Back to Journals » Drug Design, Development and Therapy » Volume 17
Review on the Diverse Biological Effects of Glabridin
Authors Zhang J, Wu X, Zhong B, Liao Q, Wang X, Xie Y, He X
Received 12 August 2022
Accepted for publication 5 January 2023
Published 10 January 2023 Volume 2023:17 Pages 15—37
DOI https://doi.org/10.2147/DDDT.S385981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Jianhong Zhang,1,2,* Xinhui Wu,3,* Baiyin Zhong,1,* Qicheng Liao,1 Xin Wang,1 Yuankang Xie,1 Xiao He1
1Department of Hepatobiliary Surgery, First Affiliated Hospital of Gannan Medical University, Ganzhou, 341000, People’s Republic of China; 2Ganzhou Key Laboratory of Hepatocellular Carcinoma, First Affiliated Hospital of Gannan Medical University, Ganzhou, 341000, People’s Republic of China; 3Department of General Surgery, The First Affiliated Hospital of Gannan Medical University, Ganzhou, 341000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao He, Email [email protected]
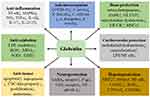
Abstract: Glabridin is a prenylated isoflavan from the roots of Glycyrrhiza glabra Linne and has posed great impact on the areas of drug development and medicine, due to various biological properties such as anti-inflammation, anti-oxidation, anti-tumor, anti-microorganism, bone protection, cardiovascular protection, neuroprotection, hepatoprotection, anti-obesity, and anti-diabetes. Many signaling pathways, including NF-κB, MAPK, Wnt/β-catenin, ERα/SRC-1, PI3K/AKT, and AMPK, have been implicated in the regulatory activities of glabridin. Interestingly, glabridin has been considered as an inhibitor of tyrosinase, P-glycoprotein (P-gp), and CYP2E1 and an activator of peroxisome proliferator-activated receptor γ (PPARγ), although their molecular regulating mechanisms still need further investigation. However, poor water solubility and low bioavailability have greatly limited the clinical applications of glabridin. Hopefully, several effective strategies, such as nanoemulsions, microneedles, and smartPearls formulation, have been developed for improvement.
Keywords: glabridin, tyrosinase inhibitor, P-gp, anti-inflammation, anti-oxidation, anti-microorganism
Graphical Abstract:
Introduction
Glycyrrhiza glabra Linne (common name: licorice) is widely used worldwide as one of herbal medicines, treating various diseases and benefiting our health. The genus Glycyrrhiza contains about 30 species, including Glycyrrhiza glabra L., Glycyrrhiza uralensis Fisch., and Glycyrrhiza inflata Bat. Of which, Glycyrrhiza inflata Bat. is only found in China.1 The main bioactive constituents of Glycyrrhiza plants involve flavonoids and triterpenoid saponins. Many flavonoids, including liquiritin, glycyrrhizic acid, glabridin, liquiritigenin, and isoliquiritigenin have been identified as bioactive compounds in treating diseases.2 Particularly, a crude drug containing at least 0.5% liquiritin and 2.0% glycyrrhizic acid is standardized in quality control in the Pharmacopoeia of the People’s Republic of China. These effective compounds have triggered great scientific interest and shown medicinal potentials. Recently, the pharmacological properties of liquiritin,3 glycyrrhizic acid,4 and liquiritigenin/isoliquiritigenin5 have been comprehensively discussed. The phytochemical characterization of glabridin, including the occurrence and biosynthesis, extraction and isolation methods, chemical synthesis, and analytical quantitation, have been reviewed in 2013.6 Both the Glycyrrhiza glabra L. root extract and glabridin have posed great impact on medicinal, dietary supplements, food, and cosmetic markets. In this article, we mainly updated the pharmacological properties of glabridin.
Glabridin (4-[(3R)-8,8-dimethyl-3,4-dihydro-2H-pyrano[2,3-f]chromen-3-yl]benzene-1,3-diol, C20H20O4) (Figure 1), a natural occurring prenylated isoflavan, accounting for 0.08–0.35% of the roots’ dry weight in Glycyrrhiza glabra L.7 Another study shows that glabridin presents about 20% of the extract of Glycyrrhiza glabra L.8 The structural stability of glabridin can be influenced by temperature, illumination, humidity, pH, solvent, and oxidant. Of which, illumination can be the main factor to promote the degradation of glabridin. Glabridin may be degraded at room temperature, and it should be kept under a dry, dark, and low oxygen condition.8 Structure-activity relationship research indicates that the two phenol hydroxyl groups (C2’ and C4’) in the B ring are important for inactivating cytochromes P450 enzymes (CYPs) and triggering pharmacological activity. However, these two hydroxyl groups are the accessible sites for glucuronidation, which is associated with elimination processes in vivo.9 The hydroxyl group at C4’ position may block the glucuronidation reaction at C2’ site.10 The importance of hydroxyl groups at C2’ and C4’ positions has been displayed,10,11 and several synthetic derivatives of glabridin have been designed, particularly those for anti-obesity activity11 and tyrosinase inhibitors.12 Various biological activities of glabridin have been reported, and they are including anti-inflammation,13,14 anti-oxidation,15,16 anti-tumor,17,18 anti-microorganism,19 bone protection,20 cardiovascular protection,21 hepatoprotection,22 neuroprotection,23 anti-obesity,24 and anti-diabetes.25 In this article, we will mainly discuss the health-benefiting effects of glabridin in these fields.
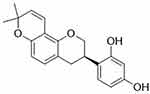 |
Figure 1 The chemical structure of glabridin. |
Anti-Inflammation
Glabridin has been reported to be one of the active ingredients in Schengen Mixture, which is a traditional Chinese medicine for treating inflammatory diseases. The virtual docking study finds that glabridin may interact with PTGS2 (cyclooxygenase-2, COX-2) protein. This is verified by a surface plasmon resonance (SPR) assay, and the dissociation rate constant (Kd) of 44.5 μM indicates strong binding affinity14 (Table 1). In both murine macrophages J774A.1 and human neutrophil HL-60 cells, glabridin can significantly inhibit the expression of COX downstream factors prostaglandin E2 (PGE2) (IC50 = 11 μM) and thromboxane B2 (TXB2) (IC50 = 11.3 μM) and lipoxygenase (LOX) downstream factor leukotriene B4 (LTB4) (IC50 = 5.3 μM).26 In lipopolysaccharide (LPS)-treated RAW264.7 cells, glabridin at the dose of 10 μg/mL decreases 33% of the nitric oxide (NO) level and inhibits interleukin 1β (IL-1β) production with an IC50 value of 30.8 μM. However, glabridin does not produce any inhibitory effects on IL-6 expression at all the tested concentrations.27 In LPS-induced rat acute respiratory distress syndrome, glabridin significantly suppresses the expression of tumor necrosis factor α (TNFα) and IL-18, decreases the production of malondialdehyde (MDA), NO, and SPA, and inhibits the activity of p38MPAK/ERK signaling pathways28 (Table 1).
 |
Table 1 The Biological Effects of Glabridin in Different Models |
Glabridin has been reported to attenuate the degradation of IκBs, inhibit the nuclear translocation of p65, reduce the DNA binding and transcriptional activity of NF-κB/Rel (Figure 2), and decrease the expression of inducible nitric oxide synthase (iNOS) and the generation of reactive oxygen species (ROS) in LPS-stimulated RAW264.7 cells. Additionally, glabridin also exhibits protective activity against the enhanced production of plasma NADPH oxidase (NOX) and TNFα in LPS-treated mice.29 Inflammation may evidently induce metabolic changes. LPS can significantly alter the profiles of the amino acid, energy, and lipid metabolism in RAW264.7 cells. Interestingly, glabridin may reverse the detrimental changes induced by LPS partially.30 In LPS-stimulated THP-1 monocytes, glabridin can decrease the chronic glucose stress-induced iNOS expression and nitrotyrosine formation. Similar protective activities of glabridin are also observed in the offspring of saturated fatty acid-fed mice mothers, which have developed metabolic dysregulation, including hyperglycemia, inflammation, and oxidative stress31 (Table 1).
Atopic dermatitis is a hereditary allergic skin disease, characterized by chronic inflammation, IgE-stimulated hyperreactivity, and epidermal barrier damage. Glabridin has been shown the protective activity against LPS-induced pathological changes in atopic dermatitis by decreasing the activity of TLR4/MyD88/NF-κB signaling pathway in HaCat cells and in mice32 (Figure 2). Psoriasis, a chronic inflammatory and autoimmune-regulated skin disease, is featured by epidermal hyperplasis and inflammation cell infiltration. In imiquimod-induced mice model, glabridin can decrease the PASI scores, improve histopathological changes, and down regulate the expression of p65, IL-6, IL-1β, IL-17A, IL-22, and IL-23. In HaCaT cells, glabridin also inhibits LPS-induced iNOS/NO, p65, IL-6, and IL-1β expression and decreases TNFα-stimulated IL-17A, IL-22, and IL-23 expression33 (Table 1).
In dextran sulphate sodium (DSS)-stimulated rat ulcerative colitis, glabridin can effectively alleviate ulceration and inflammatory cell infiltrations, decrease the productions of TNFα, myeloperoxidase (MPO), and NO/iNOS, and increase the concentrations of anti-inflammatory factor cAMP.34 Consistently, glabridin has been shown to ameliorate the disruption of the colonic architecture and the productions of inflammatory mediators, including NO and PGE2, in DSS-induced mice colitis.35 Dendritic cells (DCs) play an important role in the innate and adaptive immune activities. Maturation-promoting stimuli, such as inflammatory cytokines and LPS, have been associated with the enhancement of adhesion and co-stimulatory factors expression. Glabridin can decrease the expression of pro-inflammatory cytokines and increase antigen capture ability in LPS- or zymosan-induced DCs. The protective activity of glabridin might be associated with inhibition of NF-κB and MAPK signaling pathways36 (Figure 2) (Table 1).
Anti-Oxidation
Oxidative stress has been considered as a contributor to the oxidation of low-density lipoprotein (LDL), and oxidized LDL promotes foam cell formation and induces damage in the blood vessel wall. A random and double-blind study has been conducted and found the inhibitory effects of glabridin against LDL oxidation, contributing to its health-benefiting property.37 The protective activity of glabridin against LDL oxidation has been previously reviewed in 2015.38 At the dose of 30 μM, glabridin inhibits the generation of 7-hydroxycholesterol, 7-ketocholesterol, and 5,6-epoxycholesterol within 6 h of AAPH-induced LDL oxidation by 55%, 80%, and 40%, respectively, and within 6 h of Cu2+-induced LDL oxidation by 73%, 94%, and 52%, respectively.16 In both mouse peritoneal macrophages (MPMs) and J-774A.1 macrophage-like cells, glabridin can be accumulated up to 1.5 μg/mg of cell protein within 2-h incubation, and this may lead to inhibition of 80% cell-based LDL oxidation. In addition, glabridin also inhibits the release of superoxide by 60% in CuSO4-treated MPMs and the translocation of P-47, a cytosolic component of NADPH oxidase to the plasma membrane39 (Table 1).
