Back to Journals » Clinical Ophthalmology » Volume 15
Ripasudil Endgame: Role of Rho-Kinase Inhibitor as a Last-Ditch-Stand Towards Maximally Tolerated Medical Therapy to a Patient of Advanced Glaucoma
Authors Naik M , Kapur M, Gupta V, Sethi H, Srivastava K
Received 7 May 2021
Accepted for publication 31 May 2021
Published 24 June 2021 Volume 2021:15 Pages 2683—2692
DOI https://doi.org/10.2147/OPTH.S318897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mayuresh Naik,1 Monika Kapur,2 VishnuSwarup Gupta,1 HarinderSingh Sethi,3 Kartikeya Srivastava3
1Department of Ophthalmology, H.I.M.S.R & H.A.H. Centenary Hospital, New Delhi, India; 2Department of Ophthalmology, School of Medical Sciences & Research, Sharda University, Greater Noid, Uttar Pradesh, India; 3Department of Ophthalmology, V.M.M.C & Safdarjung Hospital, New Delhi, 110029, India
Correspondence: Mayuresh Naik
Department of Ophthalmology, H.I.M.S.R & H.A.H. Centenary Hospital, Room No. 3 of Eye OPD, 1st Floor of OPD Building, Near GK-2, Alaknanda, New Delhi, 110062, India
Tel +91-8287344576
Email [email protected]
Purpose: To elucidate the use of Ripasudil in patients of advanced glaucoma on maximally tolerated medical therapy who could not be offered the option of surgery due to the global pandemic lockdown.
Materials and Methods: Only patients with primary open angle glaucoma (POAG), who had a cup-disc ratio (CDR) of 0.9 or a near total cupping on maximum tolerated medical therapy for at least 4 weeks and yet could not meet the target IOP were included. Target IOP was defined as ≤ 12 mm Hg. A total of 30 patients were enrolled. All patients in study cohort were started on E/D Ripasudil BD. Patients were followed up at 1 week, 2 weeks, 4 weeks and then monthly for 6 months for their best corrected visual acuity (BCVA), intraocular pressure (IOP), disc changes (slit lamp biomicroscopy), perimetry, and retinal nerve fibre layer analysis using optical coherence tomography (OCT-RNFL).
Results: Mean pre-treatment IOP on five drugs was 18.3 ± 2.1 mm Hg (range 14 to 22mmHg) on maximally tolerated medical therapy. At 1 week follow-up, mean post-treatment IOP was 15.1 ± 1.7 mm Hg (range 12 to 18mmHg) and at 2 week follow-up, mean post-treatment IOP was 12.5 ± 1.9 mmHg (range 10 to 16mmHg). Thus, target IOP ≤ 12mmHg was attained in 28 patients at 2 weeks. This target IOP was maintained throughout the 6 months of follow-up period. Of the 2 patients who could not meet target IOP, 1 patient needed rearrangement of their fixed-drug-combinations to achieve target IOP at 4 weeks. The second patient required unfixing of all fixed-drug-combinations to achieve target IOP at maximally tolerated medical therapy at 6 weeks.
Conclusion: Ripasudil not only provides a better IOP control but also has a high safety profile even when started as an add-on drug to already-existing yet inadequate maximally tolerated medical therapy.
Keywords: advanced glaucoma, ripausdil, ROCK1 inhibitor, maximally-tolerated-medical-therapy, COVID-19 lockdown
Introduction
As of 2020, glaucoma is the most important cause of irreversible vision loss globally.1 Based on prevalence studies, it is estimated that 79.6 million individuals will have glaucoma in 2021. Of these, it is estimated that more than 11 million individuals will be bilaterally blind due to glaucoma in 2021 (around 13% of the cases).2 At least 50% of those with glaucoma are unaware that they are affected. In some developing countries, 90% of glaucoma is undetected and thus nearly one fifth of the patients become blind in either or both eyes.3 Intraocular pressure (IOP) is the single most important modifiable risk factor for efficient glaucoma control. Conventionally, the initial standard of care for primary open angle glaucoma is medical with surgical options being reserved for instances when there is documented structural or functional progression despite adequate IOP control or inability to control IOP on medical management.
