Back to Journals » Psychology Research and Behavior Management » Volume 13
Rewarding Effect of Catha edulis (Khat) and the Sex Differences to the Responses in Swiss Albino Mice
Authors Limenie AA , Tolessa T , Makonnen E , Seifu D
Received 12 December 2019
Accepted for publication 11 March 2020
Published 20 March 2020 Volume 2020:13 Pages 279—289
DOI https://doi.org/10.2147/PRBM.S242036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Abebaye Aragaw Limenie, 1 Tesfaye Tolessa, 1 Eyasu Makonnen, 2 Daniel Seifu 3
1Department of Physiology, Addis Ababa University, College of Health Sciences, Addis Ababa, Ethiopia; 2Department of Pharmacology, Addis Ababa University, College of Health Sciences, Addis Ababa, Ethiopia; 3Department of Biochemistry, Addis Ababa University, College of Health Sciences, Addis Ababa, Ethiopia
Correspondence: Abebaye Aragaw Limenie
Department Medical Physiology, Addis Ababa University, P.O. Box 9086, Addis Ababa, Ethiopia
Email [email protected]
Background: Burden of substance abuse is becoming a worldwide problem. One of the psychostimulant plants widely consumed in Ethiopia and other East African countries is Catha edulis Forsk (khat). Most of the users claim that its stimulatory effect is the determinant factor that makes them use. However, its rewarding and reinforcing potential and variation between sexes have not been investigated. This study was, therefore, designed to measure the rewarding effect of khat extract (ke) in the addiction mice model of both sexes.
Materials and Methods: Forty-eight Swiss albino mice of both sexes (age 6– 7 weeks) weighing 21– 33 gm were used. The mice were conditioned to ke (100 mg/kg, 200 mg/kg and 300 mg/kg b.w). The control group was conditioned to tween 80 (2%, v/v) in distilled water. The reinforcing effect of khat was evaluated using the conditioned place preference paradigm. The classical pairing to the extract was made using the place conditioning box. Post-conditioning tests have been conducted four times and the average values were taken for analysis using SPSS version 21.0.
Results: Time spent in the khat-paired compartment was significantly higher for mice conditioned to ke 200 mg/kg (p< 0.05) and ke 300 mg/kg (p< 0.001). The rewarding effect of khat was strong in females at a higher dose when compared to the same sex of mice conditioned to the vehicle (p< 0.001) or male mice conditioned to the same dose of khat extract (p< 0.05). Repeated administration increased khat rewarding sensitization at all doses. Though the crude khat extract did not affect the food consumption and total body weight, water consumption was significantly less in mice received ke 100 mg/kg (p< 0.01), where it was significantly higher in mice received ke 300 mg/kg (p< 0.01). Sniffing (p< 0.05) and climbing (p< 0.05) psychomotor activities of mice were also affected by the crude khat extract.
Conclusion: Mice showed place conditioning to khat extract, and the response was significantly higher in female mice. The crude khat extract did not affect food consumption and total body weight. The mechanisms behind the rewarding response of khat extract and sexual differences should be investigated.
Keywords: conditioned place preference, khat, place conditioning score, time spent in the khat paired compartment
Introduction
Background
Substance use disorders are prevalent and becoming the major public health concerns in the global setting1 Substance abuse and addiction are huge public health concerns that affect society and public policy including health care, education, and worker productivity.2 Amphetamines and amphetamine-like substances are currently known to present major drug-abuse concerns.3
Catha edulis Forsk, commonly called khat, is one of the psychostimulant plants with an active component of cathinone reported to have a similar response with amphetamines.4 Khat is a flowering evergreen plant under Celastraceae.5 Cathinone in khat leaves has amphetamine like-structure and functions.6
Ethiopia, Zimbabwe, Somalia, Kenya, Uganda, Tanzania, Madagascar, and Djibouti are among East African countries where khat chewing is common.7 Nowadays, the prevalence of people chewing khat leaves is increasing.8 The increase in the prevalence of khat use is because of the knowledge gap and inconsistent findings in research.5,9 Indeed, the use of psychostimulants is not dependent on the knowledge of the substance user. However, most of the individuals start chewing khat is because of the limited knowledge they have on the consequence of khat chewing. From this point of view, the knowledge gap could contribute to an increase in the prevalence of khat chewing. An increase in its farm and availability could also be another reason for the increase in the prevalence of new users and demand.
Although few studies indicated that synthetic cathine showed rewarding responses,10,11 no studies have been conducted to measure the rewarding effect of the crude khat extract using the conditioned place preference (CPP) paradigm in animal models. Substance use was considered to be a male problem, and many substance abuse studies are conducted with a preponderance of male participants.12 Studies indicated that males and females differ in their biological and subjective responses to drugs of abuse.12 However, studies have not been conducted if a differential rewarding response to the crude khat extract could be observed between sexes. The present study, therefore, was aimed to evaluate the rewarding effect of the crude khat extract and the impact of biological sex on its effect.
Materials and Methods
Chemicals
Diethyl ether and chloroform (Sigma-Aldrich, Germany), Tween 80, and 70% ethanol were obtained from the local supplier (Afro-German) in Addis Ababa, Ethiopia.
