Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Risk analysis of systemic levels of estrogen and adipokines as well as estrogen receptors from PBMCs in childbearing and perimenopausal women with obesity
Authors Fan H, Chen S, Gao B, Ding S, Zhao Q, Li C, Asakawa T
Received 20 February 2019
Accepted for publication 4 July 2019
Published 1 August 2019 Volume 2019:12 Pages 1287—1295
DOI https://doi.org/10.2147/DMSO.S206069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Huijuan Fan,*,1 Shujiao Chen,*,1,2 Bizhen Gao,1 Shanshan Ding,1 Qiang Zhao,1 Candong Li,1 Tetsuya Asakawa1,3
1Research Base of Traditional Chinese Medicine Syndrome, Fujian University of Traditional Chinese Medicine, Fuzhou, Shangjie Minhou 350122, People’s Republic of China; 2Department of Internal Medicine, The Third People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Fuzhou, Shangjie Minhou 350122, People’s Republic of China; 3Department of Neurosurgery, Hamamatsu University School of Medicine, Hamamatsu-city, Shizuoka 431-3192, Japan
*These authors contributed equally to this work
Purpose: We aimed to evaluate the clinical value of systemic levels of estrogen and adipokines as well as estrogen receptors from peripheral blood mononuclear cells (PBMCs) in childbearing and perimenopausal women with obesity.
Subjects and methods: We observed 292 women, including 160 perimenopausal women (80 with obesity and 80 without obesity) and 132 women of childbearing age (67 with obesity and 65 without obesity). Body parameters, such as body mass index and waist circumference, were measured. Fat distribution was evaluated using a computerized tomography scanner. The levels of serum estrogen, leptin, visfatin, and adiponectin were measured using an enzyme-linked immunosorbent assay. The expression of circulating ERs was evaluated by Western blot analysis.
Results: Perimenopausal women and childbearing women with obesity exhibited lower levels of estrogen and adiponectin, in addition to a distribution of visceral fat with higher levels of leptin and visfatin. These findings reflect the current data of menopausal women, which confirms the reliability of this experimental system. However, the expression of ERα in peripheral blood was significantly enhanced in women with obesity of both childbearing and perimenopausal age. This result is contrary to the common understanding of adipose tissue, namely that ERα is protective. The expression of ERβ in the women without obesity of both childbearing and perimenopausal age was higher than in women with obesity, which coincides with the results of a previous study on adipose tissue.
Conclusion: Our data fundamentally contradicts the utility of circulating ERα and ERα/ERβ evaluations in obesity studies. Because estrogen exerts pleiotropic effects on multiple tissues in the body through differential regulation of ERs, although the expression of ERβ coincides with the results of a previous study on adipose tissue, the expression levels of ERs in blood cannot be used as a diagnostic of informative tool for obesity in women.
Keywords: estrogen receptors, obesity, estrogen, fat distribution, adipokines, perimenopause
Introduction
Obesity and obesity-related diseases, such as hypertension, type 2 diabetes mellitus, and stroke, are important public health concerns.1 The percentage of individuals suffering from obesity in China is increasing substantially with the nation’s economic growth. Obesity is closely related to several diseases. Therefore, many treatments, including western medicine and herbs based on traditional Chinese medicine, have been developed to manage obesity. However, obesity treatment remains limited due to uncertain efficacy and notable adverse events.2 Thus, a better understanding of the mechanisms underlying obesity is crucial. Obesity has traditionally been regarded as an excessive energy state associated with diet and lifestyle. However, recent investigations indicate that obesity is a metabolic syndrome of complicated pathogenesis associated with genetic3–5 and environmental5–7 factors. Recently, the roles of estrogen, estrogen receptors (ERs) and the G protein-coupled estrogen receptor (GPER, previously termed GPR30) have garnered increasing attention.
