Back to Journals » International Journal of Women's Health » Volume 15
Review of Current Insights and Therapeutic Approaches for the Treatment of Refractory Postpartum Hemorrhage
Authors Liu LY, Nathan L, Sheen JJ, Goffman D
Received 4 October 2022
Accepted for publication 3 February 2023
Published 1 June 2023 Volume 2023:15 Pages 905—926
DOI https://doi.org/10.2147/IJWH.S366675
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Lilly Y Liu, Lisa Nathan, Jean-Ju Sheen, Dena Goffman
Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Columbia University Irving Medical Center, New York, NY, USA
Correspondence: Lilly Y Liu, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Columbia University Irving Medical Center, 622 West 168th Street, New York, NY, 10032, USA, Tel +1 919-389-2193, Email [email protected]
Abstract: Refractory postpartum hemorrhage (PPH) affects 10– 20% of patients with PPH when they do not respond adequately to first-line treatments. These patients require second-line interventions, including three or more uterotonics, additional medications, transfusions, non-surgical treatments, and/or surgical intervention. Multiple studies have suggested that patients with refractory PPH have different clinical characteristics and causes of PPH when compared to patients who respond to first-line agents. This review highlights current insights into therapeutic approaches for the management of refractory PPH. Early management of refractory PPH relies on both hypovolemic resuscitation and achievement of hemostasis, with an emphasis on early blood product replacement and massive transfusion protocols. Transfusion needs can be more rapidly and accurately identified through point-of-care tests such as thromboelastography. Medical therapies for the treatment of refractory PPH involve treatment of both uterine atony as well as the underlying coagulopathy, with the use of tranexamic acid and adjunct therapies such as factor replacement. The principles guiding the management of refractory PPH include restoring normal uterine and pelvic anatomy, through the evaluation and management of retained products of conception, uterine inversion, and obstetric lacerations. Intrauterine vacuum-induced hemorrhage control devices are novel methods for the treatment of refractory PPH secondary to uterine atony, in addition to other uterine-sparing surgical procedures that are under investigation. Resuscitative endovascular balloon occlusion of the aorta can be considered for cases of critical refractory PPH, to prevent or decrease ongoing blood loss while definitive surgical interventions are performed. Finally, for patients with critical hemorrhage resulting in hemorrhagic shock, damage control resuscitation (a staged surgical approach focused on restoring normal physiologic recovery and maximizing tissue oxygenation prior to proceeding with definitive surgical management) has been shown to successfully control refractory PPH, with an overall mortality decrease for obstetric patients.
Keywords: refractory postpartum hemorrhage, maternal mortality, critical care obstetrics
Introduction
Obstetric hemorrhage remains the leading cause of maternal mortality worldwide, accounting for 27% of maternal deaths.1 Occurring after 5% of all live births,2 PPH is a leading cause of severe maternal morbidity, resulting in significant short-term and long-term consequences to maternal health.3 These consequences include the acute effects of hemorrhagic shock, such as multiorgan failure, transfusion-related morbidity, worsening of chronic anemia, and intensive care unit admission.4 Furthermore, rates of PPH have continued to rise globally over the last decade.5–7 Between 2010 and 2014 PPH rates increased by 13% in the United States, with uterine atony accounting for 79% of cases.8 In addition to the numerous patient characteristics that independently increase the risk for PPH, studies have examined mode of birth and induction of labor as potential causes for the significant increase in rates of PPH; however, changes in known risk factors over this time period have yet to fully account for this trend.9
Despite a global rise in the incidence of PPH, maternal mortality from obstetric hemorrhage continues to disproportionately affect patients of lower socioeconomic status and from low-resource countries, suggesting that a vast majority of these cases would be preventable with improved resources.1,4 With early diagnosis, appropriate resources, and skilled providers and care teams, management of refractory PPH can be optimized to prevent severe maternal morbidity and mortality.4
Definitions
Postpartum hemorrhage is defined as a cumulative blood loss of 1000mL or greater, or blood loss accompanied by signs or symptoms of hypovolemia within 24 hours of birth, regardless of the mode of birth. This definition was introduced by the American College of Obstetricians and Gynecologists’ reVITALize program in 2014 in order to standardize obstetric definitions for PPH.10
The majority of patients respond appropriately to first-line treatments for PPH, which include uterotonics, uterine massage, and tranexamic acid. However, 10–20% of these patients ultimately do not respond adequately to these initial interventions. This subgroup of refractory PPH cases comprises the majority of PPH-related morbidity and mortality.11 Refractory PPH is generally defined as hemorrhage requiring second-line interventions, including three or more uterotonics, bimanual uterine compression, uterine balloon tamponade/vacuum-induced hemorrhage control devices, or surgical treatment such as cervical or high vaginal laceration repair, uterine cavity exploration, surgical devascularization procedures, uterine compression sutures, or hysterectomy.11
Causes of Refractory PPH
Patients with refractory PPH appear to have different clinical characteristics and risk factors for PPH in comparison to those responsive to first line agents for PPH.11 Common risk factors for PPH include advanced age, multiple gestation, polyhydramnios, uterine fibroids, coagulation disorders, antepartum hemorrhage, chorioamnionitis, operative delivery, history of PPH, abnormal placentation and prolonged labor course, among others. However, with refractory PPH, Widmer et al found that patients with labor induction or augmentation, with episiotomies or perineal lacerations requiring suturing, and with neonatal birthweights ≥ 3500g were at significantly higher risk for refractory PPH in comparison to patients with PPH responsive to first-line agents.11
Patients with refractory PPH also appear to have different underlying risk factors and etiologies for PPH than those with responsive PPH. According to a secondary analysis of the WHO CHAMPION trial, uterine atony only accounted for 31.5% of refractory PPH cases as the sole cause, while accounting for 53.2% of PPH cases responsive to first-line agents.11 Other studies have similarly demonstrated that uterine atony accounts for up to 80% of all cases of PPH,12 but only approximately 33–50% of cases of refractory PPH.13 Widmer et al demonstrated that the remainder of refractory PPH cases in their study were attributed to obstetric lacerations in 28%, compared to 12.8% of PPH-responsive cases, as well as abnormal placentation in 11% of refractory PPH cases, compared to 5.6% of PPH-responsive cases.11 Mousa et al similarly reported trauma as the second most common cause of refractory PPH after uterine atony, in up to 50% of cases.13 Positive correlations between the severity of hemorrhage and obstetric trauma as the leading cause of refractory PPH have been described in the literature.14,15 Thus, management of refractory PPH aims to address both uterine atony as well as obstetric trauma and associated coagulopathy.