Advanced glaucoma, defined as near total cupping with or without severe visual field (VF) loss within 10 degree of fixation, tends to have a worse visual and overall prognosis.4 Lowering the intraocular pressure (IOP) to the lower teens as well as reducing IOP fluctuations has the strongest evidence of protecting the optic nerve and preserving the remnant VF.5,6 The conventional treatment protocol dictating the use of anti-glaucoma medications till maximally tolerated medical therapy before the surgical intervention, does not apply to the advanced glaucoma stages. Recent guidelines from National Institute of Health and Clinical Excellence of UK as well as the American Glaucoma Society (AGS) recommend primary glaucoma surgery in such cases.7 However, with the advent of the COVID-19 pandemic and the resultant lockdown starting March 2020, elective surgeries and procedures were halted and indefinitely deferred until it was safe for both the health care workers and the patients. In such a scenario, there was no option but to resort to alternative strategies including modifying fixed-drug-combinations within the tenets of previously existing maximally tolerated medical therapy. Ripasudil was FDA approved for the treatment of glaucoma in 2014 but the use was limited to Japan and Korea(PMDA) since then. However, the recent advent of its availability in India ushered in a new arena of opportunity that could be harnessed for lowering IOP via a new pathway, ie the selective Rho-associated coiled-coil-containing protein kinase 1 (ROCK1) inhibition.
Our study attempted to elucidate the use of Ripasudil in patients of advanced glaucoma on maximally tolerated medical therapy who could not be offered the option of surgery due to the global pandemic lockdown.
Materials and Methods
Written and informed consent was taken from all patients. This study was approved by the Institutional Ethics Committee and Institutional Review Board (IEC.IRB/HIMSR/HAHCH/03/2020-17) of H.I.M.S.R & H.A.H. Centenary Hospital, New Delhi. All procedures performed in our study involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments.
This retrospective, observational study was conducted on patients visiting Speciality Glaucoma Clinic at Tertiary healthcare hospital, ie H.I.M.S.R & H.A.H. Centenary Hospital, Department of Ophthalmology from 1 March 2020 to 30 November 2020.
Inclusion Criteria
Only patients with primary open angle glaucoma were selected.
All patients who had cup-disc ratio (CDR) of 0.9 or a near total cupping and/or a mean deviation of −12dB (Hodapp-Anderson-Parrish criteria) on the standard Humphrey Visual Field 24–2 program (Carl Zeiss Meditec)9 despite aggressive maximum tolerated medical therapy for IOP control including a prostaglandin analogue, α-agonist, β-blocker, carbonic anhydrase inhibitor, cholinergic agonist for at least 4 weeks and yet could not meet the target IOP were included. Target IOP (main outcome measure) was defined as ≤ 12 mm Hg.
A total of 30 patients were enrolled.
Exclusion Criteria
Patients with history of any previous surgery, uveitis, ocular infection, having hypersensitivity to Ripasudil, on any immunosuppressive therapy (topical/systemic), with history of herpetic keratitis and contact lens wearers were excluded from the study.
Patients who were not on maximally tolerated medical therapy as elaborated above or those who could attain and maintain their target IOP on maximally tolerated medical therapy were excluded from the study.
Procedure and Data Collection
All patients in study cohort were started on E/D Ripasudil BD.
Patients were followed up at 1 week, 2 weeks, 4 weeks and then monthly for 6 months.
