1
24. Nakamura S, Azuma M, Maruhashi T, et al. Steroid pulse therapy in patients with encephalopathy associated with severe fever with thrombocytopenia syndrome. J Infect Chem. 2018;24(5):389–392. doi:10.1016/j.jiac.2017.11.004
25. Oh WS, Yoo JR, Kwon KT, et al. Effect of Early Plasma Exchange on Survival in Patients with Severe Fever with Thrombocytopenia Syndrome: A Multicenter Study. Yonsei Med J. 2017;58(4):867–871. doi:10.3349/ymj.2017.58.4.867
26. Lee J, Jeong G, Lim JH, et al. Severe Fever with Thrombocytopenia Syndrome Presenting with Hemophagocytic Lymphohistiocytosis. Infect Chem. 2016;48(4):338–341. doi:10.3947/ic.2016.48.4.338
27. Jung IY, Ahn K, Kim J, et al. Higher Fatality for Severe Fever with Thrombocytopenia Syndrome Complicated by Hemophagocytic Lymphohistiocytosis. Yonsei Med J. 2019;60(6):592–596. doi:10.3349/ymj.2019.60.6.592
28. Nakano A, Ogawa H, Nakanishi Y, et al. Hemophagocytic Lymphohistiocytosis in a Fatal Case of Severe Fever with Thrombocytopenia Syndrome. Int Med. 2017;56(12):1597–1602. doi:10.2169/internalmedicine.56.6904
29. Jin C, Liang M, Ning J, et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci U S A. 2012;109(25):10053–10058. doi:10.1073/pnas.1120246109
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.