Risk Assessment and Early Identification
Early detection and management of refractory PPH relies upon identification of antenatal risk factors for PPH, with ongoing clinical risk assessments during labor, birth, and postpartum. Multiple risk assessment tools and protocols, such as the Safe Motherhood Initiative Risk Assessment Checklist, the California Maternal Quality Care Collaborative Obstetric Hemorrhage Risk Assessment Guide, or the Association of Women’s Health, Obstetric and Neonatal Nurses PPH Risk Assessment Table can be used at the time of labor unit admission to identify patients at higher risk for developing severe PPH, based on obstetric history and antepartum risk factors.16,17,116
Patients deemed to be at higher risk for PPH based on antenatal risk factors can be identified prenatally, triaged to an appropriate level of care for labor and birth, and preparations made with cross-matched blood prepared and hemorrhage kits available accordingly. Those with high hemorrhage risk due to suspected morbidly adherent placenta can be scheduled for surgery ahead of time in a facility with appropriate resources, and with consulting teams and blood bank preparations available for immediate assistance.18
Quantification of Blood Loss
Early identification of PPH relies upon accurate quantification of ongoing blood loss as well as frequent assessment of patient risk factors, vital signs, and clinical signs and symptoms of hemorrhage. Quantitative blood loss (QBL) has been shown to have multiple advantages over estimated blood loss (EBL) in providing a more accurate assessment of ongoing blood loss during obstetric hemorrhage. In addition to improved accuracy of calculated blood loss, QBL decreases the likelihood of underestimating blood loss by visual inspection alone, potentially leading to earlier diagnosis and interventions for PPH.19–21 The California Maternal Quality Care Collaborative has emphasized the need for cumulative QBL through an obstetric hemorrhage toolkit with the use of under-buttock drapes in vaginal births and scales for real-time weight measurements of blood-soaked materials.22 However, other studies have identified limitations in the utility of QBL for decreasing PPH-related risk. These studies have suggested that QBL measurements have not prevented worsening PPH, nor have they been shown to improve maternal outcomes associated with PPH.23,24 To date, QBL has not been shown to accurately correlate with or predict postpartum hemoglobin measurements.25 Despite the lack of definitive data supporting improved outcomes, our professional organizations have moved towards recommendations for cumulative QBL as a tool to support a shared mental model and communication between multidisciplinary teams on the labor unit.
Recently, new mobile monitoring techniques such as the Triton system have been utilized for more accurate measurements of ongoing blood loss. The Triton system specifically uses a platform to measure hemoglobin blood loss by capturing images of blood-soaked surgical sponges and transferring them to a remote server, where Feature Extraction Technology then provides a precise hemoglobin measurement.26,27 However, the clinical utility of this technology is still being studied for larger subsets of patients to determine long term efficacy in the obstetric population.
Clinical Signs for Early Detection of PPH
Management of PPH also requires ongoing evaluation of clinical signs and symptoms of acute blood loss anemia. As vital sign derangements are often a late manifestation of significant blood volume loss and hypovolemic shock, they should not be relied upon for early recognition of PPH. However, once apparent, initial vital sign derangements may include a heart rate of over 100 beats per minute (bpm), decreased pulse pressure, and/or a respiratory rate of 20–30 breaths/minute, correlating with a 15% loss in total blood volume. Ongoing blood loss can result in hypotension, profound tachycardia over 120bpm, or a respiratory rate of 30–40 breaths/minute, correlating with a 30–40% loss in total blood volume, according to the Advanced Trauma Life Support (ATLS) criteria.28 The ATLS classification of hypovolemic shock is further detailed in Table 1.
 |
Table 1 Advanced Trauma Life Support (ATLS) Classification of Hypovolemic Shock28 |
Because the association between vital sign cutoffs and acute hemorrhage are more variable in trauma and obstetric patients, initial vital sign derangements are not always predictive of the severity of ongoing blood loss.29,30 The Maternal Early Warning Criteria (MEWC) establishes an obstetric-specific list of abnormal parameters that indicate the need for urgent bedside evaluation for the early identification and recognition of obstetric emergencies such as hemorrhage. These criteria include changes in systolic or diastolic blood pressure, heart rate, respiratory rate, oxygen saturation, urine output, and maternal confusion or unresponsiveness.31 The shock index (SI), calculated as maternal heart rate divided by systolic blood pressure, has also been proposed as a predictor of hemodynamic changes in the setting of acute blood loss even with normotensive blood pressures. Elevated SI values over 0.9 have been associated with an increased risk of massive transfusion, ICU admission, and adverse PPH-related outcomes.30,32,33
Importantly, as defined by the SMI Obstetric Hemorrhage Checklist, abnormal vital signs, lab values, and oliguria are not present until stage 3 PPH, which is otherwise characterized by continued bleeding greater than 1500cc, transfusion of 2 or more units of PRBCs, or continued suspicion for occult bleeding or coagulopathy.16 This highlights that classic signs of hypovolemia, such as hypotension and tachycardia, may not be apparent in a healthy obstetric patient until approximately 25% of total blood volume has been lost.30 Thus, prompt management and treatment of clinically suspected PPH should not be delayed in the setting of normal vital signs.34
Protocols and Bundles for Early Intervention
In order to respond efficiently and effectively to severe PPH, the use of obstetric hemorrhage bundles has been proposed to improve patient outcomes. Systematic PPH protocols have resulted in earlier resolution of bleeding, decreased transfusion rates, and decreased need for invasive procedures including uterine artery embolization and cesarean hysterectomy.35,36 The Council on Patient Safety in Women’s Health Care and the SMI in 2015 developed obstetric bundles in order to target areas of improvement for key causes of maternal morbidity and mortality.12,37 These obstetric hemorrhage bundles highlighted the importance of system and unit readiness, including immediate access to hemorrhage medications and supplies, established massive transfusion and blood bank protocols, unit education, and the establishment of a hemorrhage response team including consult teams such as gynecologic oncology surgery and interventional radiology as needed.12 Familiarity with the stages of PPH and the appropriate management steps and actions according to the Safe Motherhood Initiative (SMI) are paramount for the early identification and treatment of severe PPH (Figure 1).16
Figure 1 Continued. Figure 1 SMI hemorrhage bundle for the classification of the 4 stages of PPH with corresponding management recommendations, including initial steps in management, medications, blood bank utilization, and action items. Reprinted with permission from American College of Obstetricians and Gynecologists. Obstetric Hemorrhage Checklist. Accessed March 23, 2023. https://www.acog.org/-/media/project/acog/acogorg/files/forms/districts/smi-ob-hemorrhage-bundle-hemorrhage-checklist.pdf.117

Initial interventions for suspected refractory PPH center around early recognition and patient stabilization. Intravenous access should be confirmed with two large bore IVs (typically 18 gauge), and stat blood samples should be sent for type and crossmatch, complete blood count, and coagulation profile, including fibrinogen. Fluid resuscitation should be initiated with crystalloid or colloid solution, and a Foley catheter should be considered to empty the bladder and to facilitate accurate assessment of hourly intake and output. Optimal timing of resuscitation should be immediate to prevent the onset of hemorrhagic shock, metabolic acidosis, and coagulopathy.