Plant Materials Collection
Khat leaves (500 gm) were collected from “Aweday”, 525 km South-East of Addis Ababa, in July 2017. The leaves specimen voucher number was given (August 2017, AA001) and stored in the Natural Herbarium, Addis Ababa University.
Plant Material Extraction
The edible parts (shoots and tender leaves) of the leaves were separated and gently washed with tap water. Then the leaves were freeze-dried at −20°C for 2 days and crushed using a mortar and pistil.13 200 gm of freeze-dried crushed leaves were placed into the conical flask wrapped with aluminum foil.6 A total of 400 mL organic solvents (300 mL diethyl ether and 100 mL chloroform (3:1v/v ratio) were added into the flask to cover the whole minced leaves. The mixture was shaken under the dark condition for 48 hrs using a rotary shaker (New Brunswick Scientific Co, USA) at 72g and 20°C. The mixture was filtered initially using cotton gauze followed by grade-I Whatman filter paper (WhatmanTM 1001–150). The organic solvents were then removed through evaporation using Rota-vapor under a controlled temperature of 36°C, rotation of 3g and Pascal negative pressure of 240. The water in the extract was removed through lyophilization to get the dry extract. Since the study aimed to determine the rewarding effect of the crude khat extract, the dry extract obtained after lyophilization was not further fractionated. Further determination of the compounds found in the crude khat extract and concentration of cathinone to be administered was not required to be done. This is because, naturally, humans take the whole leaves into their mouth and hold it for some time to allow the juice to be extracted from the leaves in the oral cavity where it begins to be absorbed. It is mainly the cathinone that is extracted and absorbed from the leaves in the mouth to induce behavioral changes even though other compounds are also believed to be extracted and absorbed similarly. It is the cathinone that is responsible for the central effect of khat leaves whereas other compounds could have a minor effect.
Animal Preparation
A total of forty-eight Swiss albino mice (6–7 weeks) of both sexes weighing between 21–33g were used. Six mice, the same sex, were housed per a cage under a natural light and dark (12:12) cycle at room temperature, 21±1°C. The pellets and water were supplied with no restriction, ad libitum. The amount of food (pellets) and water each group of mice consumed per day was measured during the last 8 consecutive days of the experiment. Mice were weighed twice per week. The experiment was carried out under the guidelines for the care and use of laboratory animals prepared by the National Academic Sciences.14 The research was approved by the Institutional Review Board (IRB) committee, Addis Ababa University with a protocol number of 012/15/Physio.
Grouping of Mice and Dosing
The mice were randomly assigned into four groups (n=12/group). The first group was conditioned to the vehicle (Tween 80 in distilled water, 2% v/v; T80W). Instead of saline, Tween 80 in distilled water was used to dissolve the khat extract as khat extract did not completely dissolve in normal saline. The reason why we used 2% Tween 80 in distilled water as a control was because the crude khat extract was dissolved in it to make a solution. Other groups were conditioned to the graded doses of crude khat extract (ke) (100 mg/kg, 200 mg/kg and 300 mg/kg) using oral gavage. The doses of the khat extract were selected based on the previous report.15
Solution Preparation and Volume Determination
The fresh solution of crude khat extract and vehicle were prepared only during administration. The crude khat extract was dissolved in the vehicle (T80W). The dose of the extract for each mouse was calculated from selected doses (100 mg/kg, 200 mg/kg and 300 mg/kg) and the total body weight (b.w) of each mouse. The appropriate standard vehicle volume (10 mL/kg b.w of mice) was used to determine how much volume was required to dissolve the calculated amount of khat extract. Each mouse in its respective group was administered orally once every other day from the stock solution or an equivalent volume of vehicle (0.5 mL). The final volume used for all mice was 0.5 mL.
Conditioned Place Preference (CPP) Test
A wooden CPP box with two equal-size conditioning (46.5cm×12.7cm) and one central non-conditioning (7.2cm×12.7cm) compartments was used. The peculiarity of the apparatus and the experimental procedures were according to the previous studies.16,17 The box was placed in the sound attenuating neurobehavioral animal study room (2m x 2m). The three compartments of the box had movable frontal Plexiglas.
Internal and external cues were used constantly throughout the study. Considering the internal contextual cues, the first conditioning compartment had a white wall with a rough floor. The other conditioning compartment had a black wall and a smooth floor. The middle non-conditioning compartment was with a brown color. The compartments were separated by sliding doors. The box was cleaned with a mild soap solution after each trial.
During the experiment, each mouse was acclimatized with the experimental box for four consecutive days for 20 min per trial per day. Each mouse was placed at the non-conditioning compartment and allowed to explore all open compartments freely. The pre-conditioning test was made 24 hrs after the last acclimatization day. Each mouse received T80W 30 min before its placement in the non-conditioning compartment to explore the compartments freely for 10 min period. The time spent in each compartment was measured.
