Back to Journals » Drug Design, Development and Therapy » Volume 14
Ribbon Synapses and Hearing Impairment in Mice After in utero Sevoflurane Exposure
Authors Yuan X, Liu H, Li Y , Li W, Yu H, Shen X
Received 9 March 2020
Accepted for publication 18 June 2020
Published 8 July 2020 Volume 2020:14 Pages 2685—2693
DOI https://doi.org/10.2147/DDDT.S253031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Xia Yuan,1,* Hongjun Liu,1,* Yufeng Li,1 Wen Li,2 Huiqian Yu,3 Xia Shen1
1Department of Anesthesiology, Shanghai Eye, Ear, Nose, and Throat Hospital, Fudan University, Shanghai 200031, People’s Republic of China; 2Research Center, Shanghai Eye, Ear, Nose, and Throat Hospital, Fudan University, Shanghai 200031, People’s Republic of China; 3Department of Otorhinolaryngology, Shanghai Eye, Ear, Nose, and Throat Hospital, Fudan University, Shanghai 200031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huiqian Yu
Department of Otorhinolaryngology, Shanghai Eye, Ear, Nose, and Throat Hospital, Fudan University, Shanghai 200031, People’s Republic of China
Tel +86-21-64377134
Fax +86-21-64373416
Email [email protected]
Xia Shen
Department of Anesthesiology, Shanghai Eye, Ear, Nose, and Throat Hospital, Fudan University, Shanghai 200031, People’s Republic of China
Tel +86-21-64377134
Fax +86-21-64373416
Email [email protected]
Introduction: In utero, exposure to sevoflurane (a commonly used inhalation anesthetic) can lead to hearing impairment in offspring mice, but the underlying impairment mechanism is not known.
Materials and Methods: Day-15 pregnant mice were treated with 2.5% sevoflurane for 2 h to investigate sevoflurane ototoxicity. Cochleae from offspring mice were harvested for hair-cell and ribbon-synapse assessments. Hearing in offspring mice was assessed at postnatal day 30 using an auditory brainstem-response (ABR) test. Cochlear-explant cultures from offspring mice were exposed to 2.5% sevoflurane for 6 h. Immediately after treatment, explants were assessed for hair-cell morphology, mitochondrial oxidative stress, and autophagy.
Results: In utero, sevoflurane exposure impaired hearing in the offspring is demonstrated by a decrease in ABR wave I amplitudes, a marker for ribbon-synapse functionality. Sevoflurane exposure caused no obvious damage to hair cells, but cochlear ribbon synapses were reduced in postnatal day 15 offspring, and partially recovered by postnatal day 30. Sevoflurane treatment also increased mitochondrial reactive-oxygen species stress and decreased autophagy in the cochlear explants.
Conclusion: These results suggest that oxidative stress and reduced autophagy may underly ribbon-synapse involvement in sevoflurane-induced hearing loss.
Keywords: sevoflurane, hearing impairment, ribbon synapse, hair cells, cochlea
Introduction
With the continued development of medical techniques, an increasing number of pregnant women are requiring non-obstetric surgery. In the United States, approximately 75,000 pregnant women require surgery related to pregnancy or to other medical problems each year.1 In general, obstetric-related surgery is complex, and accordingly, requires more time under anesthesia. General anesthesia can include inhaled anesthetics, intravenous anesthetics, or a combination of these, and sevoflurane is one of the most common inhaled anesthetics. In an earlier study,2 we demonstrated that the offspring of pregnant mice exposed to sevoflurane developed hearing damage, but the underlying molecular mechanisms by which this anesthetic caused this hearing loss remains unclear.
Normal hearing requires accurate sound conduction to the central nervous system and accurate signal coding. Drug ototoxicity is a main cause of sensorineural hearing loss, often through the loss of hair cells,3,4 degeneration of spiral ganglion nerves,5 or damage to ribbon synapses.6 Ribbon synapses, located between inner hair cells and spiral ganglion neurons, are considered crucial for maintaining normal hearing function. Changes to these inner hair-cell synapses are thought to occur due to changes in cochlear environmental stimulation, but these synapses are also more sensitive to ototoxicity than hair cells.7,8 Here, we examined potential mechanisms underlying sevoflurane-related ototoxicity in the mouse cochlea.
