Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Risk assessment of aggressive behavior in Chinese patients with schizophrenia by fMRI and COMT gene
Authors Tang X, Jin J, Tang Y, Cao J, Huang J
Received 1 November 2016
Accepted for publication 19 December 2016
Published 7 February 2017 Volume 2017:13 Pages 387—395
DOI https://doi.org/10.2147/NDT.S126356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Xiaoli Tang,1 Jun Jin,1 Yi Tang,1 Jinbo Cao,1 Junjie Huang2
1Department of Radiology, Shekou People’s Hospital, Shenzhen, 2Department of Medicine, Shenzhen University, Guangdong, People’s Republic of China
Background: Blood–oxygen-level dependent functional magnetic resonance imaging (BOLD-fMRI) maps cerebral activity by the hemodynamic response. Catechol-O-methyltransferase (COMT) gene is involved in the metabolism of dopamine. It is reported that both of these can be used to assess the aggression risk in patients with schizophrenia. However, these methods to assess the aggression risk patients with schizophrenia have not been established in China. Therefore, we deliver here a systematic review and meta-analysis based on the studies dealing with Chinese patients.
Method: Nine fMRI studies and 12 gene studies were included. The data of each study were extracted and summarized. Odds ratios with 95% confidence intervals were estimated on allele, dominant, and recessive models. Publication bias was evaluated by Begg’s funnel plot.
Results: Positive BOLD-fMRI values in the lower central neural system (CNS) and negative values in the high-level CNS were observed in the patients with aggression risk. A strong association was derived from the recessive gene model of COMT polymorphism rs4680 and risk in aggression behavior (odds ratio =2.10). No significant publication bias was identified.
Conclusion: Aggression behavior in patients with schizophrenia can be indicated by positive BOLD-fMRI values in the lower CNS and negative values in the high-level CNS and by a recessive gene model in COMT polymorphism rs4680. A combined test of fMRI and COMT gene could increase the predictive value.
Keywords: aggression, schizophrenia, Chinese, fMRI, COMT, meta-analysis
Introduction
Physical aggression behavior is one of the most common clinical manifestations in patients with schizophrenia. It can lead to awful clinical and social consequences.1 A significant association was identified between physical aggression and a high risk of longer hospital stays and a criminal conviction.2 In China, a large-scale review shows that the prevalence of schizophrenia was approximately 951 per 100,000.3 The risk of aggression was ~20%–40% in Chinese patients with schizophrenia, and there is an urgent need to establish operational systems for preventing the aggression of these patients.4
Functional magnetic resonance imaging (fMRI) is a functional neuroimaging procedure using MRI technique to evaluate neural activation by detecting the corresponding changes of blood flow.5 This technology is based on the fact that brain blood flow and neuronal activity are linked.6 When the hemodynamic response increases in an area, this area of the brain is being activated.7 Blood–oxygen-level dependent (BOLD) contrast is a type of specialized scan adopted to map cerebral activity by imaging the hemodynamic response associated with energy use by brain cells.8 Previous studies were reported to describe the neural characteristics on the aggressive behavior using BOLD-fMRI.9–12
Human catechol-O-methyltransferase (COMT) gene is located on the long arm of chromosome 22 (22q 11.2).13 It is one of the genes involved in the metabolism of dopamine and noradrenaline in the brain.14 There is a common single nucleotide polymorphism containing a Val (valine) to Met (methionine) substitution at codon 158 of the COMT gene (rs4680).15 COMT activity can be regulated by COMT gene polymorphism.16 The Val allele at this locus results in inactive COMT gene expression, whereas the Met allele decreases the level of COMT gene activity.17 In recent years, some studies show that the schizophrenia candidate gene COMT plays an important role in violent attacks.18–22
There are a few Chinese-based researches investigating the role of fMRI23–31 and COMT polymorphism rs468032–39 in schizophrenia with regard to aggressive behavior. However, the results of these studies were inconsistent and can be affected by the small sample sizes and the differences in sex, age, ethnicity, region, source of control, evaluation tool, and the study quality. Trying to clarify this issue, we provide a systematic review and a quantitative synthesis of data from different studies. To the best our knowledge, this is the first review and meta-analysis of the association between fMRI, COMT gene polymorphism rs4680, and violent behavior focused on Chinese population.
