Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 15
Rho-Associated Protein Kinase Inhibitor and Hypoxia Synergistically Enhance the Self-Renewal, Survival Rate, and Proliferation of Human Stem Cells
Authors Alsobaie S , Alsobaie T, Mantalaris S
Received 10 March 2022
Accepted for publication 22 June 2022
Published 2 July 2022 Volume 2022:15 Pages 43—52
DOI https://doi.org/10.2147/SCCAA.S365776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Bernard Binetruy
Sarah Alsobaie,1 Tamador Alsobaie,2 Sakis Mantalaris3
1Department of Clinical Laboratory Science, King Saud University, Riyadh, 11451, Saudi Arabia; 2Biological Systems Engineering Laboratory, Department of Chemical Engineering, Imperial College London, London, SW7 2AZ, UK; 3Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, 30322, USA
Correspondence: Sarah Alsobaie, Department of Clinical Laboratory Science, King Saud University, Prince Turki Alawal Street, Riyadh, 11451, Saudi Arabia, Tel +966 507191011, Fax +966 114677580, Email [email protected]
Introduction: High-efficacy single-cell cloning of human-induced pluripotent cells (IPSCs) remains a major challenge. The development of a culture method that supports single-cell passaging while maintaining reproducibility, homogeneity, scalability, and cell expansion to clinically relevant numbers is necessary for clinical application.
Methods: To address this issue, we combined the use of the rho-associated protein kinase (ROCK) inhibitor Y-27632 and hypoxic conditions in culture to produce a novel, efficient single-cell culture method for human IPSCs and embryonic stem cells.
Results: Through immunocytochemistry, alkaline phosphatase assays, and flow cytometry, we demonstrated that our method enabled high single-cell proliferation while maintaining self-renewal and pluripotency abilities.
Discussion: We showed the beneficial effect of the interaction between hypoxia and ROCK inhibition in regulating cell proliferation, pluripotency, and single-cell survival of pluripotent cells.
Keywords: pluripotent cells, rho-associated protein kinase, stem cells
A Letter to the Editor has been published for this article.
Introduction
The ability of human-induced pluripotent stem cells (IPSCs) to differentiate and develop into various types of tissues has garnered considerable attention.1,2 Although the culture methods for human embryonic stem cells (ESCs) and IPSCs have advanced, several challenges still prevent the clinical application of these methods. The challenges include the lack of standardization of maintenance and differentiation protocols in the context of serum- and xeno-free conditions, inadequacy of analysis in monitoring cellular stress and signaling, poor growth conditions, spontaneous differentiation, apoptosis during cell processing, and heterogeneous cell populations.3
To reduce heterogeneity and improve the efficiency of human IPSC differentiation, their high pluripotency states must be maintained. Although this may be accomplished by culturing human ESCs on mouse or human feeder cell layers,3 serum- and animal-derived materials cannot be used in clinical applications. Thus, a feeder- and serum-free culture system is needed for the maintenance, expansion, and differentiation of human IPSCs in the clinical setting. Extracellular matrices, such as undefined Matrigel and Geltrex, and animal-free extracellular proteins, such as recombinant vitronectin and Laminin-511, have been used to culture human IPSCs,4 but these options are limited in terms of homogeneity and reproducibility.5 The use of more defined serum- and feeder-free media such as mTeSR™1 and HAIF, is a feasible option for high-quality maintenance of human ESCs and IPSCs.6,7
The production of more phenotypically defined and karyotypically stable cell populations requires their maintenance as single cells to sustain their pluripotent state and high differentiation potential, which enables the use of 3D cultures and high-throughput techniques.7,8 However, single-cell cultures of human pluripotent stem cells (PSCs) could undergo apoptosis.9,10 To prevent this, inhibition of Rho-associated protein kinase (ROCK), a serine-threonine kinase that phosphorylates and activates the myosin II pathway, has been utilized to enable single-cell survival via inhibition of the E-cadherin-dependent apoptotic pathway.8,11–16
Hypoxia maintains the pluripotent state of stem cells, enhances their proliferation, and reprograms somatic cells into pluripotent cells.17,18 This is because oxygen is an efficient signaling molecule that acts on the fundamental characteristics of different stem cell types.19 Low oxygen tension (2%) culture conditions allow robust recovery of human ESC clones20 and reduction of chromosomal abnormalities. Additionally, growing stem cells in 5–7% oxygen results in an increase in blastocyst development rate and cell number.21
Single-cell passaging promotes homogeneity, offers easy accessibility to single molecules, and increases the efficiency of cellular responses; additionally, it has been found to be a critical differentiation factor for cardiomyocyte and lung epithelial lineages.22–24 Several studies have demonstrated the individual benefits of ROCK inhibitors and hypoxic conditions on cell adhesion, proliferation, survival, self-renewal, and differentiation.25–28 Thus, to create an optimal environment that enables single-cell survival, growth, and pluripotency maintenance, for the first time, we combined the use of an ROCK inhibitor and hypoxic conditions for self-renewal maintenance.