The parameters evaluated were:
Best corrected visual acuity (BCVA), intraocular pressure (IOP) using Goldmann’s Applanation tonometry, disc changes (slit lamp biomicroscopy using 90-D lens), perimetry for visual field progression (Humphrey Visual Fields ©Carl Zeiss Meditec), and retinal nerve fibre layer analysis using Optical Coherence Tomography (OCT-RNFL using Cirrus™ HD-OCT© Carl Zeiss Meditec). Structural changes were identified by slit lamp biomicroscopy and staged using disc damage likelihood scale (DDLS).8
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD. Quantitative variables were compared using paired t-test was used for comparison between pre and post. Qualitative variables were compared using Chi-Square test/Fisher’s Exact test. A p-value of <0.05 was considered statistically significant. The data was entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
Demographic Data
Out of the 30 patients, 28 patients were ≥ 55 years of age (mean 63.8 yrs). Eighteen of the patients were males, and 12 were females. There was no racial disparity. None of the patients underwent any previous surgery (Table 1).
 |
Table 1 Demographic Data at Baseline Showing the Mean Deviation in HVF 30–2 and the Fixed-Drug-Dose-Combinations in the Study Population |
Best Corrected Visual Acuity
Pre-treatment Snellen’s BCVA was > 6/12 in 22 patients and 6/60–6/12 in 8 patients.
At follow-up, the BCVA remained stable in 28 patients. It dropped by 1 Snellen line in 2 patients and 2 Snellen lines in 2 patients over a period of 6 months and later improved to 6/6P following uneventful phacoemulsification.
Intraocular Pressure (IOP)
 |
Figure 1 Graphical representation of the Mean IOP at each patient visit in the study population. |
Mean pre-treatment IOP on five drugs was 18.3 ± 2.1 mm Hg (range 14 to 22 mmHg) on maximally tolerated medical therapy (Figure 1).
At 1 week follow-up, mean post-treatment IOP was 15.1 ± 1.7 mm Hg (range 12 to 18 mmHg) and at 2 week follow-up, mean post-treatment IOP was 12.5 ± 1.9 mmHg (range 10 to 16 mmHg).
Thus target IOP ≤ 12 mmHg was attained in 28 patients at 2 weeks and was maintained throughout the 6 months of follow-up period. The mean post-treatment IOP at 6 months was 10.4 ± 1.3 mmHg (range 9 to 12 mmHg). This represented a statistically significant reduction in IOP (p<0.05) as compared to the pre-treatment IOP.
Of the 2 patients who could not meet target IOP, 1 patient needed rearrangement of their fixed-drug-combinations to achieve target IOP at 4 weeks. The second patient required unfixing of all fixed-drug-combinations to achieve target IOP at maximally tolerated medical therapyat 6 weeks. No drugs were changed, substituted or modified during the 6 months of the follow-up period.
Disc Changes
Structural changes were identified by slit lamp biomicroscopy and staged using disc damage likelihood scale (DDLS). No structural deterioration was noted in a follow up period of 6 months.
Field Changes
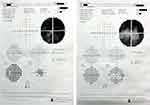
Pre-treatment all patients had visual fields with grossly depressed total deviation plots. Mean of mean deviation (MD) plot was −26.78 ± 13.76dB (Figures 2 and 3).
 |
Figure 3 Humphrey’s visual field analyser charts of a patient with cup-disc ratio 0.9 showing a biarcuate scotoma in 30–2 visual field charting in left eye. |
Visual fields showed bi-arcuate scotoma in 12 of the patients with sparing of a central and temporal island of vision without a macular splitting on 10–2 field charting while 5 patients had bi-arcuate scotoma with annular vision as well as a macular split on 10–2 field charting. Inferior arcuate scotomas were seen in 5 patients and superior arcuate scotomas were seen in 5 patients. Three patients had a severely depressed fields with macular split on 10–2 visual field.
Deepened or enlarged defect was defined as worsening of two or more points within,adjacent or contiguous to the existing scotoma by more than 10 dB. There was no defined progression in any of the 30 patients; however, mean deviation (MD) was found to be increased in 4 patients which improved substantially following cataract surgery.
OCT-RNFL
The OCT-RNFL was employed at the following instances:
i. At enrolment as a baseline OCT-RNFL for further reference
ii. After target IOP was achieved and slit-lamp biomicroscopy did not show any further structural deterioration and perimetry did not show any further functional deterioration, so as to pick up the earliest instances of microscopic nerve loss/damage.
The OCT-RNFL was outside normal limits in all patients of the cohort but did not show any statistically significant documented progression of further nerve loss as compared to the baseline OCT-RNFL done at baseline.