The prevalence of obesity is enhanced in menopausal women8 and the regulation of their adipose metabolism is quite complex. It is modulated by leptin, visfatin, adiponectin, estrogen, and ERs. Adipokines, such as leptin and visfatin, exhibit positive correlations with obesity, while adiponectin is protective, showing a negative correlation with obesity.9–11 Thus, the mechanisms of obesity are complicated and affected by the interactions between estrogen, ERs, and adipokines. In a study from 2014, Grantham found that men in developing countries are exposed to environmental estrogen-like substances that reduce the risk of obesity.7 The results of an animal study also suggest that estrogen signaling may decrease the effects of obesity on obesity-related diseases in men.12 These findings provide evidence that estrogen is protective against obesity.13 ERs, including ERα and ERβ, play a crucial role in the effects of estrogen. Liu 2010 demonstrated that ERs modulate insulin sensitivity and energy homeostasis closely associated with obesity.13 Both ERα and ERβ are expressed in subcutaneous and visceral adipose tissues. Park 2011 found that the knockout of ERα in mice leads to central obesity, while nonclassical ERα signaling may reduce the risks associated with obesity.14 Lizcano 2014 believed that ERα plays a more important role in fat distribution and adipocyte activity.2 A subsequent report suggested that selective activation of ERα may be a novel therapeutic target for promoting the beiging of white adipose tissues.15 In this respect, ERα is protective against obesity.16 The roles of ERβ, however, are poorly understood. Evidence suggests that ERβ plays an important role in metabolism.17 Recently, Gonzalez-Granillo et al reported that ERβ is protective in ovariectomized female mice.18 Other studies show that the index of ERα/ERβ ratios is more sensitive and it has been used in several studies. Our previous findings demonstrate that enhancement of the hippocampal ERα/ERβ ratio is neuroprotective against perimenopausal anxiety and depression in rats.19 Recently, the role of GPER in regulation of metabolism has been increasingly attended because it has multifold effects on many systems (immune system, nervous system, cardiovascular system, etc.). It has been documented that GPER play a crucial role in regulation of the body weight, lipid homeostasis, glucose metabolism and inflammation by evidence from in vivo and in vitro investigations.20 But the underlying mechanisms of GPER to regulate metabolism remains unclear.
However, previous studies concerning ERs and obesity investigated samples of adipose tissue in animal obesity models, while most studies of ERs in humans investigated samples of pathological tissue. Clinically, this method is invasive and difficult to perform in women who cannot undergo surgery. Measuring the ERs in blood sample is lower invasive and easy to be accepted for the patients with obesity. We found only one study involved in investigation of ERs in blood samples, but that was for patient with hemopathy;21 and no study is found for investigating obesity. Thus, we sought to evaluate whether the levels of circulating ERs in peripheral blood samples could be used for examining the ER changes of perimenopausal women. We also investigated the utility of a peripheral blood ERα/ERβ ratio index in obesity research. Here, we attempted to reveal a new method for investigating ERs with low invasion that is easily applicable in a clinical setting.
Subjects and methods
Participants
A total of 292 women were enrolled based on voluntary participation. The criteria for perimenopause were an age of 40–55 years and the presence of menstrual disorders or amenorrhea for ≥3 and <12 months.22 The criteria for women of childbearing age were an age of 20–39 years and normal menstruation. Women were considered to have simple obesity according to the Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults;23 waist circumference (WC) ≥80 cm or body mass index (BMI) ≥28 kg/m2 was defined as simple obesity. Central obesity was defined by body fat parameters measured via computerized tomography (CT) scans. A visceral fat area ≥100 cm2 was defined as central obesity.24 We excluded subjects with the following conditions: 1) type 1 diabetes, gestational diabetes, secondary hypertension, or hyperlipemia, 2) serious heart, liver, kidney, or other complications, 3) psychiatric disorders, 4) secondary or drug-induced obesity, and 5) pituitary tumors or Cushing syndrome. Data were collected between October 2016 and March 2018. Finally, 160 perimenopausal women (80 with obesity and 80 without obesity) and 132 women of childbearing age (67 with obesity and 65 without obesity) were enrolled. The study was designed and performed according to the Declaration of Helsinki of the World Medical Association (2000). It was approved and supervised by the Ethics Committee of the Fujian University of Traditional Chinese Medicine (approval number: SQ2014-007-01). The investigation protocol was explained to all study participants and their relatives. Informed consent was obtained from each participant and a relative before study initiation.