Volume Replacement and Massive Transfusion
Volume Replacement
Fluid resuscitation for large volume blood loss secondary to PPH has traditionally focused on aggressive volume replacement, based on the principle that 1 liter of blood loss requires 4–5 liters of fluid replacement.38 The goal of aggressive fluid resuscitation is to restore circulating blood volume and normalize blood pressure rapidly with a large volume infusion of crystalloid fluid.39 However, recent studies have proposed a hypotensive fluid resuscitation approach, otherwise known as permissive hypotension, during the early stages of hemorrhagic shock.40,41 Hypotensive reanimation focuses on early, aggressive blood product placement rather than large-volume crystalloid replacement. Restrictive IV fluid resuscitation consists of small 500mL boluses of fluid to decrease the risk of dilutional coagulopathy, the disintegration of pre-formed blood clots through high intravascular hydrostatic pressures, and hypothermia.42,43 Small-volume boluses also decrease the risk of third spacing and fluid extravasation, which can result in worsening hemodynamics, cardiac dysfunction, and decreased renal perfusion through increased intra-abdominal pressure.43 Henriquez et al demonstrated that increased IV fluid administration was associated with decreased fibrinogen and hemoglobin; patients who received more than 4L of fluid for acute blood loss experienced increased subsequent bleeding and adverse maternal outcomes.44 Thus, permissive hypotension is recommended for patients with a goal MAP between 50–60mmHg or systolic blood pressure between 80–90mmHg until the source of bleeding has been controlled.45 Balanced crystalloids such as lactated ringers are preferred over saline-containing solutions due to the risk of hyperchloremic acidosis and impaired kidney function.
Massive Transfusion
Hemostatic resuscitation attempts to limit fluid resuscitation, and focuses primarily on early blood product replacement and massive transfusion protocols (MTP).42 Massive transfusion is defined as transfusion of greater than 10 units of RBCs over 24 hours, replacement of total blood volume within 24 hours, or replacement of 50% total blood volume within 3 hours.42,46 Massive transfusion protocols include an automatic release of RBCs, FFP, and platelets. This is typically released in a predefined 1:1:1 ratio of 1 unit RBCs to 1 unit FFP to 1 unit pooled (6 packs) platelets. Cryoprecipitate is another key component of MTP, as cryoprecipitate provides a more concentrated form of fibrinogen, and should be administered to patients with disseminated intravascular coagulation (DIC) or fibrinogen levels less than 200–300 mg/dL.38,71,118
The MTP can be activated by a physician or nurse, although this may differ depending on the institution, and blood products should be continuously released by the blood bank until the MTP is deactivated.47 Massive transfusion has been shown to decrease overall hemorrhage-related mortality through aggressive correction of coagulopathy.48,49 The PROMTT trial from trauma literature demonstrated that high ratios of transfusion were associated with decreased mortality within the first 6 hours of transfusion, and that early administration of plasma within the first 3 hours was associated with decreased mortality at 24 hours and 30 days.50 Meanwhile, the PROPPR trial supported that a 1:1:1 transfusion ratio was associated with adequate hemostasis and decreased mortality from exsanguination at 24 hours.47,51 Therefore, while standard initiation cutoffs for MTP vary from institution to institution, many institutions activate massive transfusion for an EBL of 1500mL with ongoing bleeding. MTP should be activated without hesitation for clinically significant and rapid bleeding, to decrease maternal morbidity and mortality.52
Markers of Successful Resuscitation
Markers of successful resuscitation include maintenance of normal mental status, mean arterial pressure (MAP) over 65mmHg, adequate urine output, and normal serum pH and bicarbonate.53 Importantly, serial measurements of serum lactate should be trended during ongoing resuscitation as a marker of tissue perfusion, as the half-life of lactic acid is 20 minutes. Persistently elevated serum lactic acid levels indicate tissue hypoperfusion and the need for continued resuscitation.54 Care should also be taken during MTP and large volume resuscitation to correct electrolyte derangements, which commonly include hyperkalemia, hypocalcemia, and hypomagnesemia from RBC overload, in order to avoid cardiac arrhythmia.55
Monitoring Transfusion Needs: Point of Care Testing
Thromboelastography (TEG), or rotational thromboelastometry (ROTEM), are valuable non-invasive diagnostic tools for the evaluation and correction of coagulopathy in ongoing hemorrhage. These rapid point-of-care tests are now increasingly utilized in obstetric ICUs and on L&D units for guiding transfusion needs in refractory PPH.
Standard coagulation assays are limited in their ability to assess clinically significant coagulopathy from ongoing blood loss, as well as certain coagulation factors such as factor XIII, platelet function, and the activity of the fibrinolytic system.56,57 While standard coagulation assays are performed on plasma, TEG is performed on whole blood and can evaluate individual cellular components, including platelet function, as well as the timing and extent of fibrinolysis. This allows for rapid and accurate assessment of coagulopathy in order to guide blood component transfusion requirements in real time for patients with ongoing hemorrhage.58–60 TEG has been shown to decrease transfusion requirements and transfusion-related mortality, as well as the incidence of postoperative ICU admission and hospital length of stay.61–66
Limitations to TEG include the lack of standardized normal reference ranges between institutions, and differences in baseline parameters for certain obstetric populations; further research is needed to ensure accurate interpretation. Limited data is available on the overall mortality benefit for obstetric patients.67 However, the advantages of TEG for the rapid evaluation and determination of transfusion needs for refractory PPH support its use for high risk obstetric populations.
Medical Therapies
Uterotonics: Limitations
Medical management of PPH has traditionally targeted uterine atony, and includes uterotonics such as oxytocin (Pitocin), methylergonovine (Methergine), 15-methylprostaglandin F2α (Hemabate), and misoprostol (Cytotec). However, this approach does not address the associated coagulopathy often seen in cases of severe PPH.
Medical therapies targeting correction of coagulopathy in refractory PPH include tranexamic acid (TXA), fibrinogen concentrates, prothrombin complex concentrates, desmopressin, and in rare, select circumstances, recombinant factor VII (Table 2).40,75 The use of agents such as recombinant FVII is currently limited due to their increased risk of thrombosis, as well as the need for further research to establish their safety profile and efficacy for the treatment of PPH. However, their potential as adjunct therapies to massive transfusion protocols in extenuating circumstances remains promising, especially for the rapid correction of coagulopathy in settings where blood bank access may be limited.