Methods
Cell Culture
The embryonic stem cell line H9 and the induced pluripotent stem cell line IMR90-1 (obtained by Viral transfection (Lentivirus: Oct4, Sox2, Nanog, Lin28)) were used in this study. Both cell lines were obtained from WiCell Research Institute Inc. (Madison, WI, USA). Both cell lines were cultured in Matrigel-coated six-well plates with 2 mL of complete mTeSR™1 medium per well and incubated for the experimental period at 37°C under 5% CO2 in a humidified incubator (Nuaire 5500E) and either using 5% (hypoxic condition) or 21% O2 (normoxic).
The cultures were examined using microscopy, and the culture media were replaced with fresh media daily for 4–5 d depending on colony size. The colonies were then dissociated with 1 U/mL dispase (a protease suitable for cell dissociation) in DMEM/F-12 medium (Stem Cell Technology, Canada) and transferred onto newly coated culture plates with fresh medium.
Imaging
Phase-contrast and fluorescence microscopy images were acquired with one of the following two microscopes: (1) an IX70 inverted microscope (Olympus) equipped with a color Cool Pix 950 digital camera (Nikon) and a black and white, high-resolution charge-coupled device (CCD)-camera (Soft Imaging Systems); the results were analyzed using analySISB and analySISD software; and (2) a BX60 upright microscope (Olympus) equipped with an Axiocam digital camera (ImageJ); the results were analyzed using KS-300 software. For fluorescence imaging, the excitation wavelengths used were 490 nm for fluorescein isothiocyanate (FITC) and green fluorescent protein (GFP), 570 nm for tetramethylrhodamine isothiocyanate (TRITC) and Texas Red, and 360 nm for the nuclear counterstain 4’-6-diamidino-2-phenylindole (DAPI).
Single Cell Suspension
The mTeSR™1 culture medium was added with 10 µM Y-27632 at 2 h before the use of accutase (containing proteolytic and collagenolytic enzymes) to dissociate the cells into single-cell suspensions. The cells in the suspensions were then recovered by centrifugation, washed with phosphate-buffered saline (PBS), gently homogenized with a pipette, and passed through a 40-micron cell strainer. The dissociated cells were then seeded onto Matrigel-coated, flat-bottomed 96-well plates at low densities of 50000 cells/0.32 cm2 well.8
Immunocytochemistry
Immunocytochemistry was performed with the cell cultures in a slide chamber according to a previously reported protocol Supplementary Table S1. 29
MTS Assay
The cells were assayed at 12, 48, 72, and 96 h and compared against a standard curve (Supplementary Figure S1). MTS substrate diluted in cell culture medium was added to the cell cultures at 0.2–0.5 mg/mL and incubated for 1–4 h. The quantity of formazan was measured by recording the changes in the absorbance at 490 nm using a plate spectrophotometer.
Flow Cytometry
Flow cytometry was used to quantify the cells expressing pluripotency markers of human ESCs and IPSCs. This was performed using the Human Embryonic Stem Cell Kit (BD) according to the manufacturer’s instructions. The analysis was conducted using a BD LSR Fortessa (4 lasers) cytometer featuring blue (488 nm), yellow/green (561 nm), violet (405 nm), and red (640 nm) lasers.