Materials and Methods
Animals
All procedures involving animals were approved by the Animal Care and Use Committee of Fudan University and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In addition, the studies were designed to limit both the number of animals used and any potential suffering. Gestational day 15 (G15) C57BL/6J female mice (Shanghai SLAC laboratory animal Co., Ltd.) were used. All mice were maintained in temperature-controlled rooms (22–23°C) using a 12-hour light/dark cycle, with lights on daily at 7:00 am. Standard mouse chow and water were accessible ad libitum. Mouse pups were weaned 23 days after birth.
Sevoflurane Exposure
G15 pregnant mice were assigned randomly into two groups: an anesthesia group or a control (no anesthesia) group. Anesthesia group mice were placed in an anesthetizing chamber and exposed for 2 h to 2.5% sevoflurane and 60% oxygen (combined with N2). Control-group mice were placed in a similar chamber and were exposed only to 60% oxygen with 40% N2 (same flow rate) for 2 h. Mice breathed spontaneously throughout the exposure period. The sevoflurane concentration was continuously measured by a calibrated Vamos (Dräger, Lübeck, Germany) side-stream gas analyzer. Chamber temperature was regulated to keep the rectal temperature of each mouse at 37 ± 0.5°C. At the end of anesthetic exposure, mice were placed in a 60% oxygen chamber until their righting reflexes returned, and then for an additional 20 min.
Auditory Brainstem Response (ABR) Testing
The ABR test, as described previously,2 was used to measure auditory thresholds in offspring mice (n = 10 per group) at postnatal day 30 (P30). Briefly, each mouse was anesthetized (100 mg/kg ketamine and 25 mg/kg xylazine, i.p.) and placed on a regulated heating pad to keep body temperature at 37°C. The speaker was positioned 10 cm away from each mouse, and testing was performed double-blind. ABR thresholds were determined using specific acoustic stimuli, and dedicated equipment to amplify, measure, and display the evoked brainstem responses (System 3 RZ6, Tucker-Davis Technologies, Gainesville, FL, USA). Wave III thresholds were generally visible and reproducible. Evoked brainstem responses were amplified and averaged at each of four frequencies: 8, 16, 24, and 32 kHz. Auditory thresholds were obtained for each stimulus by changing the sound pressure level (SPL) by 10 dB increments (higher or lower) so as to identify the lowest level at which an ABR pattern could be recognized. ABR thresholds were determined at each stimulus frequency by identifying the lowest SPL level that produced a reproducible ABR pattern on the computer screen (with at least two consistent peaks).
Cochlear Tissue Processing
Cochlear tissue was harvested from postnatal day 15 (P15) and P30 offspring mice (n = 6 per group) for immunofluorescence staining. Prior to tissue harvest, mice were killed by cervical dislocation and decapitation. The temporal bone was removed and the cochlea was quickly dissected. The round and oval windows were opened, perfused with 4% paraformaldehyde, and left in 4% paraformaldehyde at 4°C overnight. Cochleae were then put into a 10% ethylenediaminetetraacetic acid (EDTA) solution for 2 to 3 days. The cochlear shell was then separated under a dissecting microscope in 0.01M phosphate-buffered saline (PBS) solution, the vestibular and tectorial membranes were removed, and the basilar membrane was harvested.
Immunofluorescence
Tissue samples were rinsed three times with PBS and permeabilized with 0.3% Triton X- 100 in PBS for 30 min at room temperature. Permeabilized samples were then blocked with 10% normal goat serum in PBS for 1 h and incubated with primary antibodies at 4°C overnight. The following primary antibodies were used: anti-CtBP2, a synaptic marker of ribbon synapse,9 (1:1000 dilution; BD Biosciences, New Jersey, USA), and anti-myosin VIIa (1:800 dilution; Proteus Biosciences, Ramona, CA,). After washes to remove unbound antibodies, tissues were incubated with TRITC- or Alexa Fluor 488-conjugated secondary antibodies (1:1000 dilution; Jackson Immuno Research, West Grove, PA, USA). Nuclei were labeled with 4ʹ,6-diamidino-2-phenylindole (DAPI) for 10 min at room temperature. Tissues were imaged with a confocal microscope (Leica, Heidelberg, Germany).