The conditioning test was conducted 24 hrs after the preconditioning phase. Briefly, on day 7, 9, 11, and 13, each mouse was administered with khat extract (Table 1). 30 min after administration, each mouse was placed in the least preferred compartment. The least preference was determined during the pre-conditioning test. The same mouse received the vehicle on a day 6, 8, 10 and 12 (Table 1). 30 min following administration of the vehicle, each mouse was placed in the preferred compartment. This procedure was repeated for eight consecutive conditioning days. The duration of confinement in the compartment was 20 min for each trial with one conditioning session per day.
 |
Table 1 Timeline for Conditioned Place Preference Study for the First Phase |
The first post-conditioning test was conducted 24 hrs after the last conditioning day (day 14th) in both male and female mice (Table 1). In this phase, each mouse was placed in a non-conditioning compartment. Khat was not given but the contextual and visual cues associated with khat effect during the conditioning phase were presented. Each mouse was allowed to explore all open compartments freely for 10 min period. The time each mouse spent in the khat paired compartment (KPC) and conditioning score were recorded during a 10 min period. The percentage of time spent in the KPC (time spent in the KPC/total time given for each mouse to explore the compartments*100) and conditioning score (time spent difference between khat and vehicle paired compartment) were determined for each mouse.
Once the first post-conditioning test was conducted on day 14, the subsequent post-condition tests were conducted on a day 24, 34 and 44. The results obtained during these four post-conditioning tests were made average to determine the general rewarding effect of the crude khat extract. The time spent in the KPC obtained during each of the four post-conditioning tests was used to determine the sensitization effect of khat extract. Khat extract could induce sensitization if the time spent in the KPC could increase over these repeated conditioning tests. In both pre and post-conditioning tests, the estrus cycle of female mice was determined through visual genital observation.
Statistical Analysis
The statistical analysis was done using SPSS version 21.0 and graphs were plotted using Microsoft excel. One-way ANOVA followed by post hoc Tukey’s test was used to compare the mean difference between the groups. Repeated two-way ANOVA followed by Bonferroni multiple comparison test was also used to evaluate the potential rewarding effects of khat extract over a repeated administration. Dunn’s post hoc comparison test was also used for not normally distributed data. The data were expressed as means ± standard error of the mean (SEM). Differences with p < 0.05 were considered statistically significant.
Results
Conditioned Place Preference Effects of Khat
Analysis of one way ANOVA indicated that there was a significant difference in the time spent in KPC (F (3, 44) = 13.28, p<0.001) between groups. The post hoc Tukey’s test indicated that the percentage of time spent in the KPC by mice paired with 300 mg/kg and 200 mg/kg khat extract was significantly higher than mice paired with T80W (38.76 ±1.67 vs 29.24±0.88, p<0.001,95% CI [537,13.67] and 33.63±0.99 vs 29.24±0.088, p <0.05, 95% CI [0.24,8.54], respectively; Figure 1). Besides, Dunn’s post hoc comparison showed that mice treated with ke 300 mg/kg had significantly greater median conditioning score than mice treated with T80W (p<0.05) as shown in Figure 2.
Effect of Sex Differences on CPP Response of Khat
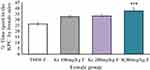
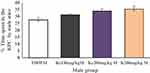
The place conditioning effect of khat extract in female mice was compared with the same-sex of mice paired with T80W or male mice paired with the same dose of khat extract. The post hoc test showed that time spent in the KPC was significantly higher in female mice paired with the higher dose of khat extract (ke 300 mg/kg) compared with the same-sex of mice paired with T80W (42.18±1.65 vs 28.75±1.51, p < 0.001, 95% CI [7.00, 19.84]; Figure 3). However, no significant difference was observed for this parameter between male mice paired with the different doses of khat extract and the same-sex of mice paired with T80W (Figure 4).
Independent t-test results indicated that time spent in KPC by female mice was significantly higher than males at the higher dose of khat extract, ke 300 mg/kg (42.18±1.65 vs 35.34±2.19, p<0.05, 95% CI [0.42, 13.26]; Figure 5).
Rewarding Sensitizing Effects of Khat
The two ways repeated measure of ANOVA indicated that treatment, number of conditioning tests and their interaction had a significant effect on the time spent in the KPC (F (3,33) = 22.14, p<0.001; F (2.02, 22.01) = 8.61, p <0.01 and F (12,132) = 5.25, p<0.001, respectively) between groups. The Bonferroni confidence interval adjusted analysis results indicated that the estimated mean of the time spent in the KPC was significantly higher in mice paired with ke 100 mg/kg (32.09±0.46 vs 28.32±0.53, p<0.01, 95% CI [1.38, 6.18]), ke 200 mg/kg (34.79±0.96 vs 28.32±0.53, p<0.001, 95% CI [3.05, 9.92]) and ke 300 mg/kg (38.69±145 vs 28.32±0.53, p<0.001, 95% CI [5.23,15.53] as shown in Figure 6.