For the calibration procedure, Calibrite™3 beads (Becton Dickinson, UK), which are suitable for PE, PerCP, and APC calibration, were used before the analysis. Unstained cells and cells stained with isotypes were used as controls to ensure that no background fluorescence/nonspecific binding was accounted for in these analyses.
Data were further processed using FlowJo software (version 10.5.3).
Further details of reagents, resources, sources, and identifiers are summarized in the Key Resource Table attached separately.
Results
Human IPSCs in naive undifferentiated states were successfully obtained as colonies with a feeder-free protocol using mTeSR™1 medium and Matrigel™ matrix substrate. By adding 10 µM Y-27632 (an inhibitor of the ROCK signaling pathway RI) to the culture medium 2 h before passaging the cells and daily for 4 days after passaging, single cells were produced and prevented from undergoing dissociation-induced apoptosis (Supplementary Figure S2).
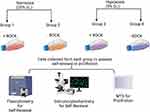
To study the effect of oxygen tension and ROCK inhibitor, the cells were separated into four different plates to generate four groups—single cells growing in the presence of 20% oxygen and ROCK inhibitor (RI), single cells maintained without ROCK in the same oxygen conditions, single cells maintained in 5% oxygen with RI, and cells grown under low oxygen conditions without RI (Figure 1).
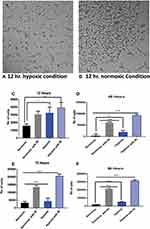
After 12 h, the cells under hypoxic conditions were able to adhere to the plate even in the absence of RI compared with cells grown under similar conditions in the presence of oxygen at normal levels (Figure 2A and B). Approximately 40 × 103 cells out of the 50 × 103 initially seeded cells survived under the RI/HYPOXIA condition, which was higher than that under other conditions (Figure 2C). Interestingly, the MTS assay of cells under various oxygen tension conditions revealed that the RI/HYPOXIA group had a significantly higher number of cells in the culture at all time points (Figure 2C–F).
At 48 h, the cell number in the RI/HYPOXIA group doubled to 100 × 103 cells, whereas the cell number in the RI/NORMOXIA group reached less than 70 × 103 cells. Similarly, at 72 h, the proliferation rate continued to rise exponentially in the RI/HYPOXIA group, reaching more than 200 × 103 cells. After 4 days, the cell numbers were significantly increased in the presence of 10 µM Y-27632 and 5% O2 compared with those in the other groups.
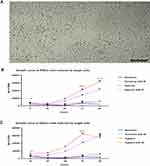
Next, we investigated whether hypoxia with ROCK inhibitor treatment affected the self-renewal capacity of the cells. The expression of the pluripotency markers Nanog, Oct3/4, and SSEA4 was assessed using flow cytometry. We found that the cells under all conditions expressed >70% of the markers, without any significant differences between the groups (Figure 3). All groups of IPSCs expressed the pluripotency surface marker SSEA-4 and the nuclear markers OCT4 and Nanog. We observed more than 75% double-positive cells (Figure 3A–C).
Finally, to validate the robustness of these experiments on other stem cell lineages, the same conditions were applied to the human ESC 9H cell line. The same trend was observed for all groups in this cell line. We compared the effects of ROCK and oxygen tension levels between human ESCs and IPSCs (Figure 4A–C).
The human IPSCs grown in hypoxia with ROCK inhibitor treatment were observed using light microscopy (Supplementary Figure S3). On day 5, the images showed a typical morphology of round pluripotent colonies, consisting of dense, flat, and smooth colonies with clear borders (Supplementary Figure S3A and B). Upon further observation, the human IPSCs displayed larger nuclei in comparison with the cytoplasm and prominent nucleoli, indicative of exponential proliferation (Supplementary Figure S3C). The spontaneous differentiation in the colonies was characterized by the presence of a compact central core, loss of defined edges, and areas of irregular cellular morphology, as indicated by the arrows in Supplementary Figure S3D. To further confirm the self-renewal ability of hIPSCs grown under ROCK and hypoxic condition, AP staining was performed with the confluent cultured human IPSCs at passage 39. All cells within the colony exhibited high AP activities, as shown in Supplementary Figure S3E.