Cardiovascular diseases may be exacerbated by oxidative stress. Cell-regulated oxidation of LDL and the content of macrophage glutathione (GSH) exhibits an inverse correlation. Glabridin has been shown to increase the accumulation of GSH and reduce lipid peroxides production in ApoE−/− mice, protecting against atherosclerosis.40 Glabridin, a phytoestrogen, substitutes estradiol enhancement of anti-oxidant enzymes under a condition of high glucose, which down regulates the expression of catalase (CAT) and paraoxonase 2 (PON2). Glabridin may exhibit as a potential anti-atherogenic candidate to increase the expression of CAT, PON2, and Mn-SOD.41 Further study shows that glabridin up regulates the expression of PON2 mRNA and protein in hyperglycemia mice. The fluorescence quenching assays indicate that glabridin can directly interact with PON2 with a binding constant of 7.61×105 M−1 and a free binding energy of −33.55 KJ/mol. In addition, glabridin protects PON2 against CuSO4-induced oxidation.42 PON1, a Ca2+-dependent enzyme binding to high-density lipoprotein (HDL), has been demonstrated to play an important role in the anti-atherogenic activity of HDL. The activity of recombinant PON1 (rePON1) may be blocked by linoleic acid hydroperoxide (LA-OOH) through oxidation of Cys284 thiol in rePON1. Glabridin has been shown to interact with rePON1 by forming hydrogen bonds with Lys338 or Val336 (a calculated binding energy of −9.5 kcal/mol), preventing from LA-OOH-induced oxidation43 (Table 1). Probably, the interaction between rePON1 or PON2 and glabridin may be a novel mechanism of glabridin in regulating oxidative stress.
Cerebral injury induced by ischemia is often associated with oxidative stress. In a rat model of middle cerebral artery occlusion (MCAO), glabridin has been shown to ameliorate the focal infarct volume, histopathological changes, and cell apoptosis with a possible molecular mechanism of decreasing the production of MDA and increasing the generation of SOD and GSH. In staurosporine-treated rat cortical neurons, glabridin can suppress the expression of Bax and caspase-3, enhance the expression of Bcl-2, and subsequently inhibit cell apoptosis44 (Table 1).
Ultraviolet B (UVB) has been shown to induce chromosome alterations and DNA damage with a possible mechanism of causing oxidative stress, leading to skin cell cycle arrest and apoptosis. Glabridin has been reported to prevent against UVB-induced DNA damage and cell apoptosis in normal human keratinocytes (NHK) by decreasing the productions of ROS directly and indirectly.45 Glabridin can inhibit the activity of tyrosinase reversibly with an IC50 value of 0.43 μM. In addition, glabridin can directly interact with tyrosinase through a fluorescence quenching assay and be considered as an inhibitor of tyrosinase. However, the docking study shows that glabridin does not bind to the active cavity of tyrosinase directly. In zebrafish, glabridin exhibits no effects on the synthesis of melanin46 (Table 1).
Anti-Tumor
The anti-tumor effects of glabridin have been explored. Glabridin has been shown to reduce the cell viability of SKNMC, A2780, and H1299 cell lines with IC50 values of 10 μM, 12 μM, and 38 μM, respectively. Intrinsic mitochondrial apoptosis pathway is involved in the mechanism of glabridin in producing cytotoxicity to SKNMC and H1299 cells. The mechanism of glabridin in promoting cell apoptosis for A2780 cells is extrinsic. In addition, glabridin may also potentiate the cytotoxic activity of doxorubicin to H1299 cells.18 The cancer stem cells (CSCs) are considered as the main contributor to cancer metastasis and recurrence, and management of CSCs can be a potential therapeutic strategy. In MDA-MB-231 and Hs578T cell lines, glabridin induces the demethylation of miR-148a gene and increases its expression, which inhibits the expression of SMAD2. In mice xenograft model, glabridin ameliorates breast cancer development, mesenchymal characteristics, and CSC-like properties47 (Table 1). Similarly, glabridin also attenuates the CSC-like properties in hepatocellular carcinoma HepG2, Huh-7, and MHCC97H cells by mediating the expression of miR-148a/SMAD2 signaling.48 Multidrug resistance (MDR) has become an impediment to chemotherapeutic management of cancers. Glabridin has been potentially indicated as a substrate of P-gp with a strong binding affinity and competitively inhibits its activity. In breast cancer MDA-MB-231/MDR1 cells, glabridin can greatly decrease the half maximal inhibitory concentration of DOX and paclitaxel and promote cell apoptosis.49
Wnt/β-catenin signaling and its downstream factor vascular endothelial growth factor (VEGF) play an important role in tumor development. Targeting Wnt/β-catenin-regulated VEGF expression has become a therapeutic target. Glabridin may ameliorate angiogenesis by down regulating the activity of miR-148/Wnt/β-catenin signaling, followed by decreased expression of VEGF in MDA-MB-231 and Hs-578T cells50 (Figure 3). In breast cancer SK-BR-3 cells, glabridin has been exhibited the inhibitory activity against cellular viability, as indicated by increased protein expression of c-PARP, cleaved-caspase-3, caspase-8, and caspase-9. The molecular mechanism might be associated with down regulation of p-ERK1/2, p-AKT, p-EGFR, and cyclin D1 expression by glabridin, which attenuates oxidative stress and increases cell apoptosis in breast cancer51 (Table 1). In glabridin-treated Ishikawa cells, DNA microarray analysis has been explored for the expression of estrogen responsive genes. Glabridin at the dose of 10 μM can activate both the genomic and non-genomic estrogen pathways. Interestingly, combination of 17β-E2 with glabridin may be a suitable and effective strategy for an estrogen replacement, which can be used for treatment of diseases in reproductive, cardiovascular, and circulatory systems.52 However, glabridin at the dose of more than 10 μM exhibits cytotoxicity in Ishikawa cells. ERα-SRC-1-co-activator signaling pathway has been involved in the regulation of glabridin in estrogenic activity.53 Consistently, combination of tamoxifen (1×10−5 M) with glabridin (1×10−6 M) exhibits estrogenic effects and inhibits cell growth in Ishikawa and MCF-7 cells by modulating the expression ESR1 and Bcl-2. Interestingly, the reduction of cell proliferation is not related to the regulatory activity of Bcl-254 (Table 1).
In human oral cancer SCC-9 and SAS cells, glabridin has been shown to inhibit cell proliferation, cause cell cycle arrest at sub-G1 phase, decrease the expression of caspase-3, caspase-8, caspase-9, and poly (ADP-ribose) polymerase cleavage, and increase the activity of p38 MAPK and JNK1/2 signaling pathways.55 In gastric cancer MN-45 cells, glabridin alone or in combination with 5-fluorouracil (5-FU) has been demonstrated to inhibit cell survival, proliferation, and invasion, as indicated by increased expression of N-cadherin, Bax, caspase-3, caspase-8, and caspase-9 and decreased expression of p16, E-cadherin, and Bcl-2.56 In both human non-small cell lung cancer A549 cells and MDA-MB-231 cells, glabridin has been exhibited the inhibitory activity against cell migration, invasion, and angiogenesis by increasing the proteosome degradation of αvβ3 integrin, decreasing the interaction between FAK and Src, and reducing the activation of AKT and RhoA and the phosphorylation of myosin light chain57,58 (Table 1).
In hepatoma carcinoma HepG2 cells, glabridin has been shown to suppress cell proliferation and cause cell cycle arrest in G1 by down regulating the expression of cyclinD3, CDK2, and CDK4. The possible underlying mechanism might be the inhibitory activity of glabridin against Braf/MEK signaling.59 Similarly, glabridin can inhibit cell proliferation and induce cell apoptosis dose-dependently by activating the expression of p38 MAPK and JNK1/2 signaling pathways in Huh7 cells.60 Another study shows that glabridin can significantly ameliorate the capacity of migration and invasion in Huh7 and Sk-Hep-1 cell lines by decreasing the phosphorylation levels of ERK1/2 and JNK1/2. Furthermore, glabridin may down regulate the expression of matrix metalloprotein-9 (MMP-9) and up regulating the expression of TIMP1 by affecting the activity of transcriptional factors NF-κB and AP-1.61 Spleen tyrosine kinase (SYK), a non-receptor protein-tyrosine kinase, mediates immunological receptor-regulated signaling and plays an important role in tumor growth and metastasis. Glabridin has been considered as a potential ligand of SYK with a fitting score of −8.2 kcal/mol using capecitabine as a control (−6.5 kcal/mol).62
Osteosarcoma, a common bone malignancy, is primarily found in children and adolescents with poor prognosis due to high activity in metastasis. Glabridin has been demonstrated to inhibit the migration and invasion, decrease the expression of MMP-2 and MMP-9, and induce cell cycle arrest at G2 phase in MG63 and HOS cell lines. Mechanistically, glabridin can decrease the phosphorylation levels of p38 MAPK and JNK, block the interaction between cAMP-response element binding protein (CREB) and AP-1, and inhibit the binding of CREB and AP-1 to DNA63 (Table 1). Acute myeloid leukemia (AML) has been considered as a cancer of blood cells and characterized by rapid growth of white blood cells abnormally. Glabridin may inhibit cell proliferation of various AML cell lines (HL-60, MV4-11, U937, and THP-1). In HL-60 cells, glabridin induces the expression of caspase-3, caspase-8, caspase-9, and PARP cleavage by increasing the phosphorylation levels of p38 MAPK and JNK1/2.64
Anti-Microorganism
The main protease (Mpro, also known as 3CLpro) of SARS-CoV-2 has become one of the most potential targets for developing new drugs. Screening study has investigated that glabridin shows strong inhibitory activity against Mpro and exhibits great binding affinity of −8.1 kcal/mol by noncovalently interacting with His41 and Met49.19 Another study reports that glabridin docks with 3CLpro of COVID-19 with estimated free binding energy of −8.25 kcal/mol by forming hydrogen bonds with Glu166, Arg188, and Gly143.65 (Table 1) Furthermore, glabridin stabilizes the active cavity of Mpro by forming hydrogen bonds with His41 and Glu166 and alkyl and Pi-alkyl interaction with Cys145 and Pro168. In the ADMET prediction, glabridin exhibits a good human intestinal absorption score and is a non-inhibitor and substrate of P-glycoprotein.66 Dengue virus, a mosquito-borne flavivirus, may cause dengue fever or dengue shock syndrome, which might be associated with the encoding of NS3 protease. Screening study has shown that glabridin can be a potential inhibitor against NS3 protease with binding affinity of −7.4 kcal/mol67 (Table 1). Sodium taurocholate co-transporting polypeptide (NTCP) has been considered as the receptor for hepatitis B virus (HBV) infection. Glabridin has been screened to be an inhibitor of HBV infection by inducing caveolar endocytosis and decreasing the expression of NTCP in primary human hepatocytes.68