Food and Water Consumption Plus Body Weight Effects of Khat
The amount of food and water consumed by each group of mice was measured in the last eight consecutive days during the experiment, and the average value was taken for analysis. The post hoc analysis did not show any significant difference in food (pellet) consumption between each group of mice consumed per day (Table 2). However, the amount of water consumed by mice that received ke 100 mg/kg was significantly less than the control (5.26±0.19 vs 6.07±0.25, p<0.01; 95% CI [0.192, 1.428]) while the consumption was significantly higher in mice that received KE 300 mg/kg (7.00±0.08 vs 6.07±0.25, p<0.01; 95% CI [−1.553, −0.317]) as shown in Table 2.
 |
Table 2 Amount of Food and Water Consumed by Each Group of Mice per Day Plus the Initial and Final Total b.w of Mice |
Regarding the total b.w, as mentioned in the methodology, the weight of each mouse was measured twice per week and the average values were taken for analysis. Significant differences were not observed between mice received the graded doses of khat extract and vehicle at each measurement week (Figure 7). However, the total b.w was increased with time and the final b.w was significantly greater than the initial b.w in mice that received T80W (t (11) = 13.68, p<0.001), ke 100 mg/kg (t (11) = 10.59, p<0.001), ke 200 mg/kg (t (11) = 8.32, p<0.001) and ke 300 mg/kg (t (11) = 7.82, p<0.001) regardless of khat consumption.
The Psychomotor Activity Effects of Khat
The conditioned place preference effect of khat could be affected by its effect on nigrostriatal dopamine-related psychomotor activities. Thus, the effect of khat extract on dopamine-related psychomotor activities was required to be evaluated in this study. There were significant differences in the number of khat paired compartment entries (KPCE), climbing and sniffing activities between groups (F (3, 44) = 11.89, p<0.001, F (3, 44) = 3.24, p<0.05 and F (3, 44) =3.34, p<0.05, respectively). The post hoc Tukey’s test analysis showed that the number of KPCE was significantly higher in mice-paired with ke 200 mg/kg (p<0.01; 95% CI [1.25, 6.75]) and ke 300 mg/kg (p<0.001; 95% CI [3.17, 8.67]) than the mice paired with T80W (Table 3). Climbing and sniffing activities in mice that received the higher dose of khat extract were significantly lower than in mice that received T80W (p<0.05; 95% CI [−20.70,-1.47] and p<0.05; 95% CI [−45.78,-2.88], respectively). However, significant differences in other dopamine-related behaviors such as T80W paired compartment entries (TCPE), total compartment entries (totCE) and rearing were not observed between mice conditioned to khat extract and T80W (Table 3).
 |
Table 3 Effect of Khat on Psychomotor Activities in Mice Paired with the Different Doses of Khat Extract and T80W |
Discussion
Conditioned Place Preference
In this study, mice paired with the middle and higher doses of crude khat extract showed significant rewarding and CPP effect in a dose-dependent manner. However, when considering the conditioning score as a measure of preference, only the higher dose of khat extract contributed to a significant place preference. This finding showed that less time was spent by mice paired with khat extract in the initially preferred compartment. This, in other words, indicates that khat extract reversed the initial natural innate place preference.
Other studies have shown that cathinone, amphetamine, and mephedrone showed a similar CPP effect to our findings.16,18,19 Similarly, synthetic cathinone and mephedrone reversed the natural innate preference in mice.18
The rewarding effect of khat extract observed in our study could be attributed to its effect on monoaminergic transmission. The dopaminergic transmission system in the brain was affected by amphetamine and other cathinone analogs.17,20
Cathinones exert their effects by acting as a substrate-type releaser at monoamine transporters.19 Administration of synthetic-cathinone analogs such as mephedrone increased the release of dopamine and serotonin from the dopaminergic nerve terminals in the brain rewarding circuits.20,21 Once, cathinone binds with the dopamine transporters, dopaminergic cells depolarized and dopamine is released into the synaptic cleft in the nucleus accumbens.21 The depolarization process is facilitated by cation influx, such as sodium, into the dopaminergic presynaptic cells. The binding of cathinone on the dopamine transporter also blocks the dopamine transporters found on the presynaptic dopaminergic cells.21,22 Thus, cathinone, not only facilitates the release of dopamine but also it blocks dopamine transporters and its reuptake.
On the other hand, the GABA and dopamine transmissions are affected by khat extract,23,24 indicating that khat extract could use GABA and dopamine for its rewarding effect. Cathinone enhancing activities are partially blocked by dopamine antagonists.25 Thus, the effects of cathinone in khat could be through its dopamine transmission enhancing response. This is supported by the finding of schizophrenic-like locomotory symptoms associated with khat extract administration in mice.26 This shows that khat extract increases the release of dopamine in the dorsal striatum of the brain that increases the psychomotor activities.
Alternatively, the rewarding effect of khat extract could be through the action of glutamate. Activation of glutamatergic cells in the ventral tegmental area of animals showed positive reinforcing behaviors.27 In support of this assumption, a higher dose of khat extract was shown to induce seizure-like manifestations in animal experimentation.28,29 It is glutamate that initiates the seizures and their propagation while GABA suppresses the initiation of seizures.30 From these findings, in addition to dopamine, it can be established that the effects of the crude khat extract on the rewarding response could be through its action on the brain GABA and glutamate transmissions.