Adverse Effect Profile
Conjunctival hyperemia was seen in 4 patients, and it was mild to moderate for the initial two hours following instillation and resolved thereafter.
Discussion
Glaucoma is the third leading cause of visual disability in the world.2 Approximately 20.9% of patients with POAG were blind in either or both eyes, in the Aravind Comprehensive Eye Study.10 There is a lack of consensus on the definition of advanced glaucoma. According to the classic Hodapp-Anderson-Parrish9 textbook, patients with a mean deviation of −12dB on the standard Humphrey Visual Field 24–2 program (Carl Zeiss Meditec) have advanced glaucoma. The International classification of Diseases 9 (365.73) and 10 (7th digit “3”) diagnostic code defines severe stage (or advanced stage or end stage glaucoma) as optic nerve abnormalities consistent with glaucoma and glaucomatous visual field abnormalities in both the hemifields and/or loss within 5º of fixation in at least one hemifield. We included patients with 0.9 or near total cupping with visual field defects encroaching or sparing the central 10º of fixation, as these are the patients with worst prognosis and in need of aggressive glaucoma control with intensive treatment.
Advanced glaucoma at presentation is a major risk factor for lifetime blindness.11 These patients are at imminent danger of losing the remnant vision with an overall worse prognosis and in desperate need of aggressive glaucoma management. Glaucoma progression is attributable not only to persistent IOP elevation but also to short and long term IOP fluctuations. At least 40% IOP reduction is required to prevent progression in advanced glaucoma cases. In the Advanced Glaucoma Intervention Study (AGIS) patients that did not show any progression had a mean IOP of 12 mm Hg.12 Also, in patients with advanced glaucoma showing functional or structural progression in spite of achievement of target IOP, assessment and control of peak diurnal IOP and IOP fluctuations should be given equal importance as absolute reduction of IOP. Hence the management of advanced glaucoma is targeted towards maximally tolerated medical therapy initiated as soon as possible so that the need of surgery can be assessed and prognosticated to the patient.
In our study, we included patients with near-total optic disc cupping with severe visual field loss within 10 degrees of fixation, ie scotoma on or encroaching fixation. Advanced glaucoma patients need a multifactorial approach wherein the vision and overall function of the patient is considered before a decision is reached regarding the preferred mode of therapy, be it medical, laser or surgical. Since a randomised clinical trial comparing the outcomes of all three modes of treatment in advanced glaucoma patients is lacking, there is no uniformly, universally accepted best treatment option for these group of patients. Recently, National Institute for Health and Clinical Excellence guideline of UK has suggested primary glaucoma surgery for patients presenting with advanced glaucoma13 while a study conducted by King et al argued that the current evidence is not supportive of this recommendation.14 Burr J. et al compared medical versus surgical interventions for POAG and concluded that there is insufficient evidence to suggest that the efficacy and cost-effectiveness of surgery was superior as compared to recently available medications in patients of advanced glaucoma.15
Amongst the newer glaucoma medications, Ripasudil has shown the most promising results. Ripasudil hydrochloride hydrate is the world’s first Rho-associated coiled-coil-containing protein-kinase (ROCK) inhibitor that lowers IOP by increasing the conventional aqueous outflow through the trabecular meshwork and Schlemm’s canal by decreasing outflow resistance. Ripasudil induces basic cellular changes such as cell contraction, cell motility, cytoskeleton rearrangement, cell adhesion and remodelling of cell to cell contact in the Trabecular meshwork- Schlemm's canal pathway. Another important aspect is that Rho-kinase inhibitors can potentially alter episcleral venous pressure which can bring down the IOP further given that the episcleral venous pressure acts as a maximum under which it is difficult to bring IOP down. The IOP lowering efficacy of Ripasudil has been well investigated in patients of POAG and OHT. In various studies, Ripasudil has demonstrated efficacy in lowering the IOP when used as monotherapy or in combination with beta-blockers or prostaglandin analogues. According to a study carried out in Japanese patients, who were on 2- or 3-drug medical therapy, IOP decreased from 2.6 mm Hg to 3.1mm Hg, ie approximately 20% to 30% IOP reduction from baseline after administration of Ripasudil.12
Our cohort study included 30 patients with diagnosed advanced glaucoma, who had been receiving maximally tolerated medical therapy for a long period of time but failed to achieve the target IOP. These patients were advised Trabeculectomy with Mitomycin C and were supposed to undergo the same, but elective operative procedures were indefinitely deferred post the advent of COVID-19. The recent availability of Ripasudil in the Indian market provided temporary respite as our study demonstrated statistically significant IOP lowering effects of Ripasudil when used as an adjunctive therapy to existing maximal medical therapy in patients of advanced glaucoma. Ripasudil was clearly a better alternative to oral therapy like Tab. Diamox (Acetazolamide ©Alembic Pharmaceuticals) especially in a situation like COVID-19 because Diamox would have required monitoring of renal function which would have been difficult to do when our prime intention was to reduce the number of hospital visits.