Glabridin has been reported to inhibit the growth of multidrug resistant Staphylococcus aureus in vitro by increasing the productions of ROS, NO, and MDA and decreasing the expression of SOD. In addition, glabridin also exhibits anti-oxidative stress activity at lower concentrations (ie, lower than 1/2 MIC).69 (Table 1) Furthermore, glabridin also inhibits the biofilm formation of S. aureus (MRSA 4423) by down regulating the expression of cell surface proteins, including fibronectin binding proteins (FnbA and FnbB), serine-aspartate repeat-containing protein D, and immunoglobulin-binding protein G, and up regulating the expression of translation elongation factors (EF-Tu and EF-G), chaperone protein, GAPDH, and pyruvate kinase.70 Enterococcus faecalis is an anaerobic Gram-positive bacterium with high resistance to various anti-microbial agents. Glabridin (25 μg/mL) is active to kill 11.2% E. faecalis embedded in a biofilm. In addition, glabridin has been shown to synergy with chlorhexidine against E. faecalis. No cytotoxicity to gingival fibroblasts for glabridin (up to 50 μg/mL) by 2-h exposure is found71 (Table 1). Interestingly, glabridin at the dose of 6.25 μg/mL does not produce any effect on the biofilm formation by E. faecalis. However, the combination of glabridin (25 μg/mL) with nisin (12.5 μg/mL) produces 61.4% killing of a pre-formed E. faecalis after a 30-min contact. The possible molecular mechanism might be associated with the attenuation of NF-κB signaling by glabridin.72 Another study shows that glabridin exhibits anti-microbial activity against both Gram-positive and Gram-negative bacteria. Glabridin has been shown to be active against both Mycobacterium tuberculosis H37Ra and H37Rv strains with a MIC value of 29.16 μg/mL.73 Streptococcus mutans is considered as the most important cariogenic bacterium. Glabridin has shown anti-bacterial activity against S. mutans, as indicated by reduced biofilm viability, decreased dextran production, attenuated adherence, and reduced acid production. Importantly, glabridin does not exhibit toxicity to oral keratinocytes.74
Glabridin has demonstrated anti-fungal activity with a MIC value of 0.0156 mg/mL against filamentous fungi Microsporum gypseum, a MIC value of 0.125 mg/mL against Trichophyton rubrum and Histoplasma capsulatum, and a MIC value of 0.25 mg/mL against Aspergillus niger, Aspergillus flavus, and Sporothrix schenckii.75 Similarly, glabridin has been shown the anti-fungal activity against Candida albicans ATCC 28366 and C. albicans LAM-1 with MIC values of 6.25 μg/mL and 12.5 μg/mL, respectively. The growth of ATCC 28366 strain is blocked by combination of nystatin (0.25 μg/mL) with glabridin (1.25 μg/mL). However, no synergistic actions on the fungicidal activity have been found.76 Interestingly, overexpression of two apoptotic genes MCA1 and NUC1 and increased apoptosis in glabridin-treated C. albicans are observed77 (Table 1). Moreover, glabridin promotes apoptosis in C. albicans by triggering the expression of apoptosis inducing factor (AIF), which is caspase-independent.78 Combined with fluconazole, glabridin exhibits strong synergistic activities against drug-resistant C. albicans, C. neoformans, and C. tropicalis. The molecular mechanism in synergistic antifungal of glabridin and fluconazole is associated with induction of cell envelope impairment, which is indicated by decreased sizes of cells and increased permeability of cell membranes.79
C. glabrata also attracts a great attention. Glabridin significantly decreases the MIC values against fluconazole-resistant (MIC50 = 8μg/mL) but not fluconazole-SDD C. glabrata and promotes apoptosis by inducing the expression of MCA1 and NUC180 (Table 1). Azole-resistant Aspergillus fumigatus has become a major medical concern. The inhibitory activity of glabridin against A. fumigatus with a MIC50 value of 16 μg/mL. In addition, combination of glabridin with voriconazole (VRC) may exhibit synergistic effects with FICI range values of 0.15–0.5 against both VRC-resistant and VRC-sensitive A. fumigatus.81 Consistently, glabridin has been reported to inhibit the proliferation, biofilm formation, and adhesive capacity of A. fumigatus. In A. fumigatus-infected mice, glabridin decreases inflammatory responses, reduces the amount of A. fumigatus, and ameliorates neutrophil infiltration in cornea.82
In another study, it has been shown that glabridin exhibits fungicidal activity against Sclerotinia sclerotiorum with an EC50 value of 6.78 μg/mL with possible mechanisms involving accumulation of ROS, loss of mitochondrial membrane potential, and destruction of cell membrane.83 Glabridin also displays fungicidal effects against Fusarium graminearum. Specifically, glabridin inhibits the mycelial growth and conidial germination of F. graminearum with EC50 values of 110.70 mg/L and 40.47 mg/L, respectively. The possible mechanism might be associated with the inhibitory activity against the expression of ergosterol biosynthesis-associated proteins and the integrity of cellular membranes, which may lead to dysfunctions of transmembrane transport, disorder of intracellular substances and energy metabolism, and initiation of cell death84 (Table 1).
Natural compounds are the potential candidates for development of new drugs against malaria. Glabridin, a main active ingredient from Glycyrrhiza glabra, has been demonstrated to inhibit the growth of Plasmodium falciparum with an IC50 value of 23.9 μM. Glabridin blocks the activity of PfLDH by interacting with NADH binding site, induces oxidative stress, and promotes mitochondrial apoptosis in parasites.85 Eimeria tenella is often found in the cecal coccidiosis in poultry, associating with hemorrhagic diarrhea and high mortality. Glabridin has been screened to inhibit the replication of E. tenella by 75%, 50%, and 30% at the doses of 21.43 μg/mL, 5.28μg/mL, and 0.96μg/mL, respectively. At the dose of 21.43 μg/mL, glabridin significantly augments the expression of stress-induced factors NADPH and EtPP5 in E. tenella sporozoites86 (Table 1).
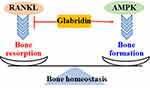
Bone Protection
The homeostasis of bone amount is maintained by a balance of osteoblast-mediated bone formation and osteoclast-regulated bone resorption. Excessive bone resorption is associated with bone loss and subsequent development of osteoporosis (Figure 4). Nuclear factor-κB ligand (RANKL) may promote osteoclastogenesis in RAW264.7 cells by up regulating the activity of TRAF6, GAB2, ERK2, JNK1, and MKK7 signaling pathways, increasing the expression of c-FOS and NFATc1, and elevating the productions of MMP-9, cathepsin K, CAII, TCIRG1, OSTM1, and CLCN7 (Figure 5). However, these RANKL-induced pathological disorders can be effectively blocked by glabridin.87 In C2C12 myotubes, glabridin can abolish dexamethasone-stimulated protein degradation by directly interacting with glucocorticoid receptor, blocking its nuclear translocation, and down regulating the expression of ubiquitin ligases MuRR1 and Cb1-b. Additionally, glabridin also inhibits dexamethasone-induced FOXO3a phosphorylation in vitro and in vivo88 (Table 1).
Metabolic disorders induce imbalance of glucose metabolism, including suppression of GLUT4-mediated glucose uptake. Glabridin has been demonstrated to increase glucose uptake by stimulating AMPK-dependent GLUT4 translocation to the plasma membrane in mice skeletal muscle L6 myotubes, leading to increased glucose uptake but decreased glycogen production and increased lactic acid generation. These effects cannot be blocked by PI3K or AKT inhibitors89 (Figures 4 and 5). Methylglyoxal (MG) is produced by glycolytic pathway to interact with protein and form glycation end products (AGEs), which play an important role in the pathological development of bone and cartilage diseases by enhancing ROS production and increasing the levels of inflammatory cytokines. Glabridin may inhibit MG-induced cytotoxicity and cell death in MC3T3-E1 cells. The molecular mechanism might be associated with the regulatory activity of glabridin in oxidative stress and inflammatory responses through NRF2/HO-1 and NF-κB pathways, respectively.90 2-deoxy-D-ribose (dRib) can promote the generation of ROS, decrease the levels of SOD and glutathione peroxidase 4 (GPX4), induce mitochondrial dysfunctions and apoptosis in MC3T3-E1 cells. The anti-oxidant N-acetyl-L-cysteine can effectively reverse the pathological damage induced by oxidative stress. Glabridin also ameliorates dRib-induced oxidative damage and increases the expression of ALP, OPN, OPG, OC, and BMPs by up regulating the expression of PI3K/AKT2 signaling pathway in MC3T3-E1 cells91 (Table 1). Antimycin A is an inhibitor of complex III in the respiratory chain. In antimycin A-treated MC3T3-E1 cells, glabridin exhibits cytoprotective activity against mitochondrial dysfunction, inhibiting mitochondria-mediated cell death. In addition, glabridin also restores antimycin A-inactivated PI3K and CREB signaling pathways.92
Estrogen plays an important role in maintenance of bone and cartilage, and estrogen deficiency is closely associated with the development of osteoporosis and osteoarthritis. The protective activity of the extract of Glycyrrhiza glabra L. against osteoporosis by an estrogen receptor (ER)-mediated mechanism has been demonstrated.20 Prenylated flavonoids are reported to modulate the activity of ERs, particularly enhancement of their affinity to ERα. Glabridin has similar chemical structure and lipophilicity to estradiol (E2). Creatine kinase (CK) activity has been considered as a convenient marker of estrogen responses. Glabridin exhibits beneficial effects by increasing CK specific activity in both diaphyseal bone and epiphyseal cartilage in rats93 (Figure 5). However, a study shows that glabridin is absence of estrogenic activity towards both ERα and ERβ at the dose range of 1×10−7 to 1×10−4 M, while it shows toxicity at the dose of more than 1×10−4 M. On the contrary, glabridin exhibits antagonistic activity against E2-activated ERα selectively by 80% at the dose of 1×10−6 M.94
Mesenchymal stem cells (MSCs) have been considered as a promising candidate for therapeutic management of many intractable diseases. Unfortunately, MSCs often lose stemness by initiating cellular senescence when they are cultured in vitro. Glabridin has been demonstrated to maintain the efficacy of MSCs in the stemness and differentiation, as shown by increased expression of Oct4, Dlx5, Runx5, Osteocalcin, and Osteopontin95 (Figure 5). Glabridin increases ALP activity, collagen content, and osteocalcin secretion and inhibits TNFα (1×10−10 M)-induced production of PGE2 and NO and apoptosis in MC3T3-E1 cells96 (Table 1).