 |
Table 2 Adjunct Medical Therapies for the Treatment of Severe PPH75 |
Tranexamic Acid
Tranexamic acid (TXA) is an antifibrinolytic agent that prevents plasmin activation and inhibits the breakdown of fibrinogen and fibrin.52 As increased fibrinolysis is seen in early hemorrhage, TXA has been proposed to decrease perioperative bleeding.68 The WOMAN trial found that patients experiencing PPH who received TXA within 3 hours of birth had a 31% decrease in maternal mortality from bleeding and a 36% decrease in the likelihood of requiring a laparotomy to control bleeding, compared to patients who did not receive TXA. Administration of TXA after this 3-hour window was not found to be beneficial. Patients who received TXA did not experience an increased risk of thrombosis.69 As a result, the World Health Organization (WHO) has since recommended the concomitant administration of TXA as a first-line agent with uterotonics for the management of PPH.70 TXA is typically given intravenously as a 1g dose over 10 minutes, and an additional dose may be given for persistent bleeding after 30 minutes, or for recurrent bleeding after 24 hours, regardless of the cause of PPH. While there is not a significant thrombotic risk at the current dose used in obstetrics, TXA should still be used with caution in patients who are at higher risk for thrombosis. Due to its renal clearance, TXA is contraindicated in patients with renal dysfunction.70
Adjunct Therapy: Fibrinogen Concentrates
Early activation of fibrinolysis in hemorrhage results in low serum fibrinogen being an early predictor of severe PPH.71 Through conversion to fibrin, fibrinogen replacement or augmentation aids in clot formation, and serves as an independent predictor of mortality in major trauma patients.72 Fibrinogen replacement is typically recommended to maintain levels above 200 mg/dL, and has traditionally been replaced with cryoprecipitate. Each unit of cryoprecipitate, containing 150–350mg of fibrinogen, is expected to raise serum fibrinogen levels by 10 mg/dL.73 A standard therapeutic dose of cryoprecipitate contains approximately 3.3g of fibrinogen, with a volume of 380 mL, while a standard therapeutic dose of fibrinogen contains approximately 2.8g of fibrinogen, with a volume of 1070 mL.119 Therefore, cryoprecipitate is typically included in massive transfusion protocols as FFP is a suboptimal source of fibrinogen replacement due to the large volume that would be needed for the same effect, increasing the risk for volume overload and pulmonary edema.73 However, limitations of cryoprecipitate include the need to thaw the product before use, as well as the potential for viral transmission. Alternatively, fibrinogen concentrates use viral inactivation as part of the manufacturing process, resulting in an improved safety profile, and are ready for immediate use at room temperature. Limited studies on the use of fibrinogen concentrates in PPH have demonstrated a decreased need for blood product transfusion and subsequent volume overload, without adverse side effects. However, further research is needed on the use of this product for the management of PPH once it becomes more widely available in the United States. Usual doses start at 2–3g, with additional doses based on serum fibrinogen levels.74
Prothrombin Complex Concentrates
In addition to early fibrinolysis, severe PPH is also characterized by consumption of clotting factors within the extrinsic pathway of the clotting cascade, due to the activation of tissue factor. Prothrombin complex concentrates (PCCs) are comprised of the vitamin K-dependent clotting factors II, VII, IX, and X, as well as proteins C and S. PCCs are available in a variety of preparations. Profilnine is a 3-factor coagulant containing factors II, IX, and X, while Kcentra and FEIBA are 4-factor coagulants containing factors II, VII, IX, and X.75 While PCCs are currently only FDA approved for the reversal of warfarin-induced bleeding, there has been limited data on their off-label use for clotting factor repletion in severe hemorrhage, which is typically at a lower dose, to decrease the risk of thrombosis.76,77 This may prove to be useful in treating obstetric hemorrhage for patients with severe liver dysfunction or acquired factor deficiencies.52 At this time, further research is needed on the safety profile and optimal dosing of PCCs before they can be routinely used to treat refractory PPH.
Desmopressin
In patients with known von Willebrand disease (typically type 1) or those with underlying renal dysfunction and uremia, desmopressin acetate (DDAVP) may be used for the treatment of refractory PPH for this specific patient population when necessary. As a synthetic analogue of vasopressin, DDAVP stimulates secretion of von Willebrand factor from endothelial cells, thus improving platelet function. DDAVP is typically administered at an intravenous dose of 0.3 µg/kg for a maximum dose of 25–30 µg/kg infused over 25–30 min, in order to avoid hyponatremia and tachyphylaxis.75
Recombinant Factor VII
Recombinant-activated Factor VII (rFVIIa) is another adjunct medication approved for patients with hemophilia A or B, and for patients with congenital FVII deficiency with acute bleeding episodes. Its off-label use includes treatment of severe postpartum hemorrhage in select cases.78,79 A randomized controlled trial by Lavigne-Lissalde et al showed that early administration of rFVIIa in patients with severe refractory PPH decreased the need for second-line therapies such as interventional hemostatic procedures and transfusions, with non-fatal venous thromboembolic events occurring in 1 of every 20 patients.80 While some studies have demonstrated that rFVIIa may decrease the need for overall blood product transfusion in acute hemorrhage, a significant survival benefit has not been shown.75 Due to its high associated thrombotic risks and questionable safety profile, rFVIIa is not currently recommended for routine use in the management of PPH, and should only be considered in extenuating circumstances at lower doses in order to decrease the risk of thrombosis.81 Fibrinogen levels must be high enough in patients who are considered for rVIIa therapy and restricted to refractory PPH cases where therapeutic hysterectomy is necessary unless ongoing bleeding can be stopped. Many obstetric units have since removed rVIIa from their massive transfusion protocols in favor of agents with more favorable safety profiles, such as TXA.75
Management of Refractory PPH
In 2012 and 2017, the WHO published recommendations for the first-line treatment of PPH, which are divided into care bundles for initial response to PPH and response to refractory PPH (Table 3).82,85 The early response PPH bundle includes the administration of IV crystalloids, uterotonics, tranexamic acid, and uterine massage.82 For the management of PPH refractory to first line treatment, the administration of additional uterotonics, a second dose of tranexamic acid, compressive measures such as the use of intrauterine balloon tamponade, aortic compression or bimanual uterine compression, and the use of non-pneumatic antishock garments (NASG), if available, are recommended to minimize ongoing blood loss prior to or while preparing to proceed to invasive surgical procedures.82
 |
Table 3 WHO PPH Care Bundles85 |
Determining Appropriate Management
When medical management of PPH has failed, further evaluation for the underlying cause of hemorrhage must be undertaken while simultaneously continuing fluid resuscitation and blood product replacement. General principles for the management of refractory PPH include restoring normal uterine and pelvic anatomy, decreasing uterine blood flow, and replicating the vascular compression that results from uterine involution.83 Restoring normal uterine and pelvic anatomy involves evaluation for and management of retained products of conception and uterine inversion, in addition to repair of obstetric lacerations. Decreasing uterine blood flow involves surgical ligation or embolization of vessels such as the uterine artery and collaterals. Vascular compression can be accomplished with uterine compression sutures such as B-lynch sutures or with mechanical tamponade such as Bakri balloons.83 An analysis by Widmer et al found that 65% of patients with severe refractory PPH required second-line management procedures, with 41% of those undergoing the most commonly used intervention of repair of cervical or high vaginal tears, followed by bimanual uterine compression, uterine balloon tamponade, and hysterectomy.11
While there are no existing data regarding the specific order in which these interventions should occur, nor the comparative effectiveness of any intervention over the other, “STASIS” is an established algorithm proposing appropriate steps for the surgical management of refractory PPH (Table 4).83 Once refractory PPH is suspected, the patient’s location should be “shifted” to the operating room for improved visualization, with vaginal packing for hemostasis during transfer if needed. A thorough pelvic exam should then be performed in the operating room to exclude the presence of retained “tissue” or lacerations (“trauma”), followed by balloon “tamponade.” The next steps involve “applying compression” through surgical compression sutures, attempting “systemic” devascularization with O’Leary, ovarian, hypogastric, quadruple, or internal iliac sutures, or consulting “interventional” radiology for uterine artery embolization if the patient is stable enough for transfer to the IR suite. Finally, the surgical team should proceed with “subtotal”/total hysterectomy if the above efforts remain futile for controlling refractory PPH.38
 |
Table 4 “STASIS” Algorithm for Management of Refractory PPH83 |
Operating Room Evaluation
The decision to mobilize the patient to the operating room for improved visualization or anesthesia administration should not be delayed in the setting of ongoing, uncontrolled bleeding. This is especially important considering that approximately 30% of refractory PPH cases are due to trauma from cervical or high vaginal lacerations that may not be identified until the patient is examined under anesthesia in the operating room.11 The appropriate ancillary support teams, including anesthesiology, gynecology specialty services, and the blood bank should be notified in preparation for moving to the operating room. A member of the team should be assigned to record the times of ongoing events and medications administered, as well as to keep track of ongoing and total blood loss throughout the case and the number and types of transfusion products. Available supplies in the OR should include a hemorrhage cart, uterotonics, uterine tamponade balloons and syringes, vaginal and abdominal retractors, packing sponges, a cesarean hysterectomy tray, an ultrasound machine, and a D&C tray, as well as a cystoscope and tower if available. Ongoing monitoring of blood loss should include quantitative drapes and a scale to weigh blood-soaked materials. Anesthesia should be tailored to the patient’s comfort at a level appropriate for anticipated OR procedures, with the avoidance of hypothermia and consideration for redosing of antibiotics based on blood loss and total operative time.83
A complete, systematic evaluation of the abdomen and pelvis for potential sources of bleeding should include a pelvic examination with adequate visualization of the cervix and the entire length of the vaginal walls, evaluation for retained products of conception with manual exploration and bedside ultrasonography, and finally laparotomy for further evaluation of pelvic and abdominal organs, for identification of potential sources of concealed hemorrhage such as broad ligament and retroperitoneal hematomas, and for surgical devascularization procedures if indicated.