The mean IOP reduction from baseline was 17.4% after 1 week and 31.6% after 2 weeks of starting Ripasudil and the predefined target IOP was achieved in all 30 patients. Thus, the adjunctive therapy with Ripasudil may be effective in deferring surgery at least for short term as all of the patients achieved the predefined target IOP. This target IOP was maintained during the 6 months of the study period. This additive IOP lowering effect of Ripasudil is attributable to the fact that its mechanism of action differs from that of other antiglaucoma medications and can be explained by two hypothesis.15–19 Firstly, Ripasudil not only induces contraction and rounding of cell bodies in the trabecular meshwork but also decreases transendothelial resistance, thus increasing transendothelial flux. Secondly, it is supposed that it takes a few weeks for intraocular distribution of Ripasudil to stabilise and thus it takes time for Ripasudil to decrease the extracellular matrix accumulation and eventually increase the conventional aqueous outflow.
The safety profile of Ripasudil is superior to prostaglandin analogues and other antiglaucoma medications as seen in various studies. The most common adverse effect with Ripasudil was conjunctival hyperemia, blepharitis and allergic conjunctivitis.16–19 Regarding the safety profile of Ripasudil in our study, conjunctival hyperemia was seen in 4 patients, and it was mild to moderate for the initial two hours following instillation and resolved thereafter. This hyperemia is hypothesized to be caused by the relaxation of vascular smooth muscles as Ripasudil is known to be a vasodilator. There was no incidence of blepharitis neither any allergic conjunctivitis nor any other adverse effect reported during the 6 month follow-up period.
Despite the noteworthy ophthalmic impact of our study, there are a few limitations. Firstly, the size of the cohort was small and there was no parallel control group for comparison. Secondly, for patients of advanced glaucoma who are already on five drugs, adding a 6th drug to the medical armamentarium for a lifetime raises concerns regarding compliance considering the socio-economic status in a developing country like India. Even in developed countries, a joint discussion between the glaucoma specialist and the patient would/may/might tend towards glaucoma surgery as against the trial of adding a 6th drug in our efforts to achieve target IOP on maximally tolerated medical therapy. The only possible counter-argument to this would be an objective assessment of the costs involved: a 5mL vial of E/D Ripatec (Ripasudil 0/4% w/v © Ajanta Pharma Ltd) costs Rs. 262 which is just about equivalent of 3$ and is nowhere comparable to the cost of surgery.
Surgery provides an edge over socioeconomic issues and adherence, with the benefit of strict IOP control and little diurnal variations. This is important in Indian setup where nearly 35% of the population falls below the international poverty line. However, in the scenario that surgery is precluded (eg a global pandemic in this case) for advanced glaucoma patients with poor IOP control and/or at risk of glaucoma progression, addition of Ripasudil is a brilliant add-on to previously existing maximally tolerated medical therapy in order to achieve target IOP at least in the immediate short-term timeline.