Osteoarthritis (OA), a chronic degenerative bone disease, is characterized by poor self-repair capacity of cartilage. The pathological development of OA can be accelerated by oxidative stress. In human OA chondrocytes, glabridin has been shown to increase the expression of Collagen II, aggrecan, SOX9, and proteoglycan 4 (PRG4). The molecular mechanism of glabridin in protecting OA cartilage against damage might be associated with attenuation of oxidative stress, promotion of mTOR-mediated autophagy, and reduction of chondrocytes apoptosis.97
Cardiovascular Protection
Maintenance of structural integrity and barrier functions in vascular endothelial cells contributes to cardiovascular homeostasis. High fat diet-induced hyperlipidemia can cause endothelial dysfunctions and increased permeability. Glabridin has been shown to ameliorate endothelial dysfunctions (Figure 5), as indicated by decreased total cholesterol and triglycerides and improved morphological structure of the arterial wall. The possible mechanism might be associated with the down regulation of myosin light chain kinase (MLCK)/phosphorylated MLC system by glabridin through MAPK signaling pathway21 (Table 1). It has been demonstrated that glabridin can trigger vasorelaxation by opening K+ channels, particularly BKCa, in a reconstructed artery (Figure 5). However, employment of the phosphodiesterase (PDE) inhibitor may increase the concentration of cellular cGMP and block the effects of glabridin on vasorelaxation.98 Consistently, glabridin exhibits vasorelaxant effects by increasing the activities of cGMP and protein kinase G (PKG) signaling pathways in phenylephrine-pretreated isolated human saphenous vein homogenates. However, glabridin does not alter the production of NO.99
Estrogen plays protective activity in vascular tissues. It has been demonstrated that glabridin, similar to E2, increases DNA synthesis in human ECV-304 cells and exhibits a biphasic effect on the proliferation of VSMC. In vivo study shows that glabridin produces the specific activity of CK in the aorta and in the heart left ventricle.100 Clinical use of doxorubicin (DOX) for managing cancers is often associated with cardiotoxicity in a dose-dependent manner. Glabridin has been found to prevent against DOX-induced dysbiosis of gut microbiota and cardiotoxicity by modulating LPS/NF-κB and butyrate-STAT6 signaling pathways101 (Table 1) (Figure 5). Transthyretin (TTR) amyloid fibrils have been implicated in patients with familial amyloid cardiomyopathy and amyloidotic polyneuropathy. Glabridin has been screened to interact with TTR by forming a hydrogen bond with Lys15 and a CH-π bond with Ala108, stabilizing the dimer-dimer interface of TTR and inhibiting TTR fibrillization.102
Hepatoprotection
The hepatoprotective activity of glabridin has been explored. Glabridin has been demonstrated to effectively reverse methotrexate-induced pathological changes and biochemical parameters in the liver by increasing the expression of NRF2 and decreasing the activity of NF-κB signaling.22 Chronic hyperglycemia can be associated with the disturbance of extracellular matrix (ECM) morphology and biochemistry, causing loss of functions in organs. Liver fibrosis is contributed by substantial alterations in the composition and amount of ECM. Glabridin has been reported to decrease streptozotocin-induced collagen fiber deposition and ameliorate microvascular abnormality and liver fibrosis in rats.103 Peroxisome proliferator-activated receptor γ (PPARγ), a ligand-activated nuclear receptor, can directly regulate the expression of target genes (Figure 5). In HepG2 cells, glabridin has been identified as an activator of PPARγ through HPLC fractionation and mass spectroscopy. Glabridin interacts with the ligand binding domain (LBD) of PPARγ with an EC50 value of 6115 nM and T0070907, an antagonist of PPARγ-LBD, inhibits the glabridin responses for full length PPARγ receptor with an IC50 value of 20 μM.104 (Table 1) Methotrexate (MTX), the first-line drugs for therapeutic management of rheumatoid arthritis, has been associated with severe side effects, such as hepatotoxicity. Combination of MTX with glabridin can significantly improve the radiological and histopathological changes, augmenting the therapeutic effects of MTX. Importantly, glabridin can prevent MTX-induced alterations in levels of serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), MDA, and GSH by inhibiting the expression of NF-κB and enhancing the activity of NRF2 pathway in the hepatic tissues.105
Cytochromes P450 (CYP) is a collection of metabolic enzymes available for most drugs. CYP2E1, an enzyme with low activity under physiological conditions, regulates many drugs’ metabolism and benefits our body by mediating oxidative responses. Higher activity of CYP2E1 often causes excessive ROS production, causing liver damage. Glabridin has been identified as a potential inhibitor of CYP2E1 by directing interacting with the active site of CYP2E1 with -CDOCKER interaction energy of −84.44 kcal/mol (Figure 5). In human liver microsomes in vitro, glabridin exhibits greater inhibitory activity against CYP2E1 enzyme (IC50 = 6.2 μM) than fisetin, epicatechin, nobiletin, and chrysin106 (Table 1). Conversion of paracetamol (PCM) to N-acetyl-p-benzoquinone imine by CYP2E1 can lead to liver toxicity. In PCM-stimulated mice liver injury model, glabridin has been shown to improve the serum biochemical parameters and oxidative stress markers. In addition, glabridin also attenuates the activity of NF-κB signaling pathway in liver tissues.107 The protecting activity of glabridin against liver damage might be associated with inhibition of CYP2E1 and suppression of oxidative stress and NF-κB pathway. Another study shows that glabridin has been screened to inhibit against CYP3A4 competitively.108 It is consistent that glabridin inactivates CYP3A4 in a time-, concentration, and NADPH-dependent manner, accompanied by a loss of the heme moiety. Furthermore, this inactivation is irreversible and demonstrated by an assay of extensive dialysis. Glabridin also inhibits the activities of CYP2B6. However, interaction between glabridin and CYP2B6 does not result in a modification of the heme moiety. HPLC analysis indicates that incubations with glabridin and NADPH do not lead to the destruction of the heme moiety. The activity of CYP2C9 can be competitively blocked by glabridin109 (Figure 5). More investigation of glabridin in interacting with CYPs is still needed, due to its wide application as a supplementary agent in foods and medicines. Particularly, those cases in which irreversible inactivation of target enzymes may occur should be prevented.
Neuroprotection
Inflammation has been involved in the pathological development of many neurodegeneration diseases, such as Parkinson’s diseases (PD), Alzheimer’s diseases (AD), and multiple sclerosis. Microglia is considered as the major cell responsible for inflammation, which is mediating neuronal toxicity. In LPS-treated BV-2 cells, glabridin has been demonstrated to decrease the productions of IL-1β, TNFα, and NO by down regulating the activity of NF-κB signaling and AP-1.23 (Figure 5) Impaired cognitive functions may be developed in patients with Alzheimer patients. Cognitive dysfunctions can be related to impaired cholinergic activity. Increased acetylcholine levels are associated with decreased cholinesterase activity, improving the memory in mice. In scopolamine-induced mice, glabridin has been shown to improve memory and reduce the brain cholinesterase activity dose-dependently110 (Table 1). Consistently, glabridin at the doses of more than 25 mg/kg may prevent against the deleterious damages of hyperglycemia on learning and memory, while glabridin at the dose of 5 mg/kg does not produce any effects.111
Glabridin has been demonstrated sedative and anxiolytic effects by potentiating GABA-mediated responses. At the dose of 1×10−12 M, glabridin increases GABA-stimulated currents by about 140%. In contrast, glabridin enhances the currents by about 580% at the dose of 3×10−6 M. The explanation might be associated with two different regulating sites in GABAA receptor for glabridin: one with low affinity with high potency and a second with high affinity but low potency.112 Further study shows that glabridin significantly potentiates the activity of GABAA α1β(1–3)γ2 receptor and decreases the EC50 values of the receptor for GABA by about 12 folds. In addition, mutation of N265 in the β2 subunit may almost abolish the potentiating activity of glabridin113 (Table 1).
Glabridin has been considered as a substrate of P-glycoprotein (P-gp), and the presence of P-gp limits the brain penetration and distribution of glabridin. In the primary rat brain microvascular endothelial cells, the efflux of glabridin has been found to be facilitated from the abluminal to the luminal side. These actions can be attenuated by co-treatment with a P-gp inhibitor verapamil. In vivo study, the concentration of glabridin in rat brain is about 27% of the plasma level, which may enhance up to 44% by co-treating with verapamil. In mdr1a knockout mice, the AUC of glabridin can be 6 times higher than that in the wild-type mice.114 5-HT3 receptor acts as the only ionotropic channel within the 5-HT receptor family and is associated with the development of nausea and vomiting. Glabridin has been demonstrated to competitively antagonize 5-HT3 receptor with an inhibiting rate of 64.7% (2.5 μM 5-HT) and 17.3% (30 μM 5-HT), respectively. The IC50 value of glabridin for inhibiting 5-HT3 receptor is 14.4 μM.115
Anti-Obesity
Metabolic syndrome is associated with abdominal obesity, dyslipidemia, diabetes, and hypertension. Obesity can be contributed by imbalance between energy intake and expenditure. The adipose tissue is the form for storing excess energy. During adipogenesis, CCAAT enhancer binding protein α (C/EBPα) and PPARγ, the two master mediators, have been known to regulate the expression of adipogenic biomarkers and lipid-associated metabolism enzymes, such as LPL. Up regulation of adipogenic biomarkers is related to enhanced uptake of glucose and fatty acids and increased production of TG hydrolysis and lipogenesis.116 The flavonoids from Glycyrrhiza glabra L. exhibit benefiting effects and reduce inflammatory and oxidative stress by suppressing the expression of PPARα and SREBP-1c.117 Treatment with different concentrations of the extract of Glycyrrhiza glabra L., the rat serum levels of cholesterol, LDL, and TG are decreased and the levels of HDL are increased.118 Glabridin has been shown to inhibit adipogenesis in 3T3-L1 cells, and glabridin-rich extract of Glycyrrhiza glabra L. also exhibits inhibitory activity against adipogenesis by decreasing the expression of C/EBPα and PPARγ, suppressing the activity of lipoprotein lipase, glucose uptake, and PGE2, and inhibiting energy metabolism, leading to amelioration of high-fat diet (HFD)-induced hepatic steatosis24 (Table 1).
AMP-activated protein kinase (AMPK), a master in energy sensation, mediates the energy homeostasis in the whole body. The acetone extract of Glycyrrhiza glabra L. has been shown the inhibitory activity against the adipogenesis of 3T3-L1 cells by inducing the phosphorylation of AMPK, as indicated that 3T3-L1 cells are arrested in the phase of G1 and the expression of cyclins and cyclin-associated kinase is inhibited in the stage of mitotic clonal expansion (MCE). In addition, the expression of lipid metabolic factors (such as UCP1 and leptin) is increased by the acetone extract of Glycyrrhiza glabra L., and the expression of SREBP1, FAS, SCD, C/EBPα, and PPARγ is decreased.119 Consistently, combination of Glycyrrhiza glabra L. extracts with Panax ginseng saponin fractions may reduce lipid accumulation, lipid composition, and adipose tissue size by activating AMPK signaling pathway and decreasing adipocyte transcriptional factors expression in 3T3-E1 cells.120 Activation of AMPK often leads to lipid dissipation by increasing fatty acid oxidation, leading to reduction of adipose tissue mass and adipocyte hypertrophy. Glabridin may activate AMPK expression and then enhance the oxidation of fatty acid by down regulating the gene expression involved in lipid metabolism, such as SREBP-1c, FAS, ACC, and SCD-1 and increasing the biological functions of mitochondria.121 It has been demonstrated that the adipose tissue can be negatively affected by 2,3,7,8-tetrachlorodibenzo-p- dioxin (TCDD), which stimulates the generation of ROS, PGE2, PLA2, COX-1, and intracellular Ca2+ concentrations, decreases the expression of insulin receptor substrate 1 and Glut4, and attenuates the expression of PPARγ and CEBPα in 3T3-L1 cells. These TCDD-induced pathological changes can be effectively ameliorated by glabridin122 (Table 1).
Anti-Diabetes
Inflammatory responses have been implicated in the pathogenesis of many diseases, including diabetes. It has been reported that the intestinal inflammation can exacerbate the progression of diabetes. The hypoglycemic effects of the extract of Glycyrrhiza glabra L. in type II diabetes (T2DM) mice have been shown, as indicated by decreased fasting blood glucose, improved insulin resistance, and decreased serum lipid. Particularly, the extract of Glycyrrhiza glabra L. can reshape the gut microbiota and ameliorate colonic inflammation by suppressing the activity of TLR4/NF-κB signaling pathway.123 Consistently, the extract of Glycyrrhiza uralensis has been demonstrated to protect high glucose-induced renal damage by suppressing the expression of TGF-β1/Smad/STAT3 signaling pathways in HK2 cells and high fructose-induced T2DM in Apoeem1/NarI/NarI mice.124 The anti-hyperglycemic potential of Glycyrrhiza glabra L. has been supported in the streptozotocin (STZ)-induced rat diabetes.125 Supplementation of functional foods to our daily diets may produce the benefiting effects and the reduction of metabolic risks. Bread infused with functional herbs, such as Glycyrrhiza glabra L., has been reported to decrease the glycaemic index.126
The hypoglycemic effects of glabridin in streptozotocin (STZ)-induced diabetic mice have been demonstrated in a dose-dependent manner with a possible mechanism of anti-oxidative stress.25 A network pharmacology approach has been conducted to investigate the bioactive ingredients of Glycyrrhiza glabra L. against diabetic nephropathy (DN). Glabridin has been screened to improve the renal pathological changes and biological functions, as indicated by increased productions of Scr, BUN, UREA, KIM-1, NGAL, and TIMP-1 in rats. In high glucose-treated NRK-52E cells, glabridin suppresses ferroptosis by increasing the activity of SOD and GSH and decreasing the production of MDA and iron concentration. Down regulation of VEGF, p-AKT, and p-ERK1/2 expression is involved in the protective activity of glabridin against DN127 (Table 1).