Simple body fat parameter collection
The indices of all participants in a fasting state, including height, body weight (BW), and WC were measured three times at 8AM and the average of the three values was recorded. The BMI was calculated as BW/height2 (kg/m2). WC was measured with tape as the circumference of the horizontal edge of the midpoint at the lower edge of the costal arch.
Central obesity assessment
As described in a previous study, a CT scanner (ECLOS-16; Hitachi, Japan) was used to perform the central obesity assessment.24 The participants were placed in the supine position, the subject was instructed to hold his or her breath during the scan, and 16-slice spiral CT scanning was performed at the waist 4-waist 5 segments (umbilical level). An experienced radiologist measured the abdominal fat area (visceral fat area [VFA]), the abdominal wall subcutaneous fat area (SFA), and the total fat area (TFA). The ratio of fat was calculated as VFA/SFA.
Blood sampling
Elbow venous blood was collected in a fasting state from 8 AM to 9 AM. For menstruating women, the blood was sampled on the 3rd–5th day after menstruation. For women whose menstruation had stopped for ≥6 months, the blood was sampled on any day according to convenience. The blood samples were divided as follows: 5 mL in a procoagulant tube, 3 mL treated with anticoagulant (EDTA-K2; Sarstedt, Niimbrecht, Germany), and 1.5 mg/mL untreated for further use.
Measurement of serum levels of estrogen, leptin, visfatin, and adiponectin
A standard enzyme-linked immunosorbent assay was used to measure the serum levels of estrogen, leptin, visfatin, and adiponectin using an estrogen kit (AB108667; Abcam, UK), a leptin kit (ELH-Leptin-1; RayBiotech, USA), an adiponectin kit (ELH-Adiponectin-1; RayBiotech, USA), and a visfatin kit (EIA-VIS-1; RayBiotech, USA). All assays were performed according to the manufacturer’s instructions, and each assay was repeated three times. All experiments were performed by the same technician who was blinded to the study participants.
Measurement of the protein expression of ERs
As described in a previous study, standard Western blot analyses were performed to evaluate the protein expression of ERα and ERβ.25 Three mL of anticoagulated blood was collected, the surface of the lymphocyte separation solution was added, and the tube was centrifuged for 25 min (1500 rpm, 23 °C; Eppendorf, USA). The second layer of milky suspension lymphocyte solution was isolated, supplemented with 10 mL of physiological saline, and centrifuged for 10 min (1500 rpm, 23 °C; Eppendorf, USA). Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation using a Histopaque density gradient (Sigma, USA) for profiling the ERs. This step was performed twice to obtain the cells that were lysed with RIPA lysis solution (P0013B; Shanghai Beyotime Biological Co, China). Once the proteins were extracted, a BCA Protein Concentration Assay Kit (P0010; Shanghai Beyotime Biological Co, China) was used to quantify them. According to the protein quantification results, the corresponding volume of the total protein sample and 5× protein gel electrophoresis loading buffer was added (Shanghai Beyotime Biological Co, China), gently mixed, denature at 95 °C for 10 min, and immediately placed on ice for future use. Fifteen mg of protein from each sample was loaded into an SDS-PAGE gel, separated by electrophoresis, and transferred to a 0.45-mm polyvinylidene fluoride membrane (Millipore, USA). For the transfer, the gel and membrane were placed into a transfer film plate marked with positive and negative electrodes, and then inserted into a transfer electrophoresis tank containing transfer buffer. The power supply was turned on and the transfer occurred under constant voltage at 65V for 2 h at 4 °C. When the protein transfer was confirmed, the membrane was placed it in a blocking solution for 1 h at room temperature (23 °C) on a slow-moving shaker. The primary antibody incubation was performed overnight at 4 °C with anti-ERα (Cat.14007–1-AP, Proteintech, USA) and anti-ERβ (Cat.21244–1-AP, Proteintech, USA) primary antibodies, respectively. The membrane was washed and incubated in a horseradish peroxidase-labeled secondary antibody (MDL Biotechnology, China) for 60 min at room temperature, shaking slowly in the dark. The membranes were imaged with a chemiluminescence imaging system (Bio-Rad, USA).