Methods
Search strategy and inclusion criteria
A systematic literature retrieve was taken from PubMed, Medline, CNKI, and the Wanfang databases (up to October 1, 2016) to obtain all eligible BOLD-fMRI studies on the violent behavior in Chinese population by adopting the search strategy: (“BOLD” OR “functional magnetic resonance imaging”) AND (“aggression” OR “violence” OR “impulsive” OR “attack”). The included publications meet the following criteria: 1) the studies on an assessment of the association between cerebral activity and aggression risk, 2) detailed information of the study is provided, 3) the experiments are based on Chinese population, and 4) the aggression behavior is defined as physical aggression against others or making threatening gestures before admission. The following studies were excluded: 1) it is not an original investigation, for example, reviews and comments; 2) the report has insufficient data; and 3) the reported data are duplicated.
To perform the meta-analyses for the association between gene polymorphisms of COMT and susceptibility to violent behavior in patients with schizophrenia in Chinese population, a further systematic literature retrieve was taken from PubMed, Cochrane, Google Scholar, CNKI, and the Wanfang databases (up to March 1, 2016) to obtain all eligible studies by adopting the search strategy: (“COMT” OR “Catechol-O-methyltransferase”) AND (“polymorphism” OR “allele” OR “mutation” OR “variants”) AND (“risk” OR “susceptibility” OR “results”) AND (“aggression” OR “violent” OR “attack”). The included publications meet the following criteria: 1) it is an assessment of the association between COMT gene polymorphisms and aggression susceptibility, 2) the experiments must be case–control study designed, 3) detailed genotype frequencies of the cases and controls are provided, and 4) the aggression behavior is defined as physical aggression against others or making threatening gestures before admission. The following studies were excluded: 1) the studies without case–control study design, for example, reviews, comments, and case-only study; 2) the studies with insufficient data; and 3) the reported data are duplicated.
Data extraction and quality assessment
The data were obtained and examined by two independent investigators. Any disagreement was discussed before a consensus was reached. The name of the first author, publication year, region of the studies, aggression evaluation tools, age, sex and ethnicity of cases, source of controls, and number of cases and controls were extracted from each study. For the fMRI studies, the diagnoses and study results were additionally reviewed. The quality of the case–control gene study was also scored by two independent investigators according to the Newcastle–Ottawa scale (NOS).40 As a result, these studies can be divided into a very high quality group (score =9), and lower quality group (score <9). Any disagreement was settled by discussions.
Statistical analysis
The studies of fMRI are summarized in a table and used in this review. The meta-analyses of COMT studies were performed using the STATA 14.0 (Stata Corporation, College Station, TX, USA). The relationship between the COMT polymorphisms and the aggression behavior susceptibility was assessed by applying the pooled odds ratios (ORs) and 95% confidence intervals (CIs) on allele (Met vs Val), dominant (Met/Met + Met/Val vs Val/Val), and recessive (Met/Met vs Met/Val + Val/Val) models. The P-values of Hardy–Weinberg equilibrium of control groups were obtained by the chi-square test for the genotype distribution. A χ2-test-based Q statistic test was used to estimate the heterogeneity within the enrolled studies. If the Q-test (P>0.1) shows homogeneity across studies, the fixed effects model would be applied.41 Otherwise, the random effects model would be selected.42 In addition, the sources of heterogeneity were explored by the subgroups of age, sex, ethnicity, region, quality, evaluation tool, and source of control. Potential publication bias was evaluated by Begg’s funnel plot.
Results
Characteristics of the studies
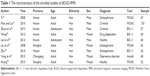
As presented in Figure 1, after a comprehensive literature retrieve from the databases, a total of 55 BOLD-fMRI studies on the brain activity of aggression were initially found. Following a scan of the abstracts, 38 irrelevant studies were excluded as they were non-fMRI studies, nonaggression studies, or reviews. Eight further articles were also excluded after reading the full article, as they were studies of magnetic resonance spectroscopy-fMRI, non-Chinese populations, or without eligible sample data. Finally, nine studies were enrolled in this review (Table 1). Of these studies, six were conducted in the regions of Hunan, two in Shanghai, and one in Chongqing in China and all the study subjects were of the Han population. Among the nine studies of BOLD-fMRI, two dealt with adolescents and others with adults. Five studies were male based and the rest were mixed-gender based. Different tools to diagnose the violent behavior are adopted, including the Modified Overt Aggression Scale (MOAS), 11-item Barratt’s Impulsivity Scale, and Amold Scale.