Miscellaneous Section
The prevalence of early age-related macular degeneration (AMD) increases with age. Pathologically, clinic AMD can be classified into dry and wet types. No effective treatments are available for dry AMD. In sodium iodate (NaIO3)-treated human retinal pigment epithelial cells (ARPE-19), glabridin ameliorates oxidative and cell apoptosis by decreasing the activity of p38 MAPK and ERK1/2 signaling pathways. In NaIO3-treated mice, glabridin inhibits retinal degeneration and reduces deposit formation, preventing retinal damage.128 In ovalbumin (OVA)-induced mice asthma, glabridin may greatly improve pulmonary function parameters, including peak inspiratory flow, peak expiratory flow, tidal volume, expiratory volume, the frequency of breathing, and enhanced pause values. Importantly, glabridin can effectively decrease the levels of OVA-induced immunoglobulin E (IgE) in mice.129 Glabridin has been shown to ameliorate fatigue in mice, as indicated by increased exhaustive exercise time, delayed elevation of blood lactic acid, and increased storage of liver and muscle glycogen.130 Areca nut chewing may cause chronic inflammation, myofibroblasts activation, and pathological fibrosis development, leading to formation of oral submucous fibrosis (OSF). Glabridin can be an effector to intervene and ameliorate myofibroblast activities, as demonstrated by reduction of collagen gel contractility, migration, invasion, and wound healing activity. Attenuation of TGFβ/Smad2 signaling pathway is involved in the protective activity of glabridin against OSF.131 In a mouse glomerular disease model (Masugi-nephritis), glabridin at the dose of 30 mg/kg for 10 days has been shown the protective activity, as found by decreased urinary protein excretion. However, the molecular mechanism of glabridin against nephritis might not be associated with the radical scavenging.132
Toxicology and Clinical Applications
Glabridin is one of the active constituents of Glycyrrhiza glabra L. and becomes a marker compound for evaluating the characteristics of Glycyrrhiza glabra L. The safety of flavonoid oil (LFO) from Glycyrrhiza glabra L. has been assessed and indicated that LFO at the dose of up to 1200 mg/day is safe without any obvious hematological or related biochemical changes in healthy subjects. In addition, a single-dose study in male subjects with 1200 mg/day of LFO shows that the pharmacokinetic parameters of glabridin involve a Cmax value of 2.65 ng/mL, Tmax of 6.0 h, and T1/2 of 10 h.133 It has been demonstrated that LFO may have abdominal fat-lowing and hypoglycemic activities by possibly regulating the expression of PPARγ in obese diabetic KK-Ay mice.134 This notion has been re-confirmed by an in vivo study in C57BL/6J mice with a period of 16 weeks. LFO consumption decreases high fat diet-induced abdominal adipose tissue mass and body weight (by 18.8%) without any obvious toxicity. A DNA microarray analysis shows that LFO may inhibit the synthesis and stimulate the catabolism of fatty acid in the liver.135 LFO has become a commercial product named as Glavonoid, which has been approved as a novel food ingredient by the European Food Safety Authority.
Traditional Chinese medicine formulas, such as Jinhua Qinggan granule, Lianhua Qingwen capsule/granule, Xuebijing injection, Qingfei Paidu decoction, HuaShiBaiDu formula, and XuanFeiBaiDu granule, and their active ingredient glabridin have been analyzed to reduce the expression of angiotensin-converting enzyme 2 (ACE2) for therapeutic management of COVID-19.136 The extract of Glycyrrhiza glabra L. has been considered to mitigate COVID-19, and the biological guided isolation of active constituents from the root of Glycyrrhiza glabra L. against factor Xa (FXa) has been investigated. Glabridin is selective as the effective compound to inhibit FXa selectively with an IC50 value of 35.3 μM.137 Glabridin shows protective activity against SARS CoV-2 with an IC50 value of 2.5 μM.138 Clinically, S. aureus has been developed to acquire multidrug resistance, and it has become a critical pathogen in hospital and community infections. Glabridin also inhibits the growth of S. aureus (MRSA 4427) by increasing the production of ROS. Combination of glabridin with norfloxacin can synergistically increase oxidative stress, which affects the integrity of macromolecules and distorts cell morphology of S. aureus.139 Pseudomonas aeruginosa has been considered as the multidrug resistant strains in the lung infection. Glabridin is one of the active ingredients from the extract of Glycyrrhiza glabra, which may be effective against P. aeruginosa-caused lung infection.140
Chronic acquired hypermelanosis (melasma) leads to hyperpigmented macules, which might be associated with dysregulation of tyrosinase activity. Forty adult Caucasian women with epidermal melasma have been involved for a clinical trial that includes a non-prescription proprietary gel formulation containing glabridin for 6 months by twice/day topical administration. This formulation has been suggested to be safe and effective in the improvement of the cosmetic appearance of epidermal melasma in Caucasian women.141 To enhance the skin permeability without irritation, partially myristoylated chitosan pyrrolidone carboxylate (PMCP) has been employed to prepared for glabridin/PMCP-polymeric micelles, which increase the absorbance of glabridin by penetrating the skin for 4 times and the inhibition of melanogenesis.142 Similarly, glabridin prepare with nanoemulsion formulation of eutectic mixture shows higher transdermal penetration (28.26 μg/cm2) than that with drug-loaded eutectic mixture (9.94 μg/cm2) or the drug solution formation (3.82 μg/cm2).143
Future Perspectives
GutGard, a flavonoid-rich extract of Glycyrrhiza glabra, has been demonstrated to exhibit anti-ulcer activity against Helicobacter pylori by inhibiting the activity of DNA gyrase and dihydrofolate reductase with IC50 values of 4.40 μg/mL and 3.33 μg/mL, respectively. Glabridin is the main ingredient in GutGard responsible for anti-Helicobacter pylori activity with a MIC value of 12.5 μg/mL.144 Biofilms have been considered as a serious issue due to their ability of resistance to anti-microbial agents. To eradicate the biofilms, the ethanol extracts (ETEX) of 155 different foodstuffs containing medicinal plants are screened. The ETEX of licorice exhibit the biofilm eradication effects against S. mutans, S. aureus, and Porphyromonas gingivalis. Glabridin (25–50 μg/mL) has been demonstrated to exhibit the greatest potential in the biofilm eradication activity. Combined with ε-poly-L-lysine, glabridin exhibits a broad activity in biofilm eradication in various bacteria, including Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa.145 The protective effects of glabridin have been demonstrated. However, most of these studies are still at the stage of screening and verification. How glabridin influences the metabolism of these potential microorganisms and affects their fates are still needed to be clearly elucidated.
The clinical effects of traditional Chinese medicines (TCM) are increasingly recognized, and their bioactive foundations are those effective compounds. Glabridin can act as the effective compound to be involved in some complexes or formulations, which are potentially used or developed for clinic management of some diseases. More importantly, combination of glabridin with other drugs in treating some diseases, such as melanin-associated or P-gp-related diseases, may be an effective strategy. The synergistic effects of glabridin provide a platform in therapeutic management of diseases. Interestingly, glabridin may augment the therapeutic effects of some drugs and ameliorate the potential adverse effects. The interactions between glabridin and other drugs still need to be further investigation, and their pharmacological effects should be further explored. Additionally, the underlying mechanisms of glabridin in regulating the potential pharmacological or physiopathological effects are still unclear. Many studies are at the initial steps to confirm the specific effects of natural compounds, such as glabridin, without or partially exploring their regulating signaling pathways.
The biological effects of flavonoids in vivo are not limited to anti-oxidant activity, due to their poor water solubility, low bioavailability, and vulnerable metabolism. The beneficial effects of flavonoids might be associated with a mechanism that is not necessarily related to their anti-oxidant activity. Probably, flavonoid can directly bind to the key proteins or enzymes that are implicated in the cellular signaling. More importantly, these effective flavonoids, such as glabridin, can be the lead compounds for further development. Chemical modifications designed by structure-activity relationship have been introduced to improve the capability of target-specificity and the activity of biological actions. Some formulations and preparations of glabridin have been improved by the pharmaceutical methods and chemical approaches. For example, microneedles, liposome, and smartPearls formulation are available for glabridin to enhance the bioavailability. More efforts are still needed.
Conclusion
Glabridin, a natural prenylated isoflavan, isolated from Glycyrrhiza glabra L., which contains many effective compounds, such as liquiritin, glycyrrhizic acid, glabridin, liquiritigenin, and isoliquiritigenin, has various pharmacological activities. In this article, we comprehensively discuss the biological activities of glabridin in anti-inflammation, anti-oxidation, anti-tumor, anti-microorganism, bone protection, cardiovascular protection, neuroprotection, hepatoprotection, anti-obesity, and anti-diabetes. Specifically, the anti-inflammatory and anti-cancer activity of glabridin might be associated with inhibition of NF-κB and MAPK signaling pathways. Glabridin may suppress LDL oxidation, increase PON2 expression, and decrease ROS generation. The protective activity of glabridin against microorganism has been demonstrated. However, the potential molecular mechanism still needs more investigation. The estrogen-like effects of glabridin benefit bone metabolism and maintain bone homeostasis by activating ER signaling. The estrogen-like effects of glabridin also contribute to cardiovascular protection. Glabridin may specifically inhibit CYP2E1 and ameliorate liver injury. More importantly, glabridin can be an inhibitor of tyrosinase and P-gp. However, the potential molecular mechanisms of glabridin in regulating pharmacological activities are still unclear.
Data Sharing Statement
The data used to support the findings of this study are included within the article.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by Natural Science Foundation of Jiangxi Province (Grant NO. 20212BAB216067), the Science and Technology research project of the Education Department of Jiangxi Province (Grant NO. GJJ211516 and GJJ201523), and the Science and Technology project of the First Affiliated Hospital of Gannan Medical University (Grant NO. YJYB202102 and YJYB202103).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ding Y, Brand E, Wang W, Zhao Z. Licorice: resources, applications in ancient and modern times. J Ethnopharmacol. 2022;298:115594. doi:10.1016/j.jep.2022.115594
2. Deng TM, Peng C, Peng DY, Yu NJ, Chen WD, Wang L. 甘草化学成分和药理作用研究进展及质量标志物的探讨 [Research progress on chemical constituents and pharmacological effects of Glycyrrhizae Radix et Rhizoma and discussion of Q-markers]. Zhongguo Zhong yao za zhi. 2021;46(11):2660–2676. Chinese. doi:10.19540/j.cnki.cjcmm.20210304.201
3. Qin J, Chen J, Peng F, et al. Pharmacological activities and pharmacokinetics of liquiritin: a review. J Ethnopharmacol. 2022;293:115257. doi:10.1016/j.jep.2022.115257
4. Mohammed EAH, Peng Y, Wang Z, Qiang X, Zhao Q. Synthesis, antiviral, and antibacterial activity of the glycyrrhizic acid and glycyrrhetinic acid derivatives. Russ J Bioorg Chem. 2022;48(5):906–918. doi:10.1134/s1068162022050132
5. Ramalingam M, Kim H, Lee Y, Lee YI. Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front Aging Neurosci. 2018;10:348. doi:10.3389/fnagi.2018.00348
6. Simmler C, Pauli GF, Chen SN. Phytochemistry and biological properties of glabridin. Fitoterapia. 2013;90:160–184. doi:10.1016/j.fitote.2013.07.003
7. Hayashi H, Hattori S, Inoue K, et al. Field survey of Glycyrrhiza plants in Central Asia (3). Chemical characterization of G. glabra collected in Uzbekistan. Chem Pharm Bull. 2003;51(11):1338–1340. doi:10.1248/cpb.51.1338
8. Ao M, Shi Y, Cui Y, Guo W, Wang J, Yu L. Factors influencing glabridin stability. Nat Prod Commun. 2010;5(12):1907–1912.