Statistical analyses
Data were analyzed with SPSS 20.0 software (IBM, USA). All measurement data are represented as means ± standard deviations. All statistical results were tested on both sides. Normality of distribution and homogeneity of variance tests were performed first. Then, analysis of variance followed by Bonferroni post-hoc correction were performed for multiple comparisons. In the row correlation analysis, the Pearson line correlation was used for normal data. A p<0.05 was considered statistically significant.
Results
Comparison of obesity between the women of childbearing and perimenopausal age
In the comparison between perimenopausal women with and without obesity, BW, BMI, WC, VFA, SFA, and levels of leptin, adiponectin, and visfatin were significantly different. However, there was a different pattern in the variation between women of childbearing age with or without obesity. The estrogen levels were significantly higher in women of childbearing age without obesity, while the visfatin levels were not significantly different between the two groups.
In the comparison between women of perimenopausal age with obesity and women of childbearing age with obesity, age, estrogen level, VFA, VFA/TFA, VFA/SFA, and the levels of adiponectin and visfatin were significantly different.
These data indicate that estrogen levels and deposition of visceral fat might be characteristic changes in perimenopausal women with obesity, and that estrogen may have a protective effect against obesity (Table 1).
 |
Table 1 Obesity-related factors in different populations |
Correlations of adipokines and obesity-related factors in women of childbearing age and perimenopausal age
As shown in Table 2, the estrogen level was negatively correlated with age and positively correlated with WC in perimenopausal women. However, in women of childbearing age with obesity, VFA/TFA and VFA/SFA exhibited a negative correlation with the estrogen level. These data suggest that the difference between the protective effect of estrogen against obesity in women of different ages is attributable to differences in the underlying mechanisms responsible for the effect. This subject requires further investigation.
 |
Table 2 Correlations of adipokines with obesity-related factors in women with obesity |
The leptin levels were positively correlated with WC and SFA in perimenopausal women with obesity and showed a positive correlation with BW, BMI, WC, and SFA in women of childbearing age.
The adiponectin levels were negatively correlated with VFA/TFA and VFA/SFA in perimenopausal women with obesity and were negatively correlated with height, BW, BMI, VFA, VFA/TFA, and VFA/SFA in women of childbearing age with obesity.
The visfatin levels were positively correlated with WC and SFA in perimenopausal women with obesity. No visfatin correlations existed in women of childbearing age with obesity (Table 2).
Our data suggest that estrogen and adiponectin are protective against obesity, while leptin and visfatin induce obesity in women of both childbearing and perimenopausal age. Leptin exhibited a higher sensitivity than visfatin.
Investigation of the ER and obesity
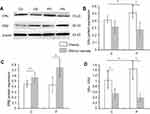
The protein expression of ERα was significantly increased in women of childbearing and perimenopausal age with obesity relative to those without obesity (Figure 1). For the perimenopausal women with obesity, the ERα expression was considerably high (Figure 1B).
The protein expression of ERβ in women of childbearing and perimenopausal age without obesity was higher than in those with obesity (Figure 1C).
The ERα/ERβ ratio exhibited the same tendency as ERα, but with a larger difference (Figure 1D).