Repair of Obstetric Trauma and Lacerations
Systematic pelvic examination includes examination of the perineum, vaginal vault, vaginal walls, and cervix. Deeper lacerations or lacerations that are actively bleeding should be repaired first, in order to minimize ongoing blood loss, using vaginal wall retractors as needed for optimal visualization. Absorbent packing such as gel foam may be used to decrease the risk of hematoma formation after the repair of large or deep sidewall lacerations. The cervix should be routinely examined with the aid of ring forceps, as deep cervical lacerations can cause significant bleeding. These are typically repaired in a running locked fashion with synthetic absorbable suture, taking care to ensure cervical patency at the end of the repair. Finally, the placement of vaginal packing at the end of a repair should be considered in the presence of coagulopathy, or when friable vaginal tissue contributes to small persistent areas of bleeding. This allows for correction of coagulopathy and spontaneous wound closure while providing hemostasis, rather than causing additional trauma from suture placement. Vaginal packing is especially helpful in providing tamponade to prevent enlargement of vaginal hematomas, and should be used with a Foley catheter to assist in voiding and to keep track of intake and output.83
Retained Products of Conception
Evaluation for retained products of conception is essential in every case of obstetric hemorrhage, as retained placenta can occur in approximately 2–3% of all births.84 Identification of retained products with bimanual exploration and bedside ultrasound, followed by their removal, is necessary in order for uterotonics to effectively treat uterine atony. Given the high risk for perforation in the postpartum uterus, suction D&C to remove retained products of conception should be performed under ultrasound guidance, which has the simultaneous benefit of confirming complete evacuation of the uterus. A single dose of antibiotics should be administered following manual placental extraction to decrease the risk of infection.82
Management of Uterine Inversion
Evaluation for uterine inversion should be considered whenever the fundus cannot be palpated in a PPH, as incomplete or partial uterine inversion may not be immediately recognized on initial exam. These patients may experience significant shock or hypotension out of proportion to the degree of blood loss, due to vasovagal stimulation.83
Once uterine inversion is identified, immediate attempts should be made to return the uterus to its anatomic position through steady, continued pressure on the uterine fundus using a closed fist; delayed efforts may result in more difficulty in reversing the inversion due to progressive uterine edema. This maneuver is most successful with the concomitant administration of nitroglycerin or terbutaline in order to relax the uterus. The placenta should be left in-situ during this maneuver if it has not already separated.83
In cases of persistent uterine inversion, surgical procedures via laparotomy must be performed. The less-invasive Huntington procedure is typically performed first: the round ligaments are grasped with Allis clamps and gentle traction is used to replace the fundus above the cervical os (Figure 2).120 Should this prove unsuccessful, the Haultain procedure should then be attempted by making a vertical posterior uterine incision to release the constriction ring, thus restoring the fundus to its anatomical position (Figure 3).120 Patients should be counseled for cesarean births in subsequent pregnancies following this procedure, as the uterine scar created is similar to a posterior classical hysterotomy. Following restoration of normal uterine anatomy, uterotonics should be administered given the high risk of uterine atony following correction of uterine inversion, in addition to preventing recurrence of inversion. A uterine tamponade balloon can be considered for cases of recurrent uterine inversion. In cases of uterine inversion refractory to the above interventions, hysterectomy is indicated due to the risk for severe, ongoing PPH.83
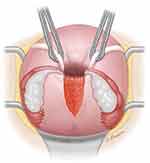 |
Figure 3 The Haultain procedure for management of uterine inversion involves making an incision in the posterior surface of the uterus to bisect the constriction ring in the myometrium, which is preventing reduction of the inversion. Reproduced from 2022 UpToDate, Inc. Puerperal Uterine Inversion. Notes: Reproduced with permission from Macones G. Puerperal uterine inversion. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on April 22, 2023.) Copyright © 2023 UpToDate, Inc. and its affiliates and/or licensors. All rights reserved.120 |
Temporizing Compressive Measures
In order to decrease uterine blood flow and compress the vasculature until appropriate care or transfer to a tertiary care facility becomes available, temporizing compressive measures have been recommended as part of some PPH guidelines to reduce blood loss for refractory PPH in low-resource settings.85 As part of the WHO recommendations for the management of refractory PPH, temporizing measures such as bimanual uterine compression, aortic compression, and non-pneumatic anti-shock garments can be considered if available to decrease uterine blood loss until the source of bleeding can be identified and treated.82
Temporizing compressive measures include bimanual uterine compression and aortic compression for refractory PPH secondary to uterine atony. Bimanual uterine compression is a two-handed procedure in which one hand is placed in the anterior vaginal fornix, while the other hand is placed behind the uterine fundus in order to compress the uterus between both hands. External aortic compression is another compressive measure used to decrease uterine blood flow by applying external compression with a closed fist at the level of the umbilicus, slightly leftward of midline.82
Non-Pneumatic Antishock Garments
The use of non-pneumatic antishock garments (NASG) has been proposed as a temporizing, first-aid compression device to decrease uterine blood loss and stabilize patients with hypovolemic shock from refractory PPH, especially in resource-limited settings.86 This consists of a lower body compression device comprised of neoprene and Velcro segments applied rapidly along the ankles, calves, thighs, pelvis, and abdomen to provide lower body circumferential counterpressure for up to 48 hours. Abdominal and pelvic compression decrease pelvic perfusion, while lower body compression and applied pressure work to raise systemic blood pressure and cardiac output to increase blood flow to the upper body.86 A review of multiple observational and international clinical trials using NASG demonstrated up to a 48% mortality benefit for patients experiencing hypovolemic shock secondary to refractory PPH, as well as a decreased need for transfer to tertiary care facilities and cesarean hysterectomies.87,88 Due to its easy application, cost effectiveness, and high safety profile, this method has been recommended by FIGO and the WHO for clinical stabilization for refractory PPH with signs of hypovolemic shock or hemodynamic instability at all levels of care, especially during transport to tertiary care facilities and to allow for more definitive surgical interventions while decreasing ongoing blood loss.85,88