9. Guo B, Fang Z, Yang L, et al. Tissue and species differences in the glucuronidation of glabridin with UDP-glucuronosyltransferases. Chem Biol Interact. 2015;231:90–97. doi:10.1016/j.cbi.2015.03.001
10. Niemeyer ED, Brodbelt JS. Regiospecificity of human UDP-glucuronosyltransferase isoforms in chalcone and flavanone glucuronidation determined by metal complexation and tandem mass spectrometry. J Nat Prod. 2013;76(6):1121–1132. doi:10.1021/np400195z
11. Choi LS, Jo IG, Kang KS, et al. Discovery and preclinical efficacy of HSG4112, a synthetic structural analog of glabridin, for the treatment of obesity. Int J Obes. 2021;45(1):130–142. doi:10.1038/s41366-020-00686-1
12. Jirawattanapong W, Saifah E, Patarapanich C. Synthesis of glabridin derivatives as tyrosinase inhibitors. Arch Pharm Res. 2009;32(5):647–654. doi:10.1007/s12272-009-1501-x
13. Parlar A, Arslan SO, Çam SA. Glabridin alleviates inflammation and nociception in rodents by activating BK(Ca) channels and reducing NO levels. Biol Pharm Bull. 2020;43(5):884–897. doi:10.1248/bpb.b20-00038
14. Ye Q, Zhang Q, Yao H, et al. Active-ingredient screening and synergistic action mechanism of shegan mixture for anti-asthma effects based on network pharmacology in a mouse model of asthma. Drug Des Devel Ther. 2021;15:1765–1777. doi:10.2147/dddt.S288829
15. Belinky PA, Aviram M, Mahmood S, Vaya J. Structural aspects of the inhibitory effect of glabridin on LDL oxidation. Free Radic Biol Med. 1998;24(9):1419–1429. doi:10.1016/s0891-5849(98)00006-9
16. Belinky PA, Aviram M, Fuhrman B, Rosenblat M, Vaya J. The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation. Atherosclerosis. 1998;137(1):49–61. doi:10.1016/s0021-9150(97)00251-7
17. Goel B, Sharma A, Tripathi N, et al. In-vitro antitumor activity of compounds from Glycyrrhiza glabra against C6 glioma cancer cells: identification of natural lead for further evaluation. Nat Prod Res. 2021;35(23):5489–5492. doi:10.1080/14786419.2020.1786830
18. Modarresi M, Hajialyani M, Moasefi N, Ahmadi F, Hosseinzadeh L. Evaluation of the cytotoxic and apoptogenic effects of glabridin and its effect on cytotoxicity and apoptosis induced by doxorubicin toward cancerous cells. Adv Pharma Bull. 2019;9(3):481–489. doi:10.15171/apb.2019.057
19. Islam R, Parves MR, Paul AS, et al. A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J Biomol Struct Dyn. 2021;39(9):3213–3224. doi:10.1080/07391102.2020.1761883
20. Azizsoltani A, Piri K, Behzad S, et al. Ethyl acetate extract of licorice root (Glycyrrhiza glabra) enhances proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells. Iran J Pharmac Res. 2018;17(3):1057–1067.
21. Wang G, Sun G, Wang Y, et al. Glabridin attenuates endothelial dysfunction and permeability, possibly via the MLCK/p-MLC signaling pathway. Exp Ther Med. 2019;17(1):107–114. doi:10.3892/etm.2018.6903
22. Dogra A, Gupta D, Bag S, et al. Glabridin ameliorates methotrexate-induced liver injury via attenuation of oxidative stress, inflammation, and apoptosis. Life Sci. 2021;278:119583. doi:10.1016/j.lfs.2021.119583
23. Park SH, Kang JS, Yoon YD, et al. Glabridin inhibits lipopolysaccharide-induced activation of a microglial cell line, BV-2, by blocking NF-kappaB and AP-1. Phytother Res. 2010;24(Suppl 1):S29–34. doi:10.1002/ptr.2872
24. Ahn J, Lee H, Jang J, Kim S, Ha T. Anti-obesity effects of glabridin-rich supercritical carbon dioxide extract of licorice in high-fat-fed obese mice. Food Chem Toxicol. 2013;51:439–445. doi:10.1016/j.fct.2012.08.048
25. Wu F, Jin Z, Jin J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol Med Rep. 2013;7(4):1278–1282. doi:10.3892/mmr.2013.1330
26. Chandrasekaran CV, Deepak HB, Thiyagarajan P, et al. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine. 2011;18(4):278–284. doi:10.1016/j.phymed.2010.08.001
27. Thiyagarajan P, Chandrasekaran CV, Deepak HB, Agarwal A. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology. 2011;19(4):235–241. doi:10.1007/s10787-011-0080-x
28. Zhang LP, Zhao Y, Liu GJ, Yang DG, Dong YH, Zhou LH. Glabridin attenuates lipopolysaccharide-induced acute lung injury by inhibiting p38MAPK/ERK signaling pathway. Oncotarget. 2017;8(12):18935–18942. doi:10.18632/oncotarget.14277
29. Kang JS, Yoon YD, Cho IJ, et al. Glabridin, an isoflavan from licorice root, inhibits inducible nitric-oxide synthase expression and improves survival of mice in experimental model of septic shock. J Pharmacol Exp Ther. 2005;312(3):1187–1194. doi:10.1124/jpet.104.077107
30. Liu K, Pi F, Zhang H, et al. Metabolomics analysis to evaluate the anti-inflammatory effects of polyphenols: glabridin reversed metabolism change caused by LPS in RAW 264.7 cells. J Agric Food Chem. 2017;65(29):6070–6079. doi:10.1021/acs.jafc.7b01692
31. Yehuda I, Madar Z, Leikin-Frenkel A, Tamir S. Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation. Mol Nutr Food Res. 2015;59(6):1041–1052. doi:10.1002/mnfr.201400876
32. Chang J, Wang L, Zhang M, Lai Z. Glabridin attenuates atopic dermatitis progression through downregulating the TLR4/MyD88/NF-κB signaling pathway. Genes Genomics. 2021;43(8):847–855. doi:10.1007/s13258-021-01081-4
33. Li P, Li Y, Jiang H, et al. Glabridin, an isoflavan from licorice root, ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int Immunopharmacol. 2018;59:243–251. doi:10.1016/j.intimp.2018.04.018
34. El-Ashmawy NE, Khedr NF, El-Bahrawy HA, El-Adawy SA. Downregulation of iNOS and elevation of cAMP mediate the anti-inflammatory effect of glabridin in rats with ulcerative colitis. Inflammopharmacology. 2018;26(2):551–559. doi:10.1007/s10787-017-0373-9
35. Kwon HS, Oh SM, Kim JK. Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2008;151(1):165–173. doi:10.1111/j.1365-2249.2007.03539.x
36. Kim JY, Kang JS, Kim HM, et al. Inhibition of bone marrow-derived dendritic cell maturation by glabridin. Int Immunopharmacol. 2010;10(10):1185–1193. doi:10.1016/j.intimp.2010.06.025
37. Carmeli E, Fogelman Y. Antioxidant effect of polyphenolic glabridin on LDL oxidation. Toxicol Ind Health. 2009;25(4–5):321–324. doi:10.1177/0748233709103034
38. Kang MR, Park KH, Oh SJ, et al. Cardiovascular protective effect of glabridin: implications in LDL oxidation and inflammation. Int Immunopharmacol. 2015;29(2):914–918. doi:10.1016/j.intimp.2015.10.020
39. Rosenblat M, Belinky P, Vaya J, et al. Macrophage enrichment with the isoflavan glabridin inhibits NADPH oxidase-induced cell-mediated oxidation of low density lipoprotein. A possible role for protein kinase C. J Biol Chem. 1999;274(20):13790–13799. doi:10.1074/jbc.274.20.13790
40. Rosenblat M, Coleman R, Aviram M. Increased macrophage glutathione content reduces cell-mediated oxidation of LDL and atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2002;163(1):17–28. doi:10.1016/s0021-9150(01)00744-4
41. Yehuda I, Madar Z, Szuchman-Sapir A, Tamir S. Glabridin, a phytoestrogen from licorice root, up-regulates manganese superoxide dismutase, catalase and paraoxonase 2 under glucose stress. Phytother Res. 2011;25(5):659–667. doi:10.1002/ptr.3318
42. Yehuda I, Madar Z, Leikin-Frenkel A, et al. Glabridin, an isoflavan from licorice root, upregulates paraoxonase 2 expression under hyperglycemia and protects it from oxidation. Mol Nutr Food Res. 2016;60(2):287–299. doi:10.1002/mnfr.201500441
43. Atrahimovich D, Vaya J, Tavori H, Khatib S. Glabridin protects paraoxonase 1 from linoleic acid hydroperoxide inhibition via specific interaction: a fluorescence-quenching study. J Agric Food Chem. 2012;60(14):3679–3685. doi:10.1021/jf2046009
44. Yu XQ, Xue CC, Zhou ZW, et al. In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice). Life Sci. 2008;82(1–2):68–78. doi:10.1016/j.lfs.2007.10.019
45. Veratti E, Rossi T, Giudice S, et al. 18beta-glycyrrhetinic acid and glabridin prevent oxidative DNA fragmentation in UVB-irradiated human keratinocyte cultures. Anticancer Res. 2011;31(6):2209–2215.
46. Chen J, Yu X, Huang Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochimica acta Part A. 2016;168:111–117. doi:10.1016/j.saa.2016.06.008
47. Jiang F, Li Y, Mu J, et al. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: an epigenetic regulation of miR-148a/SMAd2 signaling. Mol Carcinog. 2016;55(5):929–940. doi:10.1002/mc.22333
48. Jiang F, Mu J, Wang X, et al. The repressive effect of miR-148a on TGF beta-SMADs signal pathway is involved in the glabridin-induced inhibition of the cancer stem cells-like properties in hepatocellular carcinoma cells. PLoS One. 2014;9(5):e96698. doi:10.1371/journal.pone.0096698
49. Qian J, Xia M, Liu W, et al. Glabridin resensitizes p-glycoprotein-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic agents. Eur J Pharmacol. 2019;852:231–243. doi:10.1016/j.ejphar.2019.04.002
50. Mu J, Zhu D, Shen Z, et al. The repressive effect of miR-148a on Wnt/β-catenin signaling involved in Glabridin-induced anti-angiogenesis in human breast cancer cells. BMC Cancer. 2017;17(1):307. doi:10.1186/s12885-017-3298-1
51. Zhu K, Li K, Wang H, Kang L, Dang C, Zhang Y. Discovery of glabridin as potent inhibitor of epidermal growth factor receptor in SK-BR-3 cell. Pharmacology. 2019;104(3–4):113–125. doi:10.1159/000496798
52. Melissa PSW, Phelim YVC, Navaratnam V, Yoke Yin C. DNA microarray analysis of estrogen responsive genes in Ishikawa cells by glabridin. Biochem Insights. 2017;10:1178626417721676. doi:10.1177/1178626417721676
53. Su Wei Poh M, Voon Chen Yong P, Viseswaran N, Chia YY. Estrogenicity of glabridin in Ishikawa cells. PLoS One. 2015;10(3):e0121382. doi:10.1371/journal.pone.0121382
54. Jen SH, Wei MP, Yin AC. The combinatory effects of glabridin and tamoxifen on Ishikawa and MCF-7 cell lines. Nat Prod Commun. 2015;10(9):1573–1576.