Sex Difference in CPP Effects of Khat
In this study, CPP was observed in female mice paired with a higher dose of khat extract compared with the same-sex of mice paired with the vehicle. However, significant CPP was not observed in male mice when compared with the same-sex of mice paired with the vehicle. The khat paired compartment preference was significantly higher in female mice compared with their male counterparts at a higher dose of the extract. This indicated that the rewarding effect of khat, particularly at the higher dose, was stronger in females than in males.
A similar finding was reported in for female rats showing a greater preference for amphetamine than males.31 Female rats also have shown to be more sensitive to amphetamine, cocaine and are found to be more vulnerable to addiction than males.32
Few studies have revealed that females and males have a difference in their extent of the response to nicotine, cocaine, and methamphetamine.12,33 For instance, the adverse effects of methamphetamine were found to be more pronounced in females than in males.33 Drug dose-escalating is higher in females predisposing them to the more likely hood of addition so easily as compared to males.34 Females show greater mood reduction and stress responses than males during drug-withdrawal.34 The psychomotor response to methamphetamine is also strong in females showing higher psychomotor activities are more vulnerable to addiction.35,36
Unlike our study, the previous study indicated that males were more rewarded and had displayed a dose-dependent conditioned place preference while females did not show a place preference at any dose of cathinone, α-pyrrolidinopentiophenone.37 This difference could be attributed to differences in animal species used for the experiment, concentration of cathinone found in khat extract and route of administration. In our study, it was the crude khat extract that was administered to mice orally. However, in the previous study, it was the synthetic cathinone administered intraperitoneally to rats.
The rewarding and reinforcing differential responses to khat extract between sexes observed in our study could be attributed to the dopaminergic response variation to khat extract between sexes.
The mesolimbic dopaminergic transmission response to khat extract could be modulated by sex steroids. Dopaminergic projections from the ventral tegmental area and substantia nigra to nucleus accumbens, hippocampus, prefrontal cortex, amygdala, and corpus striatum are modulated by sex steroids.38,39 Ovarian hormones have access to the brain and affect drug-craving and drug-taking behavior.34 The effects of cocaine and amphetamine are more intense during the follicular phase.34 Therefore, dopaminergic transmission affected by khat extract is modulated by gonadal hormones,34,40 indicating that these hormones could be involved in the differential rewarding effect of the crude extract of khat between sexes.
The difference in the pharmacokinetics of khat extract between sexes could be also the reason for the differential rewarding responses observed between sexes in our study. Behavioral and neurobiological responses to substances relevant to addiction were affected by their pharmacokinetics.41 This indicated that the addictive potential effects of a substance depend on their absorption, distribution, metabolism, and excretion. These processes affecting the addictive potential effects of substances might be different between sexes. The differences between males and females in drug metabolism affect the drug responses between sexes.12 There is also a finding showing that methamphetamine increases metabolic activities more in males than in females.33 The amount and its rapidity with which it enters into the brain tissue may play a role in substance addiction.41 The faster the drug reaches in the brain, the more likely that it causes addiction.41
The sex differences in the brain are regulated by gonadal hormones and sex chromosomes.42,43 Thus, the sex differences in the brain may also involve in the khat extract induced rewarding difference between sexes. The chromosomal female mice showed faster food-reinforced instrumental habit formation than chromosomal male mice regardless of gonadal phenotype.43 This indicated that chromosomal genes that determined the goal-directed behaviors affect habit formation in mice than gonads or gonadal hormones itself.43
The brain differences between the sexes contributed to variation in addiction–like responses between males and females, showing that brain differences between sexes could affect the rewarding response of the crude extract of khat.34
The neurochemical and hormonal response differences between the sexes could be also involved in such differential responses induced by khat extract between males and females in our study. Females with methamphetamine abusers had larger volumes in the corpus callosum and more hyperperfused regions in the parietal and occipital areas of the brain.34,35
Rewarding Sensitizing Effects of Khat Extract
In our study, two ways repeated measure of ANOVA showed that the number of conditioning tests had a significant effect on the time spent in the khat-paired compartment. The Bonferroni confidence interval adjusted post hoc analysis also indicated that the estimated mean time spent in the khat paired compartment was significantly higher in mice paired with all doses of khat extract when compared with mice paired with T80W. This showed that time spent in the khat paired compartment was gradually increased with repeated administration of khat extract. Such a response revealed that the rewarding effect of the same dose of khat extract was increased with time. This also showed the sensitization effect of repeated doses of khat extract in mice.
Similar to the present findings, other studies also showed that the rewarding response to cocaine and amphetamine was increased through repeated exposures in rodents.44,45 Addictive substances showed locomotor sensitization and hyperfunction of the mesolimbic dopaminergic system.46 This indicates that dopaminergic response to these substances increases gradually.
Food and Water Consumption versus Bodyweight Effects of Khat
In this study, khat extract did not show any significant change in the amount of food consumption and total b.w. However, many other findings have shown that khat consumption at a higher dose and prolonged duration reduces appetite and b.w.47–49 Therefore, the discrepancy could be attributed to the duration of khat consumption, in our study the crud khat extract was given for a month on every other day.