  | Figure 1 Flowchart displaying the selection procedure of fMRI studies. |
Figure 2 illustrates the studies of COMT gene polymorphism. Totally, 87 records were initially identified as eligible. Following the scan of the abstracts, 73 irrelevant studies were excluded as they were nonpolymorphism studies, non-case–control studies, or reviews. Six further articles were also excluded after reading the full article, for they were studies of other polymorphisms in COMT, the populations were non-Chinese, or without eligible samples data. Finally, eight publications containing 12 independent case–control studies with totally 826 cases and 1,201 controls were included in this meta-analysis. As shown in Table 2, ethnicities are categorized as the Han (11 studies) and the Uigur (1 study). Of them, only one dealt with children and females, and all other studies dealt with adults and males. Two were population-based, which means that the sources of control were healthy people. Others were hospital-based, meaning the controls were nonviolent patients with schizophrenia. The evaluation tools included the Overt Aggression Scale (OAS), MOAS, Achenbach Scale, and self-designed medical chart.
  | Figure 2 Flowchart displaying the selection procedure of COMT studies. |
Results of the meta-analysis
As described in Table 3, moderate heterogeneity was observed in the allele and dominant gene models (P=0.06, P=0.05, respectively). Random effects models were adapted to calculate the overall ORs and CIs of these two models. Contrarily, no heterogeneity was identified in the recessive model (P=0.86). Therefore, the fixed effects model was used in the analysis of this gene model.
Allele (Met vs Val)
Generally, no significant association was identified between this model in patients and susceptibility of aggression (OR =0.97, P=0.754). Similarly, no difference was seen in the analysis of the variants of COMT and aggression risk in the subgroup analysis regarding to sex, age, ethnicity, region, and evaluation tools (P>0.05). However, in the subgroup analysis by the source of control, an association was found in the population-based studies (OR =0.66, P=0.026), but not in the hospital-based studies (P=0.648). Interestingly, in the subgroups analysis on study quality, an increased susceptibility to aggression was shown in the high quality (NOS =9) studies (OR =1.34, P=0.048), whereas a decreased susceptibility was found in the lower quality (NOS <9) studies (OR =0.84, P=0.040).
Dominant (Met/Met + Met/Val vs Val/Val)
A negative relationship was found between COMT polymorphism and aggression risk in this gene model by overall estimation, although it was not statistically significant (OR =0.89, P=0.410). In the subgroups by sex, age, ethnicity, and region, no significant result was found. Similar to the allele model, a correlation was identified in the subgroup by population-based controls (OR =0.53, P=0.005) and lower quality group (OR =0.67, P=0.021) in this dominant gene model. In addition, the studies using self-designed evaluation tools also showed a decreased susceptibility to aggression with the polymorphism in this model (OR =0.75, P=0.010).
Recessive (Met/Met vs Met/Val+Val/Val)
There is a positive association between mutant type of COMT and the susceptibility to violent behavior (OR =1.16, P=0.408) in this model, although it was not statistically significant. No significant result was observed in any of these subgroups by sex, age, ethnicity, region, source of control, evaluation tool, and study quality either. However, the P-value of the statistical test of high quality studies was very close to the borderline and it showed a strong association between COMT polymorphism rs4680 and aggression in this recessive gene model (OR =2.10, P=0.064).
Quality assessment and publication bias
The quality assessment of the included gene studies is summarized in Table 4. Totally, five publications with very good quality (NOS =9), two with good quality (NOS =8), and five with lower quality (NOS =7, 6) were assessed. All were qualified to be included in this analysis, suggesting the reliability of this analysis. The Begg’s funnel plot presented a symmetrical and funnel-like distribution of the dots in the diagram, indicating that the publication bias risk was low in this analysis (Met vs Val, Figure 3).