Intrauterine Balloon Tamponade
Once additional sources of PPH have been excluded, such as lacerations, retained products of conception, or uterine inversion, mechanical tamponade continues to be recommended by the Safe Motherhood Initiative and in most hemorrhage protocols for the management of refractory PPH secondary to uterine atony. The most common intrauterine balloon tamponade system is the Bakri balloon, which decreases uterine blood flow through inward compression of uterine vasculature.83 Studies have suggested that the use of mechanical tamponade decreases the risk for requiring more invasive procedures,89 and successfully controls PPH secondary to atony in up to 80% of cases.90 This success rate approaches 100% if uterine balloon tamponade is placed in a timely fashion prior to the development of hemorrhagic shock.91 A recent meta-analysis by Suarez et al examined the use and efficacy of uterine balloon tamponade for the treatment of PPH across 91 studies.92 The overall pooled success rate of balloon tamponade for management of PPH was 85.9%, with the highest success rates when used for uterine atony and placenta previa. While some studies showed no difference in the risk for invasive surgical interventions or mortality, other studies showed a significant decrease in the need for uterine artery embolization and invasive procedures.93,94 Thus, these devices appear to be most effective when a quality device is used in a timely manner following the immediate recognition of refractory PPH.39 The use of uterine balloon tamponade appeared to be safe across all studies, with a low complication rate (≤ 6.5%).92
Numerous similar devices can be used if a Bakri balloon is not available, including Foley catheters, condom catheters, and Sengstaken-Blakemore tubes, among others. The balloon can be placed either manually or with the aid of a speculum and ring forceps, and concomitant ultrasound guidance can be used to confirm correct placement at the uterine fundus. It is important to ensure that the entire balloon is situated within the uterus in order to provide adequate compression of the lower uterine segment. The Bakri balloon can be inflated with up to 500 mL of saline until adequate compression is achieved, as evidenced by decreased bleeding, and then left in place between 2 to 24 hours, depending on the amount of drainage. The drainage port of the Bakri should be flushed every 2 to 4 hours to prevent clot formation, and can then be slowly deflated every few hours to ensure continued minimal drainage prior to removal.95 Vaginal packing is typically used in conjunction with the Bakri balloon to keep it in place, as well as a Foley catheter to drain the bladder and measure strict input and output.
Intrauterine Vacuum-Induced Hemorrhage Control Device (Jada System)
The Jada system is a novel intrauterine vacuum-induced hemorrhage control device that has also shown promise in refractory PPH secondary to uterine atony, prior to proceeding with surgical intervention for compression and/or devascularization procedures. In comparison to uterine balloon tamponade, which applies outward pressure on the uterine walls for the treatment of uterine atony, the Jada system applies low-level intrauterine vacuum to cause constriction of myometrial blood vessels for hemostasis.96 The device consists of a loop with vacuum pores which facilitate the creation of an intrauterine vacuum, and a vacuum connector which can connect to a wall suction. The intrauterine loop is placed within the uterine cavity through the cervix and the cervical seal filled with sterile water to limit the vacuum suction to the intrauterine cavity. The device is then connected to wall suction and blood is collected via the suction into a canister, as the uterus contracts and collapses. The vacuum is typically applied for at least an hour following control of hemorrhage to prevent bleeding recurrence (Figure 4).121
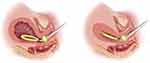 |
Figure 4 The Jada device produces a low-level vacuum that induces collapse of the atonic postpartum uterus. Contraction of the myometrium subsequently provides physiologic control of bleeding. Vacuum pressure ranges between 80 mm Hg ± 10 mm Hg. The maximum vacuum pressure is 90 mm Hg. Vacuum pressure should not be increased higher than 90 mm Hg or tissue trauma may occur. Reproduced from Jada System: US Jada Brochure 2022 https://www.thejadasystem.com/static/pdf/US_JDA_110085_Jada_Brochure2.0.pdf]. © 2021 Organon group of companies; reproduced with permission. All rights reserved.121 |
Advantages of the Jada system include real-time monitoring of effectiveness and ongoing blood loss through active collection of blood in the canister and palpable uterine tone as the uterus contracts down following vacuum activation. The efficacy of the Jada system for the treatment of PPH was evaluated through the PEARLE study, a multicentered clinical trial which demonstrated a treatment success rate of 94%.96 Definitive control of bleeding was achieved with the Jada device in this study after a median time of 3 minutes, and was recommended for treatment of PPH by 97% of users on additional analysis of device usability. There were no serious adverse events reported to be associated with the device during the study.96
An observational study using a modified intrauterine balloon connected to a vacuum device for vacuum-induced uterine tamponade showed a similar success rate between 86–100% for controlling uterine atony.97 However, many of these pilot studies were limited to patients with an EBL of 1500mL or less, with a majority of cases involving vaginal births. Thus, the generalizability of vacuum-induced hemorrhage control devices to patients with more severe PPH and cesarean births requires further investigation.
Surgical Devascularization
Should hemorrhage persist despite the above interventions, the surgical team should proceed with laparotomy in order to perform surgical devascularization and uterine compression procedures. Surgical devascularization is performed to interrupt uterine blood flow, without known adverse effects on future fertility or pregnancy outcomes.98 The O’Leary suture, otherwise known as uterine artery ligation, is performed by elevating the uterus, passing the suture 2cm medial to the uterine artery through the myometrium, and then back out through an avascular portion of the broad ligament before tying. Uterine artery ligation has been shown in some studies to successfully control bleeding in 42–88% of cases of refractory PPH, although the procedural-specific success rates largely depend upon the timing of intervention in relation to the onset of bleeding, the presence of coagulopathy, and surgeon expertise.39,99 While hypogastric artery ligation is historically described as another method of surgical devascularization for PPH, it is rarely used due to its technical difficulty and significant risk of injury to the posterior branch of the external iliac artery and to the internal iliac vein.