55. Chen CT, Chen YT, Hsieh YH, et al. Glabridin induces apoptosis and cell cycle arrest in oral cancer cells through the JNK1/2 signaling pathway. Environ Toxicol. 2018;33(6):679–685. doi:10.1002/tox.22555
56. Zhang L, Chen H, Wang M, et al. Effects of glabridin combined with 5-fluorouracil on the proliferation and apoptosis of gastric cancer cells. Oncol Lett. 2018;15(5):7037–7045. doi:10.3892/ol.2018.8260
57. Tsai YM, Yang CJ, Hsu YL, et al. Glabridin inhibits migration, invasion, and angiogenesis of human non-small cell lung cancer A549 cells by inhibiting the FAK/rho signaling pathway. Integr Cancer Ther. 2011;10(4):341–349. doi:10.1177/1534735410384860
58. Hsu YL, Wu LY, Hou MF, et al. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol Nutr Food Res. 2011;55(2):318–327. doi:10.1002/mnfr.201000148
59. Wang Z, Luo S, Wan Z, et al. Glabridin arrests cell cycle and inhibits proliferation of hepatocellular carcinoma by suppressing braf/MEK signaling pathway. Tumour Biol. 2016;37(5):5837–5846. doi:10.1007/s13277-015-4177-5
60. Hsieh MJ, Chen MK, Chen CJ, et al. Glabridin induces apoptosis and autophagy through JNK1/2 pathway in human hepatoma cells. Phytomedicine. 2016;23(4):359–366. doi:10.1016/j.phymed.2016.01.005
61. Hsieh MJ, Lin CW, Yang SF, Chen MK, Chiou HL. Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-κB and AP-1 activity in human liver cancer cells. Br J Pharmacol. 2014;171(12):3037–3050. doi:10.1111/bph.12626
62. Biswas P, Dey D, Rahman A, et al. Analysis of SYK gene as a prognostic biomarker and suggested potential bioactive phytochemicals as an alternative therapeutic option for colorectal cancer: an in-silico pharmaco-informatics investigation. J Personal Med. 2021;11(9):888. doi:10.3390/jpm11090888
63. Jie Z, Xie Z, Zhao X, et al. Glabridin inhibits osteosarcoma migration and invasion via blocking the p38- and JNK-mediated CREB-AP1 complexes formation. J Cell Physiol. 2019;234(4):4167–4178. doi:10.1002/jcp.27171
64. Huang HL, Hsieh MJ, Chien MH, Chen HY, Yang SF, Hsiao PC. Glabridin mediate caspases activation and induces apoptosis through JNK1/2 and p38 MAPK pathway in human promyelocytic leukemia cells. PLoS One. 2014;9(6):e98943. doi:10.1371/journal.pone.0098943
65. Jamali N, Soureshjani EH, Mobini GR, Samare-Najaf M, Clark CCT, Saffari-Chaleshtori J. Medicinal plant compounds as promising inhibitors of coronavirus (COVID-19) main protease: an in silico study. J Biomol Struct Dyn. 2021;1–12. doi:10.1080/07391102.2021.1906749
66. Srivastava V, Yadav A, Sarkar P. Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS-CoV2. Mater Today Proc. 2022;49:2999–3007. doi:10.1016/j.matpr.2020.10.055
67. Rahman MM, Biswas S, Islam KJ, et al. Antiviral phytochemicals as potent inhibitors against NS3 protease of dengue virus. Comput Biol Med. 2021;134:104492. doi:10.1016/j.compbiomed.2021.104492
68. Miyakawa K, Matsunaga S, Yamaoka Y, et al. Development of a cell-based assay to identify hepatitis B virus entry inhibitors targeting the sodium taurocholate cotransporting polypeptide. Oncotarget. 2018;9(34):23681–23694. doi:10.18632/oncotarget.25348
69. Singh V, Pal A, Darokar MP. A polyphenolic flavonoid glabridin: oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic Biol Med. 2015;87:48–57. doi:10.1016/j.freeradbiomed.2015.06.016
70. Gangwar B, Kumar S, Darokar MP. Glabridin averts biofilms formation in methicillin-resistant Staphylococcus aureus by modulation of the surfaceome. Front Microbiol. 2020;11:1779. doi:10.3389/fmicb.2020.01779
71. Marcoux E, Lagha AB, Gauthier P, Grenier D. Antimicrobial activities of natural plant compounds against endodontic pathogens and biocompatibility with human gingival fibroblasts. Arch Oral Biol. 2020;116:104734. doi:10.1016/j.archoralbio.2020.104734
72. Grenier D, Marcoux E, Azelmat J, Ben Lagha A, Gauthier P. Biocompatible combinations of nisin and licorice polyphenols exert synergistic bactericidal effects against Enterococcus faecalis and inhibit NF-κB activation in monocytes. AMB Express. 2020;10(1):120. doi:10.1186/s13568-020-01056-w
73. Gupta VK, Fatima A, Faridi U, et al. Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol. 2008;116(2):377–380. doi:10.1016/j.jep.2007.11.037
74. Vaillancourt K, LeBel G, Pellerin G, Ben Lagha A, Grenier D. Effects of the licorice isoflavans licoricidin and glabridin on the growth, adherence properties, and acid production of streptococcus mutans, and assessment of their biocompatibility. Antibiotics. 2021;10(2):163. doi:10.3390/antibiotics10020163
75. Fatima A, Gupta VK, Luqman S, et al. Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytother Res. 2009;23(8):1190–1193. doi:10.1002/ptr.2726
76. Messier C, Grenier D. Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses. 2011;54(6):e801–6. doi:10.1111/j.1439-0507.2011.02028.x
77. Nabili M, Moazeni M, Hedayati MT, et al. Glabridin induces overexpression of two major apoptotic genes, MCA1 and NUC1, in Candida albicans. J Glob Antimicrob Resist. 2017;11:52–56. doi:10.1016/j.jgar.2017.08.006
78. Moazeni M, Hedayati MT, Nabili M. Glabridin triggers over-expression of apoptosis inducing factor (AIF) gene in Candida albicans. Curr Med Mycol. 2018;4(3):19–22. doi:10.18502/cmm.4.3.172
79. Liu W, Li LP, Zhang JD, et al. Synergistic antifungal effect of glabridin and fluconazole. PLoS One. 2014;9(7):e103442. doi:10.1371/journal.pone.0103442
80. Moazeni M, Hedayati MT, Nabili M, Mousavi SJ, Abdollahi Gohar A, Gholami S. Glabridin triggers over-expression of MCA1 and NUC1 genes in Candida glabrata: is it an apoptosis inducer? J Mycol Med. 2017;27(3):369–375. doi:10.1016/j.mycmed.2017.05.002
81. Nabili M, Aslani N, Shokohi T, Hedayati MT, Hassanmoghadam F, Moazeni M. In vitro interaction between glabridin and voriconazole against Aspergillus fumigatus isolates. Rev Iberoam Micol. 2021;38(3):145–147. doi:10.1016/j.riam.2020.12.005
82. Gao H, Peng X, Zhan L, et al. The role of Glabridin in antifungal and anti-inflammation effects in Aspergillus fumigatus keratitis. Exp Eye Res. 2022;214:108883. doi:10.1016/j.exer.2021.108883
83. Li A, Zhao Z, Zhang S, Zhang Z, Shi Y. Fungicidal activity and mechanism of action of glabridin from Glycyrrhiza glabra L. Int J Mol Sci. 2021;22(20):10966. doi:10.3390/ijms222010966
84. Yang C, Xie L, Ma Y, et al. Study on the fungicidal mechanism of glabridin against Fusarium graminearum. Pestic Biochem Physiol. 2021;179:104963. doi:10.1016/j.pestbp.2021.104963
85. Cheema HS, Prakash O, Pal A, Khan F, Bawankule DU, Darokar MP. Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum. Parasitol Int. 2014;63(2):349–358. doi:10.1016/j.parint.2013.12.005
86. Thabet A, Alzuheir I, Alnassan AA, Daugschies A, Bangoura B. In vitro activity of selected natural products against Eimeria tenella sporozoites using reproduction inhibition assay. Parasitol Res. 2022;121(1):335–344. doi:10.1007/s00436-021-07360-z
87. Kim HS, Suh KS, Sul D, Kim BJ, Lee SK, Jung WW. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int J Mol Med. 2012;29(2):169–177. doi:10.3892/ijmm.2011.822
88. Yoshioka Y, Kubota Y, Samukawa Y, Yamashita Y, Ashida H. Glabridin inhibits dexamethasone-induced muscle atrophy. Arch Biochem Biophys. 2019;664:157–166. doi:10.1016/j.abb.2019.02.006
89. Sawada K, Yamashita Y, Zhang T, Nakagawa K, Ashida H. Glabridin induces glucose uptake via the AMP-activated protein kinase pathway in muscle cells. Mol Cell Endocrinol. 2014;393(1–2):99–108. doi:10.1016/j.mce.2014.06.009
90. Choi EM, Suh KS, Kim YJ, Hong SM, Park SY, Chon S. Glabridin alleviates the toxic effects of methylglyoxal on osteoblastic MC3T3-E1 cells by increasing expression of the glyoxalase system and Nrf2/HO-1 signaling and protecting mitochondrial function. J Agric Food Chem. 2016;64(1):226–235. doi:10.1021/acs.jafc.5b05157
91. Kim HS, Suh KS, Ko A, et al. The flavonoid glabridin attenuates 2-deoxy-D-ribose-induced oxidative damage and cellular dysfunction in MC3T3-E1 osteoblastic cells. Int J Mol Med. 2013;31(1):243–251. doi:10.3892/ijmm.2012.1172
92. Choi EM. Glabridin protects osteoblastic MC3T3-E1 cells against antimycin A induced cytotoxicity. Chem Biol Interact. 2011;193(1):71–78. doi:10.1016/j.cbi.2011.05.007
93. Somjen D, Katzburg S, Vaya J, et al. Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues. J Steroid Biochem Mol Biol. 2004;91(4–5):241–246. doi:10.1016/j.jsbmb.2004.04.008
94. Simons R, Vincken JP, Mol LA, et al. Agonistic and antagonistic estrogens in licorice root (Glycyrrhiza glabra). Anal Bioanal Chem. 2011;401(1):305–313. doi:10.1007/s00216-011-5061-9
95. Heo JS, Lee SG, Kim HO. The flavonoid glabridin induces OCT4 to enhance osteogenetic potential in mesenchymal stem cells. Stem Cells Int. 2017;2017:6921703. doi:10.1155/2017/6921703
96. Choi EM. The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochem Pharmacol. 2005;70(3):363–368. doi:10.1016/j.bcp.2005.04.019
97. Dai J, Zhang Y, Chen D, et al. Glabridin inhibits osteoarthritis development by protecting chondrocytes against oxidative stress, apoptosis and promoting mTOR mediated autophagy. Life Sci. 2021;268:118992. doi:10.1016/j.lfs.2020.118992
98. Chanda D, Prieto-Lloret J, Singh A, et al. Glabridin-induced vasorelaxation: evidence for a role of BK(Ca) channels and cyclic GMP. Life Sci. 2016;165:26–34. doi:10.1016/j.lfs.2016.09.018
99. Güven C, Parlar A. Glabridin relaxes vascular smooth muscles by activating BK(Ca) channels and inhibiting phosphodiesterase in human saphenous vein. Curr Med Sci. 2021;41(2):381–389. doi:10.1007/s11596-021-2358-6
100. Somjen D, Knoll E, Vaya J, Stern N, Tamir S. Estrogen-like activity of licorice root constituents: glabridin and glabrene, in vascular tissues in vitro and in vivo. J Steroid Biochem Mol Biol. 2004;91(3):147–155. doi:10.1016/j.jsbmb.2004.04.003