Cochlear-Explant Tissue Culture
C57BL/6J male mice were used to obtain cochlear tissue for the cultures. Cochleae from P2 mice were dissected in ice-cold PBS. Cochlear explants were cultured for 24 h on small round slides pre-coated with the cell-TAK (Discovery Labware, USA) to aid cochlear attachment. Culture medium was serum-free, containing Dulbecco’s modified Eagle medium (Gibco, Carlsbad, CA, USA) supplemented with N2 and B27 (both from Invitrogen, Carlsbad, CA, USA), and ampicillin (Sangon Biotech, Shanghai) and incubations were at 37°C in a 5% CO2 atmosphere.
Cochlear-Explant Treatments
Cultured cochlear explants were treated either with 21% O2, 5% CO2, and 2.5% sevoflurane from an anesthesia machine to a sealed plastic box for 6 h in a 37°C incubator, or with 5% CO2 plus 21% O2 (control conditions). Immediately after treatment, the samples were harvested for immunofluorescence (n = 4 per group) and Western blot analysis (n = 10 per group).
Oxidative Stress Assessment in Cochlear Explants
The Mito-Sox Red assay (Life Technologies, M36008) was used to analyze reactive-oxygen species (ROS) production in live cochlear explant cultures (n = 6) following the manufacturer’s instructions. After sevoflurane exposure, tissues were incubated in 5 μM Mito-Sox reagent for 10 min at 37°C. The reagent was then removed and the tissues were washed three times with PBS. Samples from treated and control groups were processed in parallel and immunolabeled with identical solutions. Images were acquired using a Nikon fluorescence microscope (Nikon, Japan), under identical conditions with equal laser gain settings. Fluorescence intensities were quantified using Image J software, and then normalized to DAPI fluorescence intensity to improve measurement accuracy.
Western Blot Analysis
Total protein was extracted from cultured cochlear tissue (n = 10 per group). Protein concentrations were determined using a bicinchoninic acid (BCA) protein kit (Beyotime, P0010S). Protein samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then blotted onto polyvinylidene fluoride (PVDF) membranes (Immobilon-140 P; Millipore, Bedford, MA). The membranes were blocked (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; and 0.1% Tween-20) for 1 h at room temperature and incubated overnight at 4°C with either anti-LC3B (1:1000 dilution; Sigma-Aldrich, L7543) or anti-β-actin (1:10,000 dilution; Cell Signaling Technology, abs119600) primary antibodies. The membranes were washed three times in Tris-buffered saline with Tween-20 (TBST) for 10 min each and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000 dilution; Cell Signaling Technology, A0208) for 1 h at room temperature. Blots were developed using an enhanced chemiluminescence (ECL)-Kit (Beyotime, Jiangsu, China). Each experiment was repeated at least three times.
Statistical Analysis
Outcomes were measured by a group-blinded observer. Data are shown as median (minimal, maximal), and all experiments were repeated at least three times. Statistical analyses were conducted using Microsoft Excel and GraphPad Prism 6 software. An indicated n size represents either the number of mice or independent cochleae. The Mann–Whitney U-tests were used to determine statistical significance for the two-group comparisons. A P value < 0.05 was considered statistically significant.