Discussion
In the meta-analysis, although no significant result was observed in the overall estimation of all three models, moderate heterogeneity was found in the allele and dominant gene models. Subgroup analysis was conducted to identify the sources of heterogeneity and to avoid the bias produced by the overall estimation. The source of control, evaluation tool, and study quality were considered to be the main factors that caused heterogeneity in this meta-analysis. The studies using population-based control, self-designed evaluation tool, and low quality method showed a negative association between the target polymorphism and aggression. Contrarily, positive results were observed in the hospital-based, standard tool applied, and good quality studies that were more reliable to draw conclusions. To confirm this deduction, three gene models including allele, dominant, and recessive models were compared in the subgroup analysis by study quality referred to NOS. It was found that all the studies with very high quality (NOS =9) showed an increased susceptibility to violent behavior with the mutant type COMT gene polymorphism rs4680 Met. By combining these evidences, it seems that this polymorphism is more likely to increase the aggression risk in patients with schizophrenia by the recessive model (Met/Met vs Met/Val + Val/Val, OR =2.10) as the ORs were higher than those of the other two models. However, all the results from the three models could not reach a statistically significant level (P>0.05). Multiple factors responsible for these results might be considered: the limited sample size and number of enrolled studies. This conclusion is consistent with a recent meta-analysis of this same topic in Caucasian population by Bhakta et al.43
From the results of the BOLD-fMRI studies on violent behaviors, it is shown that an increased cerebral activity was observed in the superior frontal gyrus, right superior temporal gyrus, left temporal lobe, insula lobe, cingulate gyrus, parahippocampal gyrus, thalamus, and brainstem, whereas a decreased cerebral activity was in the prefrontal lobe, temporal lobe, frontal gyrus, right middle occipital gyrus, lingual gyrus, prefrontal-temporal-limbic circuits, and pons (Table 5). Despite some exceptions, these results suggest that the aggression behavior is identified by positive BOLD-fMRI values in the lower central neural system (CNS) and negative values in the high-level CNS. Previous studies have shown that fMRI values were depend on the COMT polymorphism rs4680 in noise characteristics, working memory and plan, abstinence challenge, pain stimulation, and Parkinson’s disease.44–48 Considering the results mentioned earlier that the COMT rs4680 polymorphism increases aggression risk in patients with schizophrenia by the recessive model and the BOLD-fMRI is able to identify the aggression behavior risk by measuring the cerebral activity in the high-level and low-level CNS, it seems reasonable to combine both the methods to increase the predictive value of the risk of aggression behavior with respect to specificity and sensitivity. Certainly, a well-designed study is needed to elaborate the relationship between fMRI and COMT polymorphism rs4680 and their combined predictive value in aggression risk in patients with schizophrenia.
  | Table 5 The results of the BOLD-fMRI studies on participants with aggression |
Despite the systematic review and meta-analysis have several advantages in some aspects, the limitations are to be mentioned. 1) As the number of fMRI studies was so limited, we can only conduct a qualitative review rather than a quantitative analysis. The conclusion of this review needs to be carefully interpreted. 2) In terms of the COMT studies, we failed to analyze the gene-environmental effects since the susceptibility to physical aggression may be influenced by interactions between individual genes and environment. 3) The possible influence of some clinical variables on the association of fMRI values or COMT polymorphism with aggression in patients with schizophrenia were not considered, such as antipsychotic treatment, duration of illness, the sampling effect in different stages of disease progression, and comorbid with substance abuse including smoking or alcohol drinking. Further high-quality and larger sample-sized studies are required to confirm the conclusion of this review and meta-analysis.
In conclusion, our review and meta-analysis indicate that there is a relationship among violent behavior in patients with schizophrenia, positive BOLD-fMRI values in the lower CNS and negative values in the high-level CNS, as well as a recessive gene model in COMT polymorphism rs4680. Hence, a combined test of fMRI and COMT gene might increase the predictive value and furthermore, help develop informative strategies for preventing aggression in patients with schizophrenia.