Uterine Compression Sutures
Uterine compression sutures are an alternative surgical technique for the management of refractory PPH secondary to uterine atony. The most common compression suture is the B-lynch, which is typically placed in a series of loops with a large absorbable suture to compress the uterine fundus over the lower uterine segment. The suture is first placed across the hysterotomy, looped over the uterine fundus, then placed across and below the posterior aspect of the hysterotomy, looped back over the fundus, and then placed under the hysterotomy before tying. While the suture is tied down, the uterus must be manually compressed by another member of the surgical team for maximal effect.100 The Hayman suture employs a similar technique, although without a hysterotomy. Alternative compression sutures include box sutures, Cho’s multiple square technique, and vertical and horizontal compression sutures, with the same goal of providing external surgical compression.38 A systematic review by Doumouchtsis et al demonstrated a 91.7% overall success rate of compression sutures in achieving hemostasis for refractory PPH.101
Management of Intraoperative Bleeding
Additional surgical interventions must be considered if bleeding occurs intraoperatively during cesarean, or results from uterine rupture. Thorough evaluation of potential sources of intraoperative bleeding includes evaluation of bleeding from the placental bed, presence of uterine extensions, development of hematomas, and bleeding from the inferior epigastric vessels within the rectus muscles. Significant hemorrhage can occur from deep uterine extensions at the time of cesarean into the lower uterine segment and bilaterally into the broad ligament and associated vasculature, often during second stage arrest cesarean births. These should be repaired by identifying the apex of the extension, and repairing from the apex towards the hysterotomy with running, locked sutures. Traumatized vessels may need to be isolated and ligated, taking care to avoid injuring other structures such as the ureters. Bleeding from areas within the placental bed can often be oversewn, as long as a morbidly adherent placenta or focal accreta has been excluded. In patients with a suspected uterine rupture in the setting of a prior hysterotomy scar, bimanual examination should be initially performed to assess the integrity of the prior scar and the presence of any myometrial defects. Once confirmed, laparotomy and repair are indicated.83
Uterine Artery Embolization
In patients who are hemodynamically stable and in settings where interventional radiology services are readily available, uterine artery embolization (UAE) with absorbable material such as Gelfoam can be considered as a fertility-sparing alternative to hysterectomy. When performed in a timely manner, UAE is an effective treatment for refractory PPH, with up to a 95% success rate in some studies.102 UAE is associated with a low complication rate of 8.7%, with low-grade fever being the most common, followed by less common complications of pelvic infection and groin hematomas.103 A recent systematic review and meta-analysis comparing the safety and effectiveness of UAE to conventional hysterectomy for the management of refractory PPH found that UAE had significantly reduced continued blood loss, shorter operative time, and decreased postoperative length of stay.104 With regards to pregnancy outcomes after UAE, prior studies have not shown an association between UAE and adverse pregnancy or fertility related outcomes.102,105 A few publications have shown higher rates of preterm birth, fetal growth restriction, and especially placenta accreta spectrum in subsequent pregnancies following UAE.106 UAE is not recommended for brisk, ongoing bleeding that would not allow time to accomplish embolization, or for the hemodynamically unstable patient, for whom definitive surgical intervention is recommended. Furthermore, UAE may not be possible following an unsuccessful attempt at uterine artery ligation, in which case hysterectomy becomes required.
Novel Uterine Sparing Surgical Procedures
Additional uterine-sparing surgical procedures have been proposed for the treatment of refractory PPH. A pilot study in China investigated the use of bilateral cervical apex clamping for refractory PPH following 13 vaginal births and 5 cesarean births.107 The procedure involved clamping the anterior and posterior walls of the cervical apex bilaterally with nontraumatic forceps. The study found a 94.4% overall success rate and a significant reduction in blood loss as well as the need for UAE, although there was no change in the incidence of hysterectomy.107
Another pilot study by Shi et al investigated the use of circular sutures at placental attachment sites for 80 cases of refractory PPH.108 The procedure involved a circular suture of the uterine serosa and myometrium at the placental attachment site, and was utilized for cases of refractory PPH regardless of the underlying etiology. Active bleeding was controlled for all patients in the study with the use of the technique, without recurrence of bleeding.108 Larger comparative studies are needed to investigate the efficacy and safety of these novel surgical methods.
Resuscitative Endovascular Balloon Occlusion of the Aorta
Recently, resuscitative endovascular balloon occlusion of the aorta (REBOA) has been used for severe refractory PPH, especially for cases of placenta accreta and morbidly adherent placenta. This method involves the insertion of an endovascular balloon into the aorta through the femoral artery for proximal hemorrhage control.109 A case series by Ordonez et al successfully utilized REBOA for 12 patients at the time of cesarean for suspected placenta accreta in order to control intraoperative bleeding. The median time of aortic occlusion in this study was 22 minutes, with no associated adverse outcomes.110 A systematic review by the same author on the use of REBOA for prevention of major PPH in at-risk patients demonstrated a decreased incidence of intraoperative hemorrhage and decreased transfusion requirements.110 Another review by Morrison et al demonstrated the successful use of REBOA for bleeding control in two case series of obstetric patients with severe refractory PPH, with no cases of maternal mortality reported.111 In cases of critical refractory PPH, REBOA may be considered either prophylactically or in emergency situations to prevent or decrease ongoing blood loss while definitive surgical interventions are performed.
Hysterectomy
Definitive surgical management for refractory PPH with hysterectomy is recommended in patients who have otherwise failed conservative medical and surgical interventions. There are no clear recommendations supporting supracervical versus total hysterectomy for cesarean hysterectomies; thus, this decision should be made on a case by case basis with a goal of minimizing blood loss and operative time. Supracervical cesarean hysterectomies may often be easier to perform in pregnancy to avoid the difficulty of identifying, dissecting, and ligating the vessels around an edematous and dilated cervix from labor. However, care should be taken to ensure the absence of placental invasion into the cervix or cervical lacerations to minimize the need for reoperation.83 In cases where bleeding is identified from the lower uterine segment, cervix, or vaginal fornices, a total hysterectomy may be more effective in controlling persistent bleeding from cervical arterial blood flow.112 Cesarean hysterectomies carry an increased risk of ureteral and bladder injury, as the ureters are typically larger and more superiorly displaced in the recently gravid uterus than in the non-gravid uterus, and cystoscopy may be considered if there is a concern for ureteral injury.