Results
In utero Sevoflurane Exposure Impaired Mouse Offspring Hearing
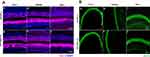
ABR testing showed that hearing thresholds at different frequencies were significantly elevated in the sevoflurane-exposure group compared to the control group at 8 kHz [25 (25, 30) dB SPL vs 35 (30,35), P = 0.013], 16 kHz [27.5 (25, 30) dB SPL vs 35 (30, 40), P = 0.028], 24 kHz [30 (25, 35) dB SPL vs 40 (35, 40), P = 0.013], and 32 kHz [35 (35, 40) dB SPL vs 50 (45, 55), P = 0.002), indicating hearing impairment (Figure 1A). We also calculated wave I amplitudes at a stimulus of at 90 dB SPL at different frequencies. Wave I amplitudes were significantly lower in the sevoflurane-exposure group compared to the control group at 8 kHz [2.47 (1.57, 3.12) uV vs 1.61 (1.55,2.46), P = 0.014], 16 kHz [2.88 (1.89, 3.39) uV vs 1.99 (1.59, 2.69), P = 0.011], 24 kHz [1.09 (0.99, 1.32) uV vs 0.96 (0.89, 1.42), P = 0.034], and 32 kHz [0.70 (0.29, 1.29) uV vs 0.29 (0.14, 0.69), P = 0.019] (Figure 1B and C).
Sevoflurane Exposure Did Not Impair Cochlear Hair Cells in Offspring Mice
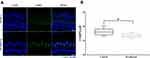
Ototoxicity often leads to morphological changes in the cochlea, such as stereocilia disruption or the loss of hair cells, resulting in hearing impairment.3,4 We examined hair-cell morphology and number using immunohistochemistry in P30 mice. Compared to control-group hair cells, no obvious morphological changes were observed in sevoflurane-exposed hair cells, or in their numbers, throughout the three regions examined (Figure 2A). We also examined these same hair cell features in cochlear-explant cultures after sevoflurane exposure. Neither hair-cell morphology, nor their numbers, changed significantly after exposure to the anesthetic (Figure 2B). These results suggest that sevoflurane-related ototoxicity is not due to hair-cell damage.
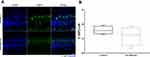
In utero Sevoflurane Exposure Caused Ribbon-Synapse Degeneration in Cochleae of P15 Offspring Mice
With the aforementioned results suggesting that hair cells were not involved in this hearing impairment, and ABR tests showing that wave I amplitudes (surrogates for ribbon-synapse function) were reduced in the sevoflurane-exposure group, we next examined the number of ribbon synapses in the cochlear middle turn in P15 offspring. At this age (Figure 3), the number of cochlear ribbon synapses in the sevoflurane-exposure group was significantly less compared to the control group [18.04 (16.57, 20.29) vs 16.79 (15.71, 17.86), P = 0.040]. This finding suggests that ribbon-synapse impairment may be the underlying mechanism for sevoflurane-related hearing loss.
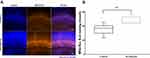
After Sevoflurane Exposure, Partial Ribbon-Synapse Restoration in Cochleae of P30 Offspring Mice
After ototoxic-drug withdrawal, there is evidence that ribbon synapses have the capacity to regenerate,10 so we also examined ribbon-synapse numbers in the middle turn of the cochlea in P30 offspring after in utero sevoflurane exposure. At this longer time point after exposure (Figure 4), the number of ribbon synapses in the sevoflurane-exposure group was similar to that found in the control group [17.82 (16.43, 19.67) vs 16.29 (11.67, 19.33), P = 0.343], and suggests a partial restoration of ribbon synapses after sevoflurane withdrawal.
Mitochondrial ROS Levels Increased in Cochlear-Explant Cultures After Sevoflurane Exposure
In a previous study,2 we found that 2 h of sevoflurane exposure of G15 pregnant mice induced swollen mitochondria in both the cochlear neurons and hair cells of their offspring. Here, we dissected and cultured cochleae from P2 mice and exposed them to 2.5% sevoflurane for 6 h. Mito-Sox Red staining, a redox fluorophore that selectively detects mitochondrial superoxide, was used to assess any changes in mitochondrial ROS levels caused by sevoflurane. Quantification of Mito-Sox Red staining intensity (Figure 5) showed that ROS levels increased significantly in the middle turn of the cochlea after sevoflurane treatment compared to control levels [18.04 (16.57, 20.29) vs 16.79 (15.71, 17.86), P = 0.040] (47.91 ± 1.88 vs 34.46 ± 2.66, P = 0.0014], suggesting a potential mechanism to account for ribbon-synapse impairment.