Acknowledgment
We thank Prof Liu Yuefei for his help in English language editing of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Swanson JW, Van Dorn RA, Swartz MS, Smith A, Elbogen EB, Monahan J. Alternative pathways to violence in persons with schizophrenia: the role of childhood antisocial behavior problems. Law Hum Behav. 2008;32(3):228–240. | ||
Kling RN, Yassi A, Smailes E, Lovato CY, Koehoorn M. Evaluation of a violence risk assessment system (the Alert System) for reducing violence in an acute hospital: a before and after study. Int J Nurs Stud. 2011;48(5):534–539. | ||
Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–2005: an epidemiological survey. Lancet. 2009;373(9680):2041–2053. | ||
Chen Q, Zhou J. [Aggression of Chinese inpatients with schizophrenia: a systematic literature review]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(7):752–756. Chinese. | ||
Ramsey NF, Kirkby BS, Van Gelderen P, et al. Functional mapping of human sensorimotor cortex with 3D BOLD fMRI correlates highly with H2(15)O PET rCBF. J Cereb Blood Flow Metab. 1996;16(5):755–764. | ||
Thulborn KR. A BOLD move for fMRI. Nat Med. 1998;4(2):155–156. | ||
Baudendistel KT, Reichenbach JR, Metzner R, Schroeder J, Schad LR. Comparison of functional MR-venography and EPI-BOLD fMRI at 1.5 T. Magn Reson Imaging. 1998;16(8):989–991. | ||
Hoogenraad FG, Pouwels PJ, Hofman MB, Reichenbach JR, Sprenger M, Haacke EM. Quantitative differentiation between BOLD models in fMRI. Magn Reson Med. 2001;45(2):233–246. | ||
Caffrey MK, Nephew BC, Febo M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology. 2010;58(1):107–116. | ||
Febo M, Ferris CF. Oxytocin and vasopressin modulation of the neural correlates of motivation and emotion: results from functional MRI studies in awake rats. Brain Res. 2014;1580:8–21. | ||
Kose S, Steinberg JL, Moeller FG, et al. Neural correlates of impulsive aggressive behavior in subjects with a history of alcohol dependence. Behav Neurosci. 2015;129(2):183–196. | ||
Brunnlieb C, Nave G, Camerer CF, et al. Vasopressin increases human risky cooperative behavior. Proc Natl Acad Sci U S A. 2016;113(8):2051–2056. | ||
Winqvist R, Lundstrom K, Salminen M, Laatikainen M, Ulmanen I. The human catechol-O-methyltransferase (COMT) gene maps to band q11.2 of chromosome 22 and shows a frequent RFLP with BglI. Cytogenet Cell Genet. 1992;59(4):253–257. | ||
Olanow CW, Obeso JA. Pulsatile stimulation of dopamine receptors and levodopa-induced motor complications in Parkinson’s disease: implications for the early use of COMT inhibitors. Neurology. 2000;55(11 Suppl 4):S72–S77. | ||
Bilder RM, Volavka J, Czobor P, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52(7):701–707. | ||
Lichtenberg P, Bachner-Melman R, Gritsenko I, Ebstein RP. Exploratory association study between catechol-O-methyltransferase (COMT) high/low enzyme activity polymorphism and hypnotizability. Am J Med Genet. 2000;96(6):771–774. | ||
Wacker J, Gatt JM. Resting posterior versus frontal delta/theta EEG activity is associated with extraversion and the COMT VAL(158)MET polymorphism. Neurosci Lett. 2010;478(2):88–92. | ||
Strous RD, Nolan KA, Lapidus R, Diaz L, Saito T, Lachman HM. Aggressive behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: a replication study. Am J Med Genet Neuropsychiatr Genet. 2003;120B(1):29–34. | ||
Flory JD, Xu K, New AS, Finch T, Goldman D, Siever LJ. Irritable assault and variation in the COMT gene. Psychiatr Genet. 2007;17(6):344–346. | ||
Vevera J, Stopkova R, Bes M, et al. COMT polymorphisms in impulsively violent offenders with antisocial personality disorder. Neuro Endocrinol Lett. 2009;30(6):753–756. | ||
Grigorenko EL, De Young CG, Eastman M, et al. Aggressive behavior, related conduct problems, and variation in genes affecting dopamine turnover. Aggress Behav. 2010;36(3):158–176. | ||
Singh JP, Volavka J, Czobor P, Van Dorn RA. A meta-analysis of the Val158Met COMT polymorphism and violent behavior in schizophrenia. PLoS One. 2012;7(8):e43423. | ||