Discussion
In the present study, we investigated the distribution of estrogen, ERs, and adipokines in women of childbearing and perimenopausal age. The estrogen levels of perimenopausal women were significantly lower relative to those in women of childbearing age. The deposition of the visceral fat was higher in perimenopausal women than in women of childbearing age. The adiponectin levels were higher in normal women and women of childbearing age and were lower in perimenopausal women with obesity. The levels of leptin and visfatin, however, exhibited an opposite tendency. The leptin and visfatin levels were higher in perimenopausal women with obesity and lower in normal and childbearing women. Similar results were found in the correlation analyses. These results are congruent with previous analogous studies of menopausal women. Interestingly, our data exhibited that the leptin levels showed a positive correlation with BW, BMI, WC, and SFA in women of childbearing age, whereas the visfatin levels did not. We therefore considered that leptin might be a more sensitive index than visfatin in the women with obesity. The previous studies supported that combined use of the indices of adipokines including adiponectin, leptin and visfatin is more reliable.26,27 Mastorakos et al reported that serum visfatin was the best negative predictor of percentage body fat in insulin resistance in normal pregnancy.28 Plati et al found that serum visfatin levels exhibits a more sensitivity than serum leptin levels in women with polycystic ovaries.29 Our results are opposite with these previous studies. We believe the changes and mechanisms of adipokines are complicated and multifold, which may be altered in different pathophysiological state. However, no more study compared the sensitivity of serum leptin and visfatin in women with obesity, which needs further investigation in our future studies.
With respect to ERs, we found that the ERα levels were higher in perimenopausal women and women with obesity, and lower in childbearing women and women without obesity. These data are contrary to the results of previous studies.2,13,15 The ERβ levels exhibited a reverse tendency, with lower values in women with obesity. ERα/ERβ ratios exhibited a similar tendency as those of ERα, but the difference was larger. Our results suggest a low utility of investigating circulating ERs in obesity-related studies.
The estrogen levels were significantly lower in perimenopausal women than childbearing women. When the estrogen levels were compared between women with and without obesity, only those of childbearing age exhibited a significant difference. There was no significant difference between perimenopausal women with and without obesity. These data are congruent with a previous report on pre- and postmenopausal women that observed lower estrogen levels only in premenopausal women.30 We speculate that estrogen levels decrease considerably at the perimenopausal age, irrespective of the presence or absence of obesity. Thus, our study did not detect a significant difference between the estrogen levels of perimenopausal women with and without obesity. We also found that VFA/TFA and VFA/SFA were significantly different between women with obesity of childbearing and perimenopausal age. However, there was no difference between the women with and without obesity at the same age. These results suggest that fat distribution is different between women with obesity of childbearing and perimenopausal age. Deposition of visceral fat may be a characteristic change in perimenopausal women with obesity. Notably, our data indicate that VFA/TFA and VFA/SFA were negatively correlated with the estrogen levels of women of childbearing age with obesity, which provides strong evidence for the relationship between estrogen and visceral fat. Several reports have demonstrated that a decrease in the estrogen levels of menopausal women may cause loss of subcutaneous fat and enhance visceral fat, and that estrogen is closely associated with the accumulation of subcutaneous fat.2,31–33 Our data of perimenopausal women are similar to the data of menopausal women. Estrogen metabolism and regulation become disrupted in the perimenopausal age. This metabolic disruption exerts a remarkable impact on fat metabolism, resulting in visceral fat accumulation that is closely associated with obesity and obesity-related diseases. Our data regarding adipokines are congruent with previous reports of menopausal women.2,34 Our correlation analyses provide further evidence that leptin and visfatin are factors that induce obesity, while adiponectin exerts a protective effect against obesity. These results of estrogen levels, fat distribution, and adipokines in perimenopausal women are in accordance with the data from previous reports of menopausal women, which confirms the reliability of the experimental system in the present study.