Autophagy Decreased in Cochlear-Explant Cultures After Sevoflurane Exposure
Previous research has shown that ROS have the ability to induce cellular defense pathways such as autophagy.11 We therefore assessed autophagy in cochlear-explant cultures using Western blot analysis of LC3B-II expression, a marker for autophagy. Ratio of LC3B-II/β-actin expression decreased significantly [0.46 (0.34, 0.68) vs 0.33 (0.26, 0.37), P = 0.030] in cochlear-explant cultures after sevoflurane exposure (Figure 6).
Discussion
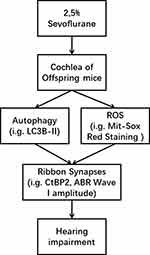
We have shown that in utero exposure to sevoflurane impairs hearing in postnatal mice by a mechanism that involves ribbon synapses rather than hair cells, and that autophagy decreased during the same time as mitochondrial oxidative stress increased in cochlear explants exposed to sevoflurane. Potential mechanism of sevoflurane induced hearing impairment, as shown as Figure 7.
Consistent with our previous findings,2 ABR testing demonstrated that exposure to sevoflurane during pregnancy caused hearing impairment in C57BL/6J offspring mice. As hair-cell damage is a common mechanism for hearing impairment,3 we assessed hair cells in P30 offspring after in utero exposure to sevoflurane and found no differences between control cochleae and those exposed to sevoflurane either in hair-cell morphology or survival. Similarly, hair cells from cochlear-explant cultures exposed to sevoflurane were also unchanged compared to control hair cells. These findings suggested that sevoflurane-related ototoxicity was caused by other mechanisms.
Other studies have proposed that acoustic overexposure can change cochlear ribbon synapses and cause temporary hearing loss despite normal cochlear morphology.6,11 Ribbon synapses are very sensitive to external insults,7,8 so we hypothesized that changes in these synapses may have contributed to sevoflurane-induced hearing impairment. As ABR testing showed that wave-I amplitudes (surrogates for ribbon-synapse function) were decreased after sevoflurane exposure, we assessed these synapses in cochleae from P15 mice and found that sevoflurane ototoxicity significantly decreased the number of ribbon synapses without changing the number and morphology of hair cells.
Cochlear ribbon synapses, located between hair cells and the terminals of bipolar primary auditory neurons (spiral ganglion neurons), represent the first afferent synaptic connections in the hearing pathway.12–14 These synapses are characterized by pre-synaptic ribbons to which many synaptic vesicles are attached, and the ribbons are anchored to the active synaptic zone where vesicle exocytosis occurs.15–17 Ribbon synapses have been recognized for their high rate of neurotransmitter release,18–20 and mammalian ribbon synapses are associated with sensory systems that require the highest rates of information transfer and sensory discrimination, such as vision and hearing.21,22 Our finding of ribbon-synapse loss in P15 mice after in utero sevoflurane exposure confirms previous reports7,8,23 that these synapses are more sensitive to external stimuli than hair cells.
Previous studies have also shown that, after ototoxic agent withdrawal, shifts in hearing thresholds were consistent with synapse repair.8,10 We also found evidence of partial cochlear synapse restoration in P30 mice, but ABR thresholds did not recover. One possible explanation is that sevoflurane, in addition to damaging these synapses, may have also damaged spiral ganglion neurons. Another possibility is that the partially-restored ribbon synapses had not yet fully recovered their functionality. Zhang et al found that sevoflurane may cause neurodevelopmental delays in the offspring mice by reducing neurogenesis through the Wnt-catenin signaling pathway.24
Our previous study demonstrated that sevoflurane exposure caused significant cochlear mitochondrial swelling and an increase in nitric-oxide synthase expression.2 Here, we found that mitochondrial oxidative stress was also elevated in cochlear-explant cultures after sevoflurane exposure, suggesting that ROS triggered this stress and made cochleae more vulnerable to mitochondrial dysfunction and subject to eventual ribbon-synapse loss. However, accumulating evidence supports the idea that ROS and autophagy are both implicated in the onset and development in many diseases, with autophagy proposed to be a cellular defense against destructive stimuli by ensuring the timely removal of damaged cells and cytotoxic substances,11,25,26 but when impaired or abnormally upregulated, autophagy can also cause cell loss.27 In the other words, ROS and autophagy may both be determinants for modulating cellular homeostasis. Here, cochlear autophagy decreased (i.g. decreased expression of LC3B-II) after sevoflurane exposure, despite an increase in cellular ROS stress (i.g. increased Mito-Sox Red staining intensity).