To confirm the pluripotency of the RI/HYPOXIA human IPSCs, immunoreactivity to pluripotency markers was evaluated after 5 days at passage 39 (Supplementary Figure S4). The cells showed a high expression of the following common proteins found in pluripotent cells: nuclear markers, Oct4, Sox2, and Nanog (Supplementary Figure S4A–C). The surface markers SSEA4, TRA-1-60, and TRA-1-81 (Supplementary Figure S4D–F) were also positively expressed.
We also performed flow cytometry analysis to quantify the expression of the pluripotency-associated markers. After passaging the cells over five times, 86% of the cells were Nanog+SSEA4+, indicating that a high percentage of cells was simultaneously positive for both markers. This indicates that these intracellular and surface markers were co-expressed in these cells.
The IPSC cultures with hypoxia/RI also accumulated undifferentiated cells over 5 passages (34–39), showing no difference in the percentage of pluripotent cells co-expressing these two markers (Supplementary Figure S5). Unstained human IPSCs and manufacturer-recommended isotypes were used as controls to set the exclusion gates that generated these results (Supplementary Figure S6 for passage 34 and Supplementary Figure S7 for passage 39).
Discussion
The synergistic effects of the ROCK inhibitor Y-27632 and 5% oxygen conditions enhanced the viability and pluripotency of single-cell suspensions of human IPSCs. The development of culture methods that allow passaging of single cells while promoting self-renewal and maximizing yield to suitable numbers is necessary to accelerate stem cell research as methods of characterization and application require single-cell populations.30 Single-cell passaging leads to an extreme reduction in cell viability, and inhibiting the ROCK pathway prevents stem cell apoptosis when disturbing colonies to single cells.31
In this study, both human ESCs and IPSCs showed a one-fold increase in cell numbers after cell culture with ROCK inhibitors under hypoxia for 72 h. ROCK exerts direct effects on single-cell proliferation in different types of cells. Nakashima et al32 found that ROCK enhances pancreatic cancer cell proliferation. Horani et al33 also observed enhanced basal-cell proliferation in cultured human tracheobronchial and mouse tracheal epithelial cells. However, our results showed that even in the absence of ROCK, hypoxia alone was able to maintain single-cell survival of human IPSCs, suggesting that hypoxia plays an important role in single-cell viability. Cell yields were also found to be >2-fold higher than that of the NO ROCK/NORMOXIA group 96 h after seeding, reflecting the effect of low oxygen tension (Figures 4).
Increased proliferation rate of stem cell colonies under low oxygen tension has also been observed in several other primitive populations, including mesenchymal stem cells and neural progenitor cells.34,35 Forristal et al18 demonstrated that ESCs under 5% oxygen showed increased cell proliferation and attachment to MEFs or feeder-free Matrigel™, and this is consistent with the results of Ludwig et al7 A previous study found that exposing chondrocytes treated with both 10 µM Y-27632 and 5% oxygen resulted in the deregulation of cell cycle-associated proteins and cellular growth and proliferation, as well as the development of tissue-like morphology. In addition, increased production of the extracellular matrix was observed under these conditions.36
There was a noticeable increase in cellular attachment after 12 h in the ROCK/HYPOXIC group (Figure 4B). ROCK inhibitors are known to increase cellular adhesion, and adding them to Matrigel increases plating efficiency by promoting ECM–integrin interactions.15 Furthermore, ROCK-treated human ESCs are more difficult to dissociate enzymatically.37 Duś-Szachniewicz et al found enhanced integrin and cadherin expression and attachment to Matrigel and stromal cells of single lymphoma cell adhesive proteins.36 However, the effect of hypoxia on single-cell adhesion in IPSCs has not been explored.
To further confirm the self-renewal ability of the cells, we measured glycoprotein markers, Tra1-60, Tra-1-81, SSEA3, and SSEA4, which are highly associated with self-renewal.1 In this study, hypoxia and ROCK did not affect self-renewal as the levels of Oct4, Sox2, and Nanog in our culture method were not significantly different from those in the standard culture method. The positive effect of hypoxia on pluripotency, as observed by maintenance of OCT-4, SOX-2, and Nanog expression in several studies, has been shown to be regulated by HIF‑2α.18,38 In addition, ROCK inhibitors have been shown to enhance pluripotency. Peerani et al39 and Harb et al40 highlighted the role of ROCK in the maintenance of human ESC pluripotency. Peerani et al39 also demonstrated that Y-27632 treatment increased Oct4 expression. Furthermore, Harb et al40 found that human ESCs added with Y-27362 to a single synthetic matrix grew without the need for niche-forming feeder layers or animal-derived matrices.