With respect to the data of the ERs, the blood sample results contrary to those from the adipose tissue. The protective role of ERα against obesity has been well documented. Recently, Arao et al found that activation of ERα prevents fat accumulation mediated by increased energy expenditure.35 Liu et al reported that ERα activation by resveratrol protects against the effects of high-fat diet-induced mouse cardiomyopathy.36 Leonetti et al showed that ERα activation improved metabolic alterations and provided vascular protection in a mouse model of obesity-related disorders.37 However, the blood sample data in the present study showed that ERα was lower in women of childbearing age and perimenopausal age without obesity. These data do not support the protective effects of ERα and, therefore, are nonsensical. Our data shows that ERβ is higher in women without obesity, but there is no significant difference between women of perimenopausal and childbearing ages. Although these findings are partially in agreement with results from a previous study of adipose tissue,18 namely that ERβ is protective, the roles of ERβ remain controversial. Miao et al found that ERβ plays a novel role in regulating the browning of adipose tissue.38 Ponnusamy et al found that ERβ activation enhances mitochondrial function, enhances energy expenditure, and browns adipose tissue.39 A recent study observed the body metabolism in non-ovariectomized female mice who were administered a high-fat diet and selective ERβ agonist. They reported that ERβ activation improved the fasting glucose levels and insulin sensitivity and reduced liver steatosis. They concluded that ERβ activation exerts a beneficial effect on whole-body metabolism related to obesity.18 Because the utility of circulating ERα measurement is not supported by the data of the present study, the ERα/ERβ ratio cannot be considered a useful measurement. An explanation of these results is that estrogen exerts pleiotropic effects on multiple tissues in the body through differential regulation of ERs, we believe that expression of ERs in the blood cells may not reflect the true levels of ERs in metabolically relevant tissues. In this regard, although the expression of ERβ coincides with the results of a previous study on adipose tissue, we believe that the expression levels of ERβ in blood also cannot be used as a diagnostic of informative tool for obesity in women.
In this study, we compared the obesity-related indexes, estrogen, fat distribution, adipokines, and circulating ERs between women of childbearing and perimenopausal age. We found that the estrogen, fat distribution, and the adipokines of these women were similar to those of menopausal women, which confirmed the reliability of our experimental system. Our data suggest that circulating ERα is not protective, which is contrary to the acknowledged conclusion that ERα derived from adipose tissue is protective. These data do not corroborate the utility of circulating ERα and ERα/ERβ ratios as indexes for obesity-related studies. Despite the finding that circulating ERβ exhibits similar tendencies to those observed in previous studies of adipose tissue, the clinical value of circulating ERβ cannot be supported. In future experiments, we will consider other invasive methods for investigating ERs as well as GPER in obesity studies.
Conclusion
In the present study, we compared the obesity-related indexes, estrogen, fat distribution, adipokines, and circulating ERs in women of childbearing and perimenopausal age. We found that the obesity-related indexes, estrogen, fat distribution, and adipokines were similar to those observed in previous studies. Our results regarding circulating ERα were opposite of those from adipose tissue. This finding fundamentally disagrees with the utility of detecting blood ERα and ERα/ERβ ratios in obesity studies. Despite the finding that circulating ERβ exhibits similar tendencies to those seen in previous studies, the utility of circulating ERβ measurement cannot be supported. The expression levels of ERs in blood cannot be used as a diagnostic of informative tool for obesity in women.