There are a few limitations in our study. First, we did not examine neurotrophin expression in the cochlea, even though anesthetics have been reported to decrease neurotrophins in the developing brain,28 and overexpression of neurotrophin-3 in cochlear supporting cells may promote the recovery of synapse number and function after noise damage.29 Second, we did not assess ribbon-synapse function in hair cells. Functionally impaired ribbon synapses relate to reduction of Ca2+ currents and exocytosis.30 Third, we did not assess the number of spiral ganglion neurons or their morphology. In addition to hair cells and ribbon synapses, these neurons play an important role in normal hearing.5 In addition, anesthetic exposure during pregnancy may cause postsynaptic changes in the offspring mice during development.31 Further study is warranted to test the postsynaptic change of the ribbon synapses.
Conclusion
We found that in utero sevoflurane exposure caused hearing impairment in C57BL/6J offspring mice and that this impairment was associated with cochlear synaptic-ribbon impairment. In addition, increased mitochondrial oxidative stress and decreased autophagy may be mechanisms that account for this synaptic impairment. However, further research is required to determine how sevoflurane exposure changed this synaptic function without changing hair-cell survival or morphology.
Author Contributions
Xia Shen and Huiqian Yu designed the study. Xia Yuan, Hongjun Liu, Yufeng Li, and Wen Li conducted the study. Xia Shen and Huiqian Yu analyzed the data. Xia Yuan carried out the in vivo study. Hongjun Liu carried out the in vitro study. Xia Shen and Huiqian Yu wrote the manuscript, and all other authors participated in manuscript revisions. All Authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Funding
Funding was provided by the National Natural Science Foundation of China (Grant #81671045). We acknowledge Professor Huawei Li for technical support and critical discussions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Reitman E, Flood P. Anaesthetic considerations for non-obstetric surgery during pregnancy. Br J Anaesth. 2001;107:i72–i78. doi:10.1093/bja/aer343
2. Shen X, Xiao Y, Li W, et al. Sevoflurane anesthesia during pregnancy in mice induces hearing impairment in the offspring. Drug Des Devel Ther. 2018;12:1827–1836. doi:10.2147/DDDT.S156040
3. McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997(1):40–51. doi:10.1016/j.brainres.2003.10.031
4. Ding D, Jiang H, Salvi RJ. Mechanisms of rapid sensory hair cell death following co-administration of gentamicin and ethacrynic acid. Hear Res. 2010;259(1–2):16–23. doi:10.1016/j.heares.2009.08.008
5. Liang Q, Shen N, Lai B, Xu C, Sun Z, Wang Z. Electrical stimulation degenerated cochlear synapses through oxidative stress in neonatal cochlear explants. Front Neurosci. 2019;13:1073. doi:10.3389/fnins.2019.01073
6. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi:10.1523/JNEUROSCI.2845-09.2009
7. ShuNa L, Zhong R, Ke L. A pattern of otoferlin expression interrupted by gentamicin exposure in ribbon synapse of inner hair cell in C57BL/6J mice. Acta Neurol Belg. 2009;109(3):221–225.
8. Liu K, Jiang X, Shi C, et al. Cochlear inner hair cell ribbon synapse is the primary target of ototoxic aminoglycoside stimuli. Mol Neurobiol. 2013;48:647–654.