Further studies are needed to elucidate the signaling pathway underlying the upregulation of self-renewal markers during cell incubation under low oxygen conditions, such as hypoxia-inducible factor (HIF) genes. Additional investigations are also required to clarify whether the crosstalk of ROCK inhibition and HIF upregulation has an effect of cell-survival enhancement in human stem cells.
Conclusions
Our findings support that culturing human IPSCs on Corning Matrigel matrix in mTeSR™1 medium maintained their stem cell properties at high expansion rates.6 Compared with conventional protocols, this culture method is simple, easy, and reproducible. Self-renewal assays involving immunocytochemistry, AP, and flow cytometry confirmed our method’s ability to maintain undifferentiated human IPSCs. The application of hypoxia and ROCK inhibitors enhanced the proliferation of single human IPSCs without affecting their self-renewal ability. Additionally, single-cell cultures using human ESCs showed results similar to those of human IPSCs.
This study provides, to the best of our knowledge, the first evidence of the synergistic effect of ROCK inhibition and hypoxia on expanding viable pluripotent single-cell suspensions of human IPSCs. Further studies are warranted to determine the underlying mechanisms associated with the combined effect of low oxygen tension and ROCK pathway inhibition.
Abbreviations
AP, alkaline phosphatase; BSA, bovine serum albumin; BSEL, Biological Systems Engineering Laboratory; ESC, embryonic stem cell; HESC, human embryonic stem cell; IPSC, induced pluripotent cell; PBS, phosphate-buffered saline; PerCP, peridinin-chlorophyll-protein; PI, propidium iodide; PSC, pluripotent stem cell.
Acknowledgment
This research was funded by King Saud University, Vice Deanship of Research Chairs.
Funding
This study was financially supported by King Saud University, Vice Deanship of Research Chairs.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi:10.1126/science.282.5391.1145
2. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi:10.1038/292154a0
3. Chen KG, Mallon BS, McKay RDG, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Stem Cell. 2014;14:13–26. doi:10.1016/j.stem.2013.12.005
4. Braam SR, Zeinstra L, Litjens S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alpha V beta 5 integrin. Stem Cells. 2008;26:2257–2265. doi:10.1634/stemcells.2008-0291
5. Lam MT, Longaker MT. Comparison of several attachment methods for human iPS, embryonic and adipose-derived stem cells for tissue engineering. J Tissue Eng Regen Med. 2012;6(Supplement 3):s80–s86. doi:10.1002/term.1499
6. Lannon C, Moody J, King D, et al. A defined, feeder-independent medium for human embryonic stem cell culture. Cell Res. 2008;18:S34–S34. doi:10.1038/cr.2008.124
7. Ludwig TE, Bergendahl V, Levenstein ME, et al. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi:10.1038/nmeth902
8. Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi:10.1038/nbt1310
9. Hasegawa K, Fujioka T, Nakamura Y, Nakatsuji N, Suemori H. A method for the selection of human embryonic stem cell sublines with high replating efficiency after single- cell dissociation. Stem Cells. 2006;24:2649–2660. doi:10.1634/stemcells.2005-0657
10. Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi:10.1006/dbio.2000.9912
11. Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Stem Cell. 2010;7:225–239. doi:10.1016/j.stem.2010.06.018
12. Emre N, Vidal JG, Elia J, et al. The ROCK inhibitor Y-27632 improves recovery of human embryonic stem cells after fluorescence-activated cell sorting with multiple cell surface markers. PLoS One. 2010;5(e12148). doi:10.1371/journal.pone.0012148
13. Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Stem Cell. 2010;7:240–248. doi:10.1016/j.stem.2010.06.017
14. Walker A, Su H, Conti MA, et al. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1(71). doi:10.1038/ncomms1074