Acknowledgments
The authors would like to thank Enago for the English language review. This study was supported by grants from the National Natural Science Foundation of China (No: 81473595), Fujian Provincial Health Commission (No: 2017FJZYLC402) and the Japanese Society for the Promotion of Science (Grant-in-Aid for Young Scientists, Type B, No. 20791025 and Grant-in-Aid for Scientific Research C, General, Nos. 24592157, 15k10358 and 18K08991).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chu DT, Minh Nguyet NT, Dinh TC, et al. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab Syndr. 2018;12(6):1095–1100. doi:10.1016/j.dsx.2018.05.004
2. Lizcano F, Guzman G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int. 2014;2014:757461. doi:10.1155/2014/757461
3. Ingelsson E, McCarthy MI. Human genetics of obesity and type 2 diabetes mellitus: past, present, and future. Circ Genom Precis Med. 2018;11(6):e002090. doi:10.1161/CIRCGEN.118.002090
4. Wong CM, Xu L, Yau MY. Alternative mRNA splicing in the pathogenesis of obesity. Int J Mol Sci. 2018;19:2. doi:10.3390/ijms19020632
5. Voss JD, Goodson MS, Leon JC. Phenotype diffusion and one health: a proposed framework for investigating the plurality of obesity epidemics across many species. Zoonoses Public Health. 2018;65(3):279–290. doi:10.1111/zph.12490
6. Moran-Ramos S, Lopez-Contreras BE, Canizales-Quinteros S. Gut microbiota in obesity and metabolic abnormalities: a matter of composition or functionality? Arch Med Res. 2017;48(8):735–753. doi:10.1016/j.arcmed.2017.11.003
7. Grantham JP, Henneberg M, Rosenfeld CS. The estrogen hypothesis of obesity. PLoS One. 2014;9(6):e99776. doi:10.1371/journal.pone.0099776
8. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi:10.1001/jama.2009.2014
9. Oh KJ, Lee DS, Kim WK, Han BS, Lee SC, Bae KH. Metabolic adaptation in obesity and type II diabetes: myokines, adipokines and hepatokines. Int J Mol Sci. 2016;18(1):8. doi:10.3390/ijms18010008
10. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. doi:10.3390/ijms15046184
11. Arnoldussen IA, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol. 2014;24(12):1982–1999. doi:10.1016/j.euroneuro.2014.03.002
12. Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab. 2014;306(10):E1188–E1197. doi:10.1152/ajpendo.00579.2013
13. Liu S, Mauvais-Jarvis F. Minireview: estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151(3):859–864. doi:10.1210/en.2010-0412
14. Park CJ, Zhao Z, Glidewell-Kenney C, et al. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese Eralpha-null mutant mice. J Clin Invest. 2011;121(2):604–612. doi:10.1172/JCI57873
15. Santos RS, Frank AP, Fatima LA, Palmer BF, Clegg DJ. Activation of estrogen receptor alpha induces beiging of adipocytes. Mol Metab. 2018. doi:10.1016/j.molmet.2018.09.002
16. Morselli E, Santos RS, Gao S, et al. Impact of estrogens and estrogen receptor-alpha in brain lipid metabolism. Am J Physiol Endocrinol Metab. 2018;315(1):E7–E14. doi:10.1152/ajpendo.00473.2017
17. Shi H, Kumar SP, Liu X. G protein-coupled estrogen receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci. 2013;114:193–250.
18. Gonzalez-Granillo M, Savva C, Li X, et al. ERbeta activation in obesity improves whole body metabolism via adipose tissue function and enhanced mitochondria biogenesis. Mol Cell Endocrinol. 2019;479(5):147–158. doi:10.1016/j.mce.2018.10.007
19. Chen S, Asakawa T, Ding S, et al. Chaihu-Shugan-San administration ameliorates perimenopausal anxiety and depression in rats. PLoS One. 2013;8(8):e72428. doi:10.1371/journal.pone.0072428
20. Sharma G, Mauvais-Jarvis F, Prossnitz ER. Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. J Steroid Biochem Mol Biol. 2018;176:31–37. doi:10.1016/j.jsbmb.2017.02.012
21. Pelekanou V, Kampa M, Kiagiadaki F, et al. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERalpha36 and GPR30/GPER1. J Leukoc Biol. 2016;99(2):333–347. doi:10.1189/jlb.3A0914-430RR
22. Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118(Suppl 12B):14–24.
23. Chen C, Lu FC, Department of Disease Control Ministry of Health PRC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17:1–36.