Conclusion
Despite the paradigm shifts that have been proposed in the management of advanced glaucoma, Ripasudil not only provides a better IOP control but also has a high safety profile even when started as an add-on drug to already-existing yet inadequate maximally tolerated medical therapy. Since it can also serve as a cost effective addition to medical armamentarium of glaucoma, it has literally been the last ditch stand to preserve salvageable vision in patients of advanced glaucoma during the COVID-19 pandemic.
Statement of Justification
The following case series represents a retrospective analysis of the safety and efficacy of Ripasudil in cases of advanced glaucoma in order to deduce whether the Rho-kinase inhibitor can be of any value in our pursuit of buying time to preserve salvageable vision in patients of advanced glaucoma.
Ethical Clearance
Obtained from Ethical Clearance Committee, Institution Review Board. (IEC.IRB/HIMSR/HAHCH/03/2020-17).
Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Authorship
All the authors were involved in the concept and design of the study, data acquisition, data analysis and interpretation, drafting manuscript, technical support and final review of the manuscript.
Funding
There is no funding to report.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Resnikoff S, Pascolini D, Etya D, et al. Global Data on Visual Impairment in the Year 2002. Vol. 82. Bulletin of the World Health Organization; 2004.
2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
3. George R, Ve RS, Vijaya L. Glaucoma in India: estimated burden of disease. J Glaucoma. 2010;19(6):391–397. doi:10.1097/IJG.0b013e3181c4ac5b
4. Gessesse GW, Damji KF. Advanced glaucoma: management pearls. Middle East Afr J Ophthalmol. 2013;20(2):131–141. doi:10.4103/0974-9233.110610
5. VanVeldhuisen Paul C, Ederer F. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440.
6. Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115(1123–1129):e3. doi:10.1016/j.ophtha.2007.10.031
7. National Institute for Health and Clinical Excellence (NICE). Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. Clinical Guidelines CG85, UK National Institute for Health and Clinical Excellence (NICE) Guidelines. Developed by the National Collaborating Centre for Acute Care; 2009.
8. Henderer JD. Disc damage likelihood scale. Br J Ophthalmol. 2006;90(4):395–396. doi:10.1136/bjo.2005.083360
9. Hoddapp E, Parrish RK
10. Ramakrishnan R, Nirmalan PK, Krishandas R, et al. Glaucoma in a rural population of southern India: the Aravind comprehensive eye survey. Ophthalmology. 2003;110(8):1484–1490. doi:10.1016/S0161-6420(03)00564-5
11. Peters D, Bengtsson B, Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol. 2014;92(5):421–425. doi:10.1111/aos.12203
12. Sato S, Hirooka K, Nitta E, Ukegawa K, Tsujikawa A. Additive intraocular pressure lowering effects of the rho kinase inhibitor, ripasudil in glaucoma patients not able to obtain adequate control after other maximal tolerated medical therapy. Adv Ther. 2016;33(9):1628–1634. doi:10.1007/s12325-016-0389-3
13. King AJ, Stead RE, Rotchford AP. Treating patients presenting with advanced glaucoma - should we reconsider current practice? Br J Ophthalmol. 2011;95(9):1185–1192. doi:10.1136/bjo.2010.188128
14. Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2005;18(2):CD004399.
15. Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. Phase 1 clinical trials of a selective rho kinase inhibitor, K-115. JAMA Ophthalmol. 2013;131(10):1288–1295. doi:10.1001/jamaophthalmol.2013.323
16. Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156(4):731–6 e732. doi:10.1016/j.ajo.2013.05.016
17. Tanihara H, Inoue T, Yamamoto T, et al.; K-115 Clinical Study Group. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94(1):e26–34. doi:10.1111/aos.12829
18. Tanihara H, Inoue T, Yamamoto T, et al. Additive intraocular pressure-lowering effects of the rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol. 2015;133(7):755–761. doi:10.1001/jamaophthalmol.2015.0525
19. Tanihara H, Inoue T, Yamamoto T, et al.; K-115 Clinical Study Group. Intra-ocular pressure-lowering effects of a rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: a Randomized, Open-Label, Crossover Study. Acta Ophthalmol. 2015;93(4):e254–60. doi:10.1111/aos.12599
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.