15. Pakzad M, Totonchi M, Taei A, et al. Presence of a ROCK inhibitor in extracellular matrix supports more undifferentiated growth of feeder-free human embryonic and induced pluripotent stem cells upon passaging. Stem Cell Rev Rep. 2010;6:96–107. doi:10.1007/s12015-009-9103-z
16. Nickerson CA, Ott CM, Wilson JW, Ramamurthy R, Pierson DL. Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev. 2004;68:345–361. doi:10.1128/MMBR.68.2.345-361.2004
17. Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Stem Cell. 2009;5:237–241. doi:10.1016/j.stem.2009.08.001
18. Forristal CE, Wright KL, Hanley NA, Oreffo ROC, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi:10.1530/REP-09-0300
19. Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005;1049:1–8. doi:10.1196/annals.1334.001
20. Forsyth NR, Musio A, Vezzoni P, et al. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells. 2006;8:16–23. doi:10.1089/clo.2006.8.16
21. Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi:10.1073/pnas.0501283102
22. Burridge PW, Holmström A, Wu JC. Chemically defined culture and cardiomyocyte differentiation of human pluripotent stem cells. Curr Protoc Hum Genet. 2015;87:
23. Chen KG, Mallon BS, Hamilton RS, et al. Non-colony type monolayer culture of human embryonic stem cells. Stem Cell Res. 2012;9:237–248. doi:10.1016/j.scr.2012.06.003
24. Wong AP, Chin S, Xia S, et al. Efficient generation of functional CFTR-expressing airway epithelial cells from human pluripotent stem cells. Nat Protoc. 2015;10:363–381. doi:10.1038/nprot.2015.021
25. Krawetz RJ, Li X, Rancourt DE. Human embryonic stem cells: caught between a ROCK inhibitor and a hard place. BioEssays. 2009;31:336–343. doi:10.1002/bies.200800157
26. Miyazaki T, Futaki S, Suemori H, et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun. 2012;3(1236). doi:10.1038/ncomms2231
27. Mollamohammadi S, Taei A, Pakzad M, et al. A simple and efficient cryopreservation method for feeder-free dissociated human induced pluripotent stem cells and human embryonic stem cells. Hum Reprod. 2009;24:2468–2476. doi:10.1093/humrep/dep244
28. Sahara M, Hansson EM, Wernet O, Lui KO, Später D, Chien KR. Manipulation of a VEGF-notch signaling circuit drives formation of functional vascular endothelial progenitors from human pluripotent stem cells. Cell Res. 2014;24:820–841. doi:10.1038/cr.2014.59
29. Siti-Ismail N, Bishop AE, Polak JM, Mantalaris A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials. 2008;29:3946–3952. doi:10.1016/j.biomaterials.2008.04.027
30. Kurosawa H. Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng. 2012;114:577–581. doi:10.1016/j.jbiosc.2012.07.013
31. Miñambres R, Guasch RM, Perez-Aragó A, Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006;119:271–282. doi:10.1242/jcs.02723
32. Nakashima M, Adachi S, Yasuda I, et al. Inhibition of rho-associated coiled-coil containing protein kinase enhances the activation of epidermal growth factor receptor in pancreatic cancer cells. Mol Cancer. 2011;10(79). doi:10.1186/1476-4598-10-79
33. Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol. 2013;49:341–347. doi:10.1165/rcmb.2013-0046TE
34. Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi:10.1016/j.bbrc.2007.05.054
35. Zhao D, Najbauer J, Garcia E, et al. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819–1829. doi:10.1158/1541-7786.MCR-08-0146
36. Duś-Szachniewicz K, Drobczyński S, Ziółkowski P, et al. Physiological hypoxia (physioxia) impairs the early adhesion of single lymphoma cell to marrow stromal cell and extracellular matrix. Optical tweezers study. Int J Mol Sci. 2018;19:1880. doi:10.3390/ijms19071880
37. Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580–589. doi:10.1093/humrep/den404
38. Covello KL, Kehler J, Yu H, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi:10.1101/gad.1399906
39. Peerani R, Rao BM, Bauwens C, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi:10.1038/sj.emboj.7601896
40. Harb N, Archer TK, Sato N. The rho-rock-myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One. 2008;3(e3001):e3001. doi:10.1371/journal.pone.0003001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.