24. Hiuge-Shimizu A, Kishida K, Funahashi T, et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med. 2012;44(1):82–92. doi:10.3109/07853890.2010.526138
25. Zhang X, Chen Q, Chen B, Wang F, Chen XH. Herb formula ZhenRongDan balances sex hormones, modulates organ atrophy, and restores ERalpha and ERbeta expressions in ovariectomized rats. Evid Based Complement Alternat Med. 2018;2018:5896398. doi:10.1155/2018/9567061
26. Kural B, Deger O, Erem C, Balaban Yucesan F, Aliyazicioglu R, Barlak Y. Is the combined use of insulin resistance indices, including adipokines, more reliable in metabolic syndrome? Turk J Med Sci. 2014;44(6):1021–1028. doi:10.3906/sag-1310-90
27. Taşdemir E, Şermet A. The relationship between plasma adipsin, adiponectin, vaspin, visfatin, and leptin levels with glucose metabolism and diabetes parameters. diabetes. 2019;15:21.
28. Mastorakos G, Valsamakis G, Papatheodorou DC, et al. The role of adipocytokines in insulin resistance in normal pregnancy: visfatin concentrations in early pregnancy predict insulin sensitivity. Clin Chem. 2007;53(8):1477–1483. doi:10.1373/clinchem.2007.085654
29. Plati E, Kouskouni E, Malamitsi-Puchner A, Boutsikou M, Kaparos G, Baka S. Visfatin and leptin levels in women with polycystic ovaries undergoing ovarian stimulation. Fertil Steril. 2010;94(4):1451–1456. doi:10.1016/j.fertnstert.2009.04.055
30. Garaulet M, Perez-Llamas F, Baraza JC, et al. Body fat distribution in pre-and post-menopausal women: metabolic and anthropometric variables. J Nutr Health Aging. 2002;6(2):123–126.
31. Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am J Physiol Endocrinol Metab. 2001;280(3):E496–501. doi:10.1152/ajpendo.2001.280.3.E496
32. Elbers JM, de Jong S, Teerlink T, Asscheman H, Seidell JC, Gooren LJ. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism. 1999;48(11):1371–1377. doi:10.1016/S0026-0495(99)90146-4
33. Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276(2 Pt 1):E317–E325.
34. Chedraui P, Perez-Lopez FR, Escobar GS, et al. Circulating leptin, resistin, adiponectin, visfatin, adipsin and ghrelin levels and insulin resistance in postmenopausal women with and without the metabolic syndrome. Maturitas. 2014;79(1):86–90. doi:10.1016/j.maturitas.2014.06.008
35. Arao Y, Hamilton KJ, Lierz SL, Korach KS. N-terminal transactivation function, AF-1, of estrogen receptor alpha controls obesity through enhancement of energy expenditure. Mol Metab. 2018;18:68–78. doi:10.1016/j.molmet.2018.09.006
36. Lu Y, Lu X, Wang L, Yang W. Resveratrol attenuates high fat diet-induced mouse cardiomyopathy through upregulation of estrogen related receptor-alpha. Eur J Pharmacol. 2019;843(15):88–95. doi:10.1016/j.ejphar.2018.10.018
37. Leonetti D, Soleti R, Clere N, et al. Extract enriched in flavan-3-ols and mainly procyanidin dimers improves metabolic alterations in a mouse model of obesity-related disorders partially via estrogen receptor alpha. Front Pharmacol. 2018;9:406. doi:10.3389/fphar.2018.00406
38. Miao YF, Su W, Dai YB, et al. An ERbeta agonist induces browning of subcutaneous abdominal fat pad in obese female mice. Sci Rep. 2016;6:38579. doi:10.1038/srep38579
39. Ponnusamy S, Tran QT, Harvey I, et al. Pharmacologic activation of estrogen receptor beta increases mitochondrial function, energy expenditure, and brown adipose tissue. Faseb J. 2017;31(1):266–281. doi:10.1096/fj.201600787RR
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.