Those mice that received the higher dose of khat extract consumed significantly higher water than the control. Although much has not been done on the effect of khat on the thirst-center of the hypothalamus, research showed that khat increases body temperature.50 The increase in the body temperature may be accompanied by water loss and is liable to affect the thirst mechanism. Alternatively, dopamine which is modulated by khat intake may increase the secretion of arginine- vasopressin that can stimulate the thirst center of the hypothalamus.51,52
The Psychomotor Activity Effects of Khat
In this study, dopamine-related psychomotor activities such as the number of compartment entries, rearing, sniffing and climbing activities were evaluated. The khat-paired compartment entries were higher in mice conditioned to the middle and higher doses of khat extract. This also indicates the presence of a rewarding effect of khat. However, rearing activities, total compartment entries and vehicle paired compartment entries were not affected. Nevertheless, previous studies indicated that khat extract increases the psychomotor activities and showed schizophrenia-like symptoms.26,53 The discrepancy could be attributed to the time of psychomotor activity measurement following khat extract. In our study, the psychomotor activities were measured 24 hrs after khat consumption while the reported measurements in other studies were conducted immediately following khat consumption. Unlike this study, synthetic cathinone-mephedrone produced greater repetitive movements and showed locomotor sensitization in rats.19 The difference could be attributed to the animal species used to measure locomotor behavioral effects of synthetic cathinone and crude khat extract and protocol differences.
In this article, we mainly focused on cathinone that could have a major effect in the rewarding response of the crude khat extract including its influences in male and female differential responses. However, the compound found in the khat leaves is not only cathinone but also other compounds such as cathine, norepseudoephdrine, essential oils, sterols, tannins, ascorbic acid and electrolytes.54,55 Nevertheless, most of the researches have been conducted on cathinone. Although, it is the cathinone that has higher lipid solubility and crosses the blood-brain barrier easily to induce its behavioral effects than other compounds,50 other compounds in khat leaves could have behavioral effects. Cathinone is more potent than cathine55 and administration of cathine and cathinone affects adrenocortical functions.56 Administration of cathine to obese individuals reduced their body weight.57 These findings indicate that cathine has also central effects.
On the other hand, tannins in food showed an aversive response.58 If tannins are found in the khat leaves and involved in the aversive response, it could contribute to the rewarding potential difference between males and females. Therefore, yet much work is required in the future, cathine and tannins could have minor effects it may induce a minor effect on the behavioral response difference between males and females.
In conclusion, taking the time spent in the khat paired compartment, conditioning score and number of khat paired compartment entries, conditioned place preference has been observed in mice conditioned to the khat extract in a dose-dependent manner. This response was stronger in female mice than males, indicating that sex difference could be one of the factors contributing to khat addiction. Khat extract also showed rewarding sensitization. Although the amount of food consumed by each group of mice and the total b.w were not affected by the crude extract of khat, the higher dose of crude khat extract increased water consumption. The numbers of khat- paired compartments were significantly higher at the middle and higher doses of the extract while sniffing and climbing activities were reduced by the higher doses of khat extract.
The cellular and molecular mechanisms behind these effects of khat need to be identified. Another effect of khat for possible difference and alteration in gene expression between sexes requires to be investigated. Understanding the basic mechanisms mediating the differential reinforcing response induced by khat extract between sexes is important for improving prevention and enhancing treatments related to khat addiction and relapse.
Acknowledgments
This research was supported by Addis Ababa University and the Department of Physiology in the school of Medicine. We authors are most indebted to Mr. Tesfaye Getachew who assisted us during the oral administration of the khat extract and Dr. Nigusie Deyisa who helped us during the statistical analysis. We would like to express our appreciation to Dr. Anne Depping who read the manuscript and provided important comments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Charlson FJ, Diminic S, Lund C, Degenhardt L, Whiteford HA. Mental and substance use disorders in sub-saharan africa: predictions of epidemiological changes and mental health workforce requirements for the next 40 years. PLoS One. 2014;9(10):1–11. doi:10.1371/journal.pone.0110208
2. Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL. Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comp Med. 2010;60(3):177–188.
3. Raffa RB, Shah S, Tallarida CS, Rawls SM. Amphetamine conditioned place preference in planarians. J Behav Brain Sci. 2013;3(1):131–136. doi:10.4236/jbbs.2013.31012
4. Dhaifalah I, Santavy J. Khat habit and its health effect. A natural amphetamine. Biomed Pap. 2004;148(1):11–15. doi:10.5507/bp.2004.002
5. Abebe M, Kindie S, Adane K. Adverse health effects of khat: a review. Fam Med Med Sci Res. 2015;4(1):2–5. doi:10.4172/2327-4972.1000154
6. Alfaifi H, Abdelwahab SI, Mohan S, et al. Catha edulis Forsk. (Khat): evaluation of its antidepressant-like activity. Pharmacogn Mag. 2017;13(50):354–357. doi:10.4103/pm.pm_442_16
7. Odenwald M. Chronic khat use and psychotic disorders: a review of the literature and future prospects. Sucht. 2007;53(1):9–22.