101. Huang K, Liu Y, Tang H, et al. Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front Pharmacol. 2019;10:107. doi:10.3389/fphar.2019.00107
102. Yokoyama T, Kosaka Y, Mizuguchi M. Crystal structures of human transthyretin complexed with glabridin. J Med Chem. 2014;57(3):1090–1096. doi:10.1021/jm401832j
103. Komolkriengkrai M, Nopparat J, Vongvatcharanon U, Anupunpisit V, Khimmaktong W. Effect of glabridin on collagen deposition in liver and amelioration of hepatocyte destruction in diabetes rats. Exp Ther Med. 2019;18(2):1164–1174. doi:10.3892/etm.2019.7664
104. Rebhun JF, Glynn KM, Missler SR. Identification of glabridin as a bioactive compound in licorice (Glycyrrhiza glabra L.) extract that activates human peroxisome proliferator-activated receptor gamma (PPARγ). Fitoterapia. 2015;106:55–61. doi:10.1016/j.fitote.2015.08.004
105. Dogra A, Kour D, Bhardwaj M, et al. Glabridin plays dual action to augment the efficacy and attenuate the hepatotoxicity of methotrexate in arthritic rats. ACS Omega. 2022;7(38):34341–34351. doi:10.1021/acsomega.2c03948
106. Bhatt S, Kumar V, Dogra A, et al. Amalgamation of in-silico, in-vitro and in-vivo approach to establish glabridin as a potential CYP2E1 inhibitor. Xenobiotica. 2021;51(6):625–635. doi:10.1080/00498254.2021.1883769
107. Bhatt S, Sharma A, Dogra A, et al. Glabridin attenuates paracetamol-induced liver injury in mice via CYP2E1-mediated inhibition of oxidative stress. Drug Chem Toxicol. 2021;1–9. doi:10.1080/01480545.2021.1945004
108. Li G, Simmler C, Chen L, et al. Cytochrome P450 inhibition by three licorice species and fourteen licorice constituents. Eur J Pharma Sci. 2017;109:182–190. doi:10.1016/j.ejps.2017.07.034
109. Kent UM, Aviram M, Rosenblat M, Hollenberg PF. The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6, and 2C9. Drug Metab Disposition. 2002;30(6):709–715. doi:10.1124/dmd.30.6.709
110. Cui YM, Ao MZ, Li W, Yu LJ. Effect of glabridin from Glycyrrhiza glabra on learning and memory in mice. Planta Med. 2008;74(4):377–380. doi:10.1055/s-2008-1034319
111. Hasanein P. Glabridin as a major active isoflavan from Glycyrrhiza glabra (licorice) reverses learning and memory deficits in diabetic rats. Acta Physiol Hung. 2011;98(2):221–230. doi:10.1556/APhysiol.98.2011.2.14
112. Jin Z, Kim S, Cho S, Kim IH, Han D, Jin YH. Potentiating effect of glabridin on GABAA receptor-mediated responses in dorsal raphe neurons. Planta Med. 2013;79(15):1408–1412. doi:10.1055/s-0033-1350698
113. Hoffmann KM, Beltrán L, Ziemba PM, Hatt H, Gisselmann G. Potentiating effect of glabridin from Glycyrrhiza glabra on GABA(A) receptors. Biochem Biophys Rep. 2016;6:197–202. doi:10.1016/j.bbrep.2016.04.007
114. Yu XY, Lin SG, Zhou ZW, et al. Role of P-glycoprotein in limiting the brain penetration of glabridin, an active isoflavan from the root of Glycyrrhiza glabra. Pharm Res. 2007;24(9):1668–1690. doi:10.1007/s11095-007-9297-1
115. Herbrechter R, Ziemba PM, Hoffmann KM, Hatt H, Werner M, Gisselmann G. Identification of Glycyrrhiza as the rikkunshito constituent with the highest antagonistic potential on heterologously expressed 5-HT3A receptors due to the action of flavonoids. Front Pharmacol. 2015;6:130. doi:10.3389/fphar.2015.00130
116. Seo MJ, Lee YJ, Hwang JH, Kim KJ, Lee BY. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J Nutr Biochem. 2015;26(11):1308–1316. doi:10.1016/j.jnutbio.2015.06.005
117. Mir SA, Shah MA, Ganai SA, Ahmad T, Gani M. Understanding the role of active components from plant sources in obesity management. J Saudi Soc Agric Sci. 2019;18(2):168–176. doi:10.1016/j.jssas.2017.04.003
118. Jafari F, Jafari M, Moghadam AT, et al. A review of Glycyrrhiza glabra (Licorice) effects on metabolic syndrome. Adv Exp Med Biol. 2021;1328:385–400. doi:10.1007/978-3-030-73234-9_25
119. Lee MH, Kim HM, Chung HC, Lee JH. Licorice extract suppresses adipogenesis through regulation of mitotic clonal expansion and adenosine monophosphate-activated protein kinase in 3T3-L1 cells. J Food Biochem. 2020;44(12):e13528. doi:10.1111/jfbc.13528
120. Zheng Y, Lee EH, Lee JH, et al. Preclinical research on a mixture of red ginseng and licorice extracts in the treatment and prevention of obesity. Nutrients. 2020;12(9):2744. doi:10.3390/nu12092744
121. Lee JW, Choe SS, Jang H, et al. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J Lipid Res. 2012;53(7):1277–1286. doi:10.1194/jlr.M022897
122. Choi EM, Suh KS, Jung WW, et al. Glabridin attenuates antiadipogenic activity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in murine 3T3-L1 adipocytes. J Appl Toxicol. 2018;38(11):1426–1436. doi:10.1002/jat.3664
123. Zhang Y, Xu Y, Zhang L, et al. Licorice extract ameliorates hyperglycemia through reshaping gut microbiota structure and inhibiting TLR4/NF-κB signaling pathway in type 2 diabetic mice. Food Res Int. 2022;153:110945. doi:10.1016/j.foodres.2022.110945
124. Lin HC, Paul CR, Kuo CH, et al. Glycyrrhiza uralensis root extract ameliorates high glucose-induced renal proximal tubular fibrosis by attenuating tubular epithelial-myofibroblast transdifferentiation by targeting TGF-β1/Smad/Stat3 pathway. J Food Biochem. 2022;46(5):e14041. doi:10.1111/jfbc.14041
125. Sen S, Singh R. Glycyrrhiza glabra alcoholic root extract ameliorates hyperglycemia, hyperlipidemia, and glycation-induced free iron-mediated oxidative reactions. J Food Biochem. 2021;45(12):e13970. doi:10.1111/jfbc.13970
126. Prabhahar M, K G, S P, et al. A study on Glycyrrhiza glabra-fortified bread: predicted glycemic index and bioactive component. Bioinorg Chem Appl. 2022;2022:4669723. doi:10.1155/2022/4669723
127. Tan H, Chen J, Li Y, et al. Glabridin, a bioactive component of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol Med. 2022;28(1):58. doi:10.1186/s10020-022-00481-w
128. Aung KH, Liu H, Ke Z, Jiang S, Huang J. Glabridin attenuates the retinal degeneration induced by sodium iodate in vitro and in vivo. Front Pharmacol. 2020;11:566699. doi:10.3389/fphar.2020.566699
129. Dogan MF, Parlar A, Cam SA, Tosun EM, Uysal F, Arslan SO. Glabridin attenuates airway inflammation and hyperresponsiveness in a mice model of ovalbumin-induced asthma. Pulm Pharmacol Ther. 2020;63:101936. doi:10.1016/j.pupt.2020.101936
130. Shang H, Cao S, Wang J, Zheng H, Putheti R. Glabridin from Chinese herb licorice inhibits fatigue in mice. Afri J Trad Complement Altern Med. 2009;7(1):17–23. doi:10.4314/ajtcam.v7i1.57225
131. Lee PH, Chu PM, Hsieh PL, et al. Glabridin inhibits the activation of myofibroblasts in human fibrotic buccal mucosal fibroblasts through TGF-β/smad signaling. Environ Toxicol. 2018;33(2):248–255. doi:10.1002/tox.22512
132. Fukai T, Satoh K, Nomura T, Sakagami H. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia. 2003;74(7–8):624–629. doi:10.1016/s0367-326x(03)00164-3
133. Aoki F, Nakagawa K, Kitano M, et al. Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. J Am Coll Nutr. 2007;26(3):209–218. doi:10.1080/07315724.2007.10719603
134. Nakagawa K, Kishida H, Arai N, Nishiyama T, Mae T. Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level in obese diabetic KK-A(y) mice. Biol Pharm Bull. 2004;27(11):1775–1778. doi:10.1248/bpb.27.1775
135. Aoki F, Honda S, Kishida H, et al. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci Biotechnol Biochem. 2007;71(1):206–214. doi:10.1271/bbb.60463
136. Niu W, Wu F, Cui H, et al. Network pharmacology analysis to identify phytochemicals in traditional Chinese medicines that may regulate ACE2 for the treatment of COVID-19. Evid Based Complement Altern Med. 2020;2020:7493281. doi:10.1155/2020/7493281
137. Ibrahim RS, Mahrous RSR, Abu El-Khair RM, Ross SA, Omar AA, Fathy HM. Biologically guided isolation and ADMET profile of new factor Xa inhibitors from Glycyrrhiza glabra roots using in vitro and in silico approaches. RSC Adv. 2021;11(17):9995–10001. doi:10.1039/d1ra00359c
138. Ngwe Tun MM, Toume K, Luvai E, et al. The discovery of herbal drugs and natural compounds as inhibitors of SARS-CoV-2 infection in vitro. J Nat Med. 2022;76(2):402–409. doi:10.1007/s11418-021-01596-w
139. Singh V, Pal A, Darokar MP. Glabridin synergy with norfloxacin induces ROS in multidrug resistant Staphylococcus aureus. J Gen Appl Microbiol. 2021;67(6):269–272. doi:10.2323/jgam.2021.06.002
140. Chakotiya AS, Tanwar A, Srivastava P, Narula A, Sharma RK. Effect of aquo-alcoholic extract of Glycyrrhiza glabra against Pseudomonas aeruginosa in Mice Lung Infection Model. Biomed Pharmacother. 2017;90:171–178. doi:10.1016/j.biopha.2017.03.055
141. Cantelli M, Ferrillo M, Donnarumma M, Emanuele E, Fabbrocini G. A new proprietary gel containing glabridin, andrographolide, and apolactoferrin improves the appearance of epidermal melasma in adult women: a 6-month pilot, uncontrolled open-label study. J Cosmet Dermatol. 2020;19(6):1395–1398. doi:10.1111/jocd.13161
142. Seino H, Arai Y, Nagao N, Ozawa N, Hamada K. Efficient percutaneous delivery of the antimelanogenic agent glabridin using cationic amphiphilic chitosan micelles. PLoS One. 2016;11(10):e0164061. doi:10.1371/journal.pone.0164061
143. Liu C, Hu J, Sui H, Zhao Q, Zhang X, Wang W. Enhanced skin permeation of glabridin using eutectic mixture-based nanoemulsion. Drug Deliv Transl Res. 2017;7(2):325–332. doi:10.1007/s13346-017-0359-6
144. Asha MK, Debraj D, Prashanth D, et al. In vitro anti-Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J Ethnopharmacol. 2013;145(2):581–586. doi:10.1016/j.jep.2012.11.033
145. Tsukatani T, Kuroda R, Kawaguchi T. Screening biofilm eradication activity of ethanol extracts from foodstuffs: potent biofilm eradication activity of glabridin, a major flavonoid from licorice (Glycyrrhiza glabra), alone and in combination with ɛ-poly-L-lysine. World J Microbiol Biotechnol. 2022;38(2):24. doi:10.1007/s11274-021-03206-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.