9. Uthaiah RC, Hudspeth AJ. Molecular anatomy of the hair cell’s ribbon synapse. J Neurosci. 2010;30(37):12387–12399. doi:10.1523/JNEUROSCI.1014-10.2010
10. Liu K, Chen DS, Guo WW, et al. Spontaneous and partial repair of ribbon synapse in cochlear inner hair cells after ototoxic withdrawal. Mol Neurobiol. 2015;52(3):1680–1689. doi:10.1007/s12035-014-8951-y
11. Yuan H, Wang X, Hill K, et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxidants Redox Signal. 2015;22(15):1308–1324. doi:10.1089/ars.2014.6004
12. Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. Gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci. 2004;24(17):4213–4223. doi:10.1523/JNEUROSCI.0223-04.2004
13. Moser T, Neef A, Khimich D. Mechanisms underlying the temporal precision of sound coding at the inner hair cell ribbon synapse. J Physiol. 2006;576(1):55–62. doi:10.1113/jphysiol.2006.114835
14. Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res. 2006;221(1–2):104–118. doi:10.1016/j.heares.2006.07.014
15. Lenzi D, Crum J, Ellisman MH, Roberts WM. Depolarization redistributes synaptic membrane and creates a gradient of vesicles on the synaptic body at a ribbon synapse. Neuron. 2002;36(4):649–659. doi:10.1016/S0896-6273(02)01025-5
16. Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell’s afferent synapse. Proc Natl Acad Sci. 2006;USA.103(14):5537–5542. doi:10.1073/pnas.0601103103
17. Johnson SL, Forge A, Knipper M, Munkner S, Marcotti W. Tonotopic variation in the calcium dependence of neurotransmitter release and vesicle pool replenishment at mammalian auditory ribbon synapses. J Neurosci. 2008;28(30):7670–7678. doi:10.1523/JNEUROSCI.0785-08.2008
18. Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002;164(1–2):115–126. doi:10.1016/S0378-5955(01)00417-8
19. Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 0022;5(2):147–154. doi:10.1038/nn796
20. Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005;435(7039):212–215. doi:10.1038/nature03567
21. Juusola M, French AS, Uusitalo RO, Weckstrom M. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19(7):292–297. doi:10.1016/S0166-2236(96)10028-X
22. Fuchs M, Scholz M, Sendelbeck A, et al. Rod photoreceptor ribbon synapses in DBA/2J mice show progressive age-related structural changes. PLoS One. 2012;7(9):e44645. doi:10.1371/journal.pone.0044645
23. Tong M, Brugeaud A, Edge ASB. Regenerated synapses between postnatal hair cells and auditory neurons. JARO. 2013;14(3):321–329. doi:10.1007/s10162-013-0374-3
24. Zhang Y, Dong Y, Zheng H, et al. Sevoflurane inhibits neurogenesis and the Wnt-catenin signaling pathway in mouse neural progenitor cells. Curr Mol Med. 2013;13(9):1446–1454. doi:10.2174/15665240113139990073
25. Congcong F, Lijuan G, Daniel S, Shanping M, Xiaoxing X. The interrelation between reactive oxygen species and autophagy in neurological disorders. Oxid Med Cell Longev. 2017;2017:1–16.
26. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi:10.1016/j.cell.2010.01.028
27. Janda E, Isidoro C, Carresi C, Mollace V. Defective autophagy in parkinson’s disease: role of oxidative stress. Mol Neurobio. 2012;46(3):639–661. doi:10.1007/s12035-012-8318-1
28. Guo S, Liu L, Wang C, Jian Q, Dong Y, Tian Y. Repeated exposure to sevoflurane impairs the learning and memory of older male rats. Life Sci. 2018;192:75–83. doi:10.1016/j.lfs.2017.11.025
29. Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife. 2014;3:e03564. doi:10.7554/eLife.03564
30. Liu H, Lu J, Wang Z, et al. Functional alteration of ribbon synapses in inner hair cells by noise exposure causing hidden hearing loss. Neurosci Lett. 2019;707:134268. doi:10.1016/j.neulet.2019.05.022
31. Zou S, Wei ZZ, Yue Y, Zheng H, Jiang MQ, Wu A. Desflurane and surgery exposure during pregnancy decrease synaptic integrity and induce functional deficits in juvenile offspring mice. Neurochem Res. 2020;45(2):418–427. doi:10.1007/s11064-019-02932-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.