8. Andargachew K, Eskindir L, Atkilt E. Prevalence of khat chewing and its effect on academic performance in Sidama zone, Southern Ethiopia. Afr Health Sci. 2017;17(1):175–185. doi:10.4314/ahs.v17i1.22
9. Al-Habori M. The potential adverse effects of habitual use of catha edulis (Khat). Expert Opin Drug Saf. 2005;4(6):1145–1154. doi:10.1517/14740338.4.6.1145
10. Schechter MD, Meehan SM. Conditioned place preference produced by the psychostimulant cathinone. Euro J Pharm. 1993;232(1):135–138. doi:10.1016/0014-2999(93)90739-5
11. Watterson LR, Kufahl PR, Nemirovsky NE, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol. 2014;19(2):165–174. doi:10.1111/j.1369-1600.2012.00474.x
12. Tuchman E. Women and addiction: the importance of gender issue in substance abuse research. J Addict Dis. 2010;29(2):127–138. doi:10.1080/10550881003684582
13. Atlabachew M, Chandravanshi BS, Redi-Abshiro M, Torto N, Chigome S, Pule Bellah O. Evaluation of the effect of various drying techniques on the composition of the psychoactive phenylpropylamino alkaloids of Khat (Catha edulis forsk) chewing leaves. Bull Chem Soc Ethiop. 2013;27(3):347–358. doi:10.4314/bcse.v27i3.3
14. National academies Sciences. Guide for the Care and Use of Laboratory Animals.
15. Shewamene Z, Engidawork E. Sub-acute administration of crude Khat (Catha edulis F.) extract induces mild to moderate nephrotoxicity in rats. BMC. 2014;14(66):1–9. doi:10.1186/1472-6882-14-66
16. Kitanaka N, Kitanaka J, Hall FS, et al. Attenuation of methamphetamine-induced conditioned place preference in mice after a drug-free period and facilitation of this effect by exposure to a running wheel. J Exper Neuro. 2012;6(1):11–19. doi:10.4137/JEN.S10046
17. Pourtaqi N, Imenshahidi M, Razavi BM, Hosseinzadeh H. Effect of linalool on the acquisition and reinstatement of morphine-induced conditioned place preference in mice. Avicenna J Phytomed. 2017;7(3):242–249. doi:10.22038/AJP.2016.15567.1615
18. Lisek R, Xu W, Yuvasheva E, et al. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2013;126(1):257–262. doi:10.1016/j.drugalcdep.2012.04.021
19. Gregg RA, Baumann MH, Partilla JS, et al. Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioral and neurochemical effects in rats. Br J Pharmacol. 2015;172:883–894. doi:10.1111/bph.12951
20. Baumann MH, Ayestas MA
21. Felice LJD, Glennon RA, Negus SS. Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97(1):20–26. doi:10.1016/j.lfs.2013.10.029
22. Glennon RA, Dukat M. Structure-activity relationships of synthetic cathinones. Curr Top Behav Neurosci. 2017;32:19–47. doi:10.1007/7854_2016_41
23. Shawqi -A-A, Hussein A-K, Mohanad S. Effect of khat-habituation on GABA levels in brain. Asian J Pharm Life Sci. 2015;3(2):74–80.
24. El-Sayed MK, Amin HK. Catha edulis chewing effects on treatment of paranoid schizophrenic patients. Neuropsych Dis Treat. 2015;11. 1067–1076. doi:10.2147/NDT.S81011
25. WHO. Assessment of Khat (Catha Edulis Forsk). 34th ECDD. 2006: 1–25.
26. Bogale T, Engidawork E, Yisma E. Subchronic oral administration of crude Khat extract (Catha edulis Forsk) induces schizophrenic-like symptoms in mice. BMC Complement Altern Med. 2016;16(153):1–12. doi:10.1186/s12906-016-1145-6
27. Yoo JH, Zell V, Gutierrez RN, et al. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 2016;7:1–13. doi:10.1038/ncomms13697
28. Al-Awdi SH, Al-Kadi HO, Shehab MM. Effect of khat habituation on secondary generalized seizure. J Pharmacol Clin Toxicol. 2013;1(2):1–4.