Yi J. The fMRI study of male achizophrenic patients with aggressive behavior in resting state. CNKI-J Ct Sth University. 2008;531(1):1–39. | ||
Mao XW, Liu JL. Facial emotion recognition in violent people-a functional magnetic resonance study. West China Med J. 2010;25(5):904–906. | ||
Zhou JS, Zhang YD, Song XJ, et al. The resting fMRI of adolescent criminals with violent behavior. Chin J Mental Medicine. 2012; 25(3):62. | ||
Wang D. A control study on cognition, impulsive characteristic and neuroimaging alterations of methamphetamine dependent subjects. CNKI-Central South University. 2013;616(89):1–103. | ||
Liu FJ, Xie B, Li X. Resting fMRI study of schizophremic patients with aggressive behavior: based on ReHo analysis. J Neurosci Mental Health. 2014;14(2):127–133. | ||
Lei H, Zhang X, Di X, et al. A functional polymorphism of the MAOA gene modulates spontaneous brain activity in pons. Biomed Res Int. 2014;2014:243280. | ||
Zhu P. Statistical study of fMRI data features for aggressive schizophrenia patients. CNKI-Hunan University. 2014;391(1):1–79. | ||
Huang Q. Research on the factors affecting reactive aggression and the related neural basis. CNKI-East China Normal University. 2015;269(1):1–63. | ||
Yong N, Wu F, Hu H, Meng H-Q. Resting-state fMRI study on alteration of amplitude of low frequency fluctuation and its relationship with aggressive behaviors in first-episode major depressive disorder patients. Acad J Sec Mil Med Univ. 2015;36(3):261–267. | ||
Liou YJ, Tsai SJ, Hong CJ, Wang YC, Lai IC. Association analysis of a functional catechol-o-methyltransferase gene polymorphism in schizophrenic patients in Taiwan. Neuropsychobiology. 2001;43(1):11–14. | ||
Li HF SZ, Ma JY, et al. Study of aggressive behavior and COM T gene polymorphisms in psychosis patients. J Clin Med. 2003;4(16):15–17. | ||
HY Jiang XX, XD Zhao, et al. Association study between aggression behavior in schizophrenics and catechol-O-methyltransferase gene polymorphism. Chin J Nerv Ment Dis. 2005;31(3):202–205. | ||
WY Liu YZ, Gao H, Wu HA. Association study of COMT gene polymorphism and schizophrenia with aggressive behaviors. Med J Chinese People’s Health. 2008;20(11):1105–1107. | ||
Gu Y, Yun L, Tian Y, Hu Z. Association between COMT gene and Chinese male schizophrenic patients with violent behavior. Med Sci Monit. 2009;15(9):CR484–CR489. | ||
X Huang NJ, ZN Lin, et al. The relationship between COMT polymorphisms and physical violence in schizophrenia. Guangdong Med J. 2010;31(1):82–83. | ||
Cao YP, Li LF, Zhao XF, Zhang YL. Association between aggressive behaviors and COMT Val158Met and 5-HTTLPR polymorphisms in children. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13(5):361–364. Chinese. | ||
SH Zou ZZ, R Ma, HB Dong, GY Tong. The association between COMT polymorphisms and violence in Han and Uigur population. Chin J Nerv Ment Dis. 2013;39(3):179–181. | ||
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. | ||
Bhakta SG, Zhang JP, Malhotra AK. The COMT Met158 allele and violence in schizophrenia: a meta-analysis. Schizophr Res. 2012;140(1–3):192–197. | ||
Winterer G, Musso F, Vucurevic G, et al. COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. Neuroimage. 2006;32(4):1722–1732. | ||
Loughead J, Wileyto EP, Valdez JN, et al. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14(8):820–826. | ||
Stokes PR, Rhodes RA, Grasby PM, Mehta MA. The effects of the COMT Val108/158Met polymorphism on BOLD activation during working memory, planning, and response inhibition: a role for the posterior cingulate cortex? Neuropsychopharmacology. 2011;36(4):763–771. | ||
Mobascher A, Brinkmeyer J, Thiele H, et al. The val158met polymorphism of human catechol-O-methyltransferase (COMT) affects anterior cingulate cortex activation in response to painful laser stimulation. Mol Pain. 2010;6:32. | ||
Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson’s disease is dependent on COMT val 158 met genotype. Brain. 2008;131(Pt 2):397–408. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.