29. Oyungu E, Kioy PG, Patel NB. Effects of Catha Edulis (Khat) on behavior and its potential to induce seizures in Sprague Dawley rats. East Afr Med J. 2007;84(5):219–225. doi:10.4314/eamj.v84i5.9529
30. Barker HM, White HS. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med. 2015;5(8):1–15. doi:10.1101/cshperspect.a022863
31. Weiss VG. Sex differences in monoamines following amphetamine and social reward in adolescent rats. Exp Clin Psychopharm. 2015;23(4):197–205. doi:10.1037/pha0000026
32. Anker JJ, Carroll M. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8(8):73–96. doi:10.1007/7854_2010_93
33. Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5(1):24–35. doi:10.1016/j.genm.2008.03.017
34. Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95(1–2):136–147. doi:10.1002/jnr.23963
35. Milesi-Halle A, McMillan DE, Laurenzana EM, ByrnesBlake KA, Owens SM. Sex differences in (+)-Amphetamine- and (+)-Methamphetamine induced behavioral response in male and female sprague dawley rats. Pharmacol Biochem Behav. 2007;86(1):140–149. doi:10.1016/j.pbb.2006.12.018
36. Phillips TJ, Pastor R, Scibelli AC, Reed C, Tarragon E. Behavioral sensitization to addictive drugs: clinical relevance and methodological aspects. Neuromet. 2010;50:267–305. doi:10.1007/978-1-60761-883-6_11
37. Nelson KH, Manke HN, Imanalieva A, Rice KC, Riley AL. Sex differences in α-pyrrolidinopentiophenone (α-PVP)-induced taste avoidance, place preference, hyperthermia and locomotor activity in rats. Pharmacol Biochem Behav. 2019;185:172762. doi:10.1016/j.pbb.2019.172762
38. Calipari ES, Juarez B, Morel C, et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8(13977):1–14. doi:10.1038/ncomms13877
39. Sinclair D, Tertia D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharma. 2014;231(8):1581–1599. doi:10.1007/s00213-013-3415-z
40. Yoset KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cen Nerv Syst Agents Med Chem. 2014;14(2):83–89.
41. Allain F, Minogianis E, Roberts D, Samaha A. How fast and how often: the pharmacokinetics of drug use is decisive in addiction. Neurosci Biobehav Rev. 2015;56:166–179. doi:10.1016/j.neubiorev.2015.06.012
42. Zagni E, Simoni L, Colombo D. Sex and gender differences in central nervous system-related disorders. Neurosci J. 2016;1–13. doi:10.1155/2016/282/7090
43. Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Jane R, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neur. 2007;10(11):1398–1400. doi:10.1038/nn1994
44. Riday TT, Kosofsky BE, Malanga CJ. The rewarding and locomotor-sensitizing effects of repeated cocaine administration are distinct and separable in mice. Neuropharma. 2012;62(4):1858–1866. doi:10.1016/j.neuropharm.2011.12.011
45. Herrmann AP, Andrejew R, Benvenutti R, Gama CS, Elisabetsky E. Effects of N-acetylcysteine on amphetamine-induced sensitization in mice. Braz J Phys. 2018;40(2):169–173. doi:10.1590/1516-4446-2017-2337
46. Carlezon WA, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse. Trend Neur. 2002;25(12):610–615. doi:10.1016/s0166-2236(02)02289-0
47. Murray C, Roux CWL, Emmanuel AV, et al. The effect of Khat (Catha edulis) as an appetite suppressant is independent of ghrelin and PYY secretion. Appet. 2008;51(3):747–750. doi:10.1016/J.APPET.2008.06.012
48. Legesse TG, Takle ZJ, Best MG. Effect of khat and associated factors on nutritional status among khat Chewers at Gulelle Sub-city, Addis Ababa, Ethiopia. IJ Food Sci N Eng. 2017;7(1):11–18. doi:10.5923/j.food.20170701.02
49. Alshagga MA, Alshawsh MA, Seyedan A, et al. Khat (Catha edulis) and obesity: a scoping review of animal and human studies. Ann Nutr Metab. 2016;69:200–211. doi:10.1159/000452895
50. Pennings EIM, Opperhuizen A, Amsterdam JGC. Risk assessment of khat use in the Netherlands: a review based on adverse health effects, prevalence, criminal involvement and public order. Regul Toxicol Pharmacol. 2008;2008(52):199–207. doi:10.1016/j.yrtph.2008.08.005
51. Forsling ML, Williams H. Central effects of dopamine on vasopressin release in the normally hydrated and water-loaded rat. J Physiol. 1984;346:49–59. doi:10.1113/jphysiol.1984.sp015006
52. Aloamaka EO, Amabebe E, Ozoene JO, Obika LFO. Thirst perception, drinking, arginine vasopressin activity and associated neurohumoral factors. J Afr Ass Physiol Sci. 2018;6(1):1–13.
53. Al-Awdi SH, Al-Kadi HO, Shahab MM. Effect of Khat habituation on psychomotor behavior in mice. J Pharmacol Clin Toxicol. 2014;2(1):1–3.
54. Algabr M, Dunia AM, Aissaoui H, et al. Flavonoids glycosides from leaves of Catha edulis (Celasteraceae). Der Pharma Chem. 2015;7(11):193–196.
55. Patel NB. Mechanism of action of Cathinone: the active ingredient of khat (Catha edulis). East Afr Med J. 2000;17:329–332.
56. Ahmed MB, El-Qirbi AB. Biochemical effects of Catha edulis, cathine and cathinone on adrenocortical functions. J Ethnopharmacol. 1993;39(30):213–216. doi:10.1016/0378-8741(93)90039-8
57. Hauner H, Hastreiter L, Werdier D, Chen-Stute A, Scholze J, Blüher M. Efficacy and safety of cathine (Nor-Pseudoephedrine) in the treatment of obesity: a randomized dose-finding study. Obes Facts. 2017;10:407–419. doi:10.1159/000478098
58. Lichtenstein G, Cassini MH. Behavioral mechanisms underlying food aversion in guinea pigs. Etología. 2001;9:29–34.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.