Damage Control Resuscitation
In patients with hemorrhagic shock requiring ICU-level care, damage control resuscitation (DCR) is a method utilized in trauma medicine with applicability to the high-risk obstetric population.109 Once a patient reaches a state of hemorrhagic shock, metabolic acidosis subsequently leads to organ hypoperfusion and coagulopathy, ultimately resulting in uncontrolled hemorrhage and multiorgan failure. DCR utilizes a staged surgical approach to minimize hemorrhage and operative time in order to restore normal physiologic recovery and maximize tissue oxygenation prior to proceeding with definitive surgical management.113 The goals of DCR thus focus on permissive resuscitation through massive transfusion, damage control surgery, and hemodynamic stabilization.109 Early implementation of DCR should be considered for patients with evidence of acidosis (pH < 7.1, base excess <8), EBL > 1500 mL, and hypothermia (body temperature < 34°C). Other parameters include systolic blood pressure < 70mmHg, persistent bleeding despite multiple transfusions (more than 6–10 units of RBCs), evidence of coagulopathy, hemodynamic instability requiring continuous vasopressor support, active ongoing venous bleeding, or duration of surgery over 90 minutes.109
For patients deemed eligible for damage control surgery (DCS), a four-step approach is proposed.109 First, source control should be achieved as soon as possible with an abdominal hysterectomy if this has not already been performed, in order to remove the source of hemorrhage. Operative time should be limited to under 90 minutes to optimize chance of survival, with a focus on pelvic packing to decrease operative time and control continued bleeding. Pelvic packing, for which at least 7–10 compresses are recommended, should be utilized along with temporary abdominal closure with or without a negative pressure system. Administration of prophylactic antibiotics may be considered while abdominal packing is in place.109,114
The second step of DCS involves transfer to the ICU to correct the patient’s underlying coagulopathy and metabolic derangements.115 Resuscitation parameters should be continuously monitored with a goal of reversing tissue hypoxia and acidosis. Ongoing intraabdominal bleeding should be monitored with a vacuum pack following partial abdominal closure. Drainage of more than 400 mL/h of blood in patients without underlying coagulopathy should signal the providers of the need for early definitive surgery.109
Definitive surgery is the third step of DCS that should occur once metabolic stabilization and normal physiology have been restored. This should ideally occur 48–72 hours after the initial surgery, although more than one surgical procedure may be required based on the patient’s intraoperative response, and based on intraoperative findings. Definitive closure of the abdominal cavity and wall should be performed as the final step once all surgical interventions have been completed.109
Complications of DCS are primarily related to the timing of abdominal fascial closure, and include wound infection in up to 28% of cases, intraabdominal collections in up to 20% of cases, and evisceration in up to 10% of cases.114 However, DCS has been shown to successfully control refractory PPH with an overall mortality benefit for critically ill obstetric patients. The largest study on obstetric DCS by Ordonez et al demonstrated that bleeding was controlled with the first surgical intervention in 60% of cases, with subsequent control in 98% of cases. A significant reduction in expected mortality from 40% to 7% was also seen. DCS therefore remains an invaluable and potentially life-saving technique for the management of critical, refractory PPH.114
Conclusions
Refractory PPH accounts for the majority of PPH-related morbidity and mortality, although most of these cases are preventable and treatable with appropriate access to resources and care. Utilization of obstetric hemorrhage bundles as proposed by the Safe Motherhood Initiative, the WHO, and the Alliance for Innovation on Maternal Health (AIM) is paramount to the timely and successful management of refractory PPH through system and unit readiness. Successful treatment of refractory PPH relies upon thorough and systematic evaluation for the underlying source of bleeding in order to utilize the most effective interventions, with the goal of decreasing maternal morbidity and mortality.
Funding
There is no funding to report.
Disclosure
Dena Goffman serves on the scientific advisory board and participated in PEARLE and RUBY trials for the Jada device through Alydia and Organon and serves on the Cooper Surgical Obstetrical Safety Council. She also previously received payment from Haymarket, Laborie and PRIME for postpartum hemorrhage related education. She is an Editor for UpToDate.
References
1. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e333. doi:10.1016/S2214-109X(14)70227-X
2. Vogel JP, Oladapo OT, Dowswell T, Gülmezoglu AM. Updated WHO recommendation on intravenous tranexamic acid for the treatment of post-partum haemorrhage. Lancet Global Health. 2018;6:e18–e19. doi:10.1016/S2214-109X(17)30428-X
3. Kilpatrick SK, Ecker JL. American college of obstetricians and gynecologists and the society for maternal fetal medicine. Severe maternal morbidity: screening and review. Am J Obstet Gynecol. 2016;215(3):B17–B22. doi:10.1016/j.ajog.2016.07.050
4. Nathan LM. An overview of obstetric hemorrhage. Semin Perinatol. 2019;43(1):2–4. PMID: 30691692. doi:10.1053/j.semperi.2018.11.001
5. Mehrabadi A, Hutcheon JA, Lee L, Liston RM, Joseph KS. Trends in postpartum hemorrhage from 2000 to 2009: a population-based study. BMC Pregnancy Childbirth. 2012;12:108. doi:10.1186/1471-2393-12-108
6. Mehrabadi A, Liu S, Bartholomew S, et al. Temporal trends in postpartum hemorrhage and severe postpartum hemorrhage in Canada from 2003 to 2010. J Obstet Gynaecol Can. 2014;36(1):21–33. doi:10.1016/S1701-2163(15)30680-0
7. Lutmoski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. MJOG. 2012;119:306–314.
8. Reale SC, Easter SR, Xinling X, Bateman BT, Farber MK. Trends in Postpartum Hemorrhage in the United States From 2010 to 2014. Anesth Analg. 2020;130(5):e119–e122. doi:10.1213/ANE.0000000000004424
9. Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449.e1–7. PMID: 23871950. doi:10.1016/j.ajog.2013.07.007
10. Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124(1):150–153. doi:10.1097/AOG.0000000000000322
11. Widmer M, Piaggio G, Hofmeyr GJ, et al. Maternal characteristics and causes associated with refractory postpartum haemorrhage after vaginal birth: a secondary analysis of the WHO CHAMPION trial data. BJOG. 2020;127:628–634. doi:10.1111/1471-0528.16040
12. Main EK, Goffman D, Scavone BM, et al. National partnership for maternal safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155–162. doi:10.1097/AOG.0000000000000869
13. Mousa HA, Cording V, Alfirevic Z. Risk factors and interventions associated with major primary postpartum hemorrhage unresponsive to first-line conventional therapy. Acta Obstet Gynecol Scand. 2008;87:652–661. doi:10.1080/00016340802087660
14. Dupont C, Rudigoz RC, Cortet M, et al. Frequency, causes and risk factors of postpartum haemorrhage: a population-based study in 106 French maternity units. J Gynecol Obstet Biol Reprod (Paris). 2014;43:244–253. doi:10.1016/j.jgyn.2013.05.003
15. Deneux-Tharaux C, Bonnet MP, Tort J. Epidemiology of post-partum haemorrhage. J Gynecol Obstet Biol Reprod. 2014;43:936–950. doi:10.1016/j.jgyn.2014.09.023
16. Goffman D, Ananth CV, Fleischer A, et al.; Safe Motherhood Initiative Obstetric Hemorrhage Work Group. The new york state safe motherhood initiative: early impact of obstetric hemorrhage bundle implementation. Am J Perinatol. 2019;36(13):1344–1350. PMID: 30609429. doi:10.1055/s-0038-1676976
17. Bingham D, Melsop K, Main E, Main EK. CMQCC obstetric hemorrhage hospital level implementation guide. The California Maternal Quality Care Collaborative (CMQCC) 2010. MCN. 2011;36(5):297–304. doi:10.1097/NMC.0b013e318227c75f
18. Spiegelman J, Sheen JJ, Goffman D. Readiness: utilizing bundles and simulation. Semin Perinatol. 2019;43(1):5–10. PMID: 30578146. doi:10.1053/j.semperi.2018.11.002
19. Toledo P, McCarthy RJ, Hewlett BJ, Fitzgerald PC, Wong CA. The accuracy of blood loss estimation after simulated vaginal delivery. Anesth Analg. 2007;105(6):1736–1740. doi:10.1213/01.ane.0000286233.48111.d8
20. Patel A, Goudar SS, Geller SE, et al. Drape estimation vs. visual assessment for estimating postpartum hemorrhage. Int J Gynecol Obstet. 2006;93(3):220–224. doi:10.1016/j.ijgo.2006.02.014
21. Al Kadri HM, Al Anazi BK, Tamim HM. Visual estimation versus gravimetric measurement of postpartum blood loss: a prospective cohort study. Arch Gynecol Obstet. 2011;283(6):1207–1213. doi:10.1007/s00404-010-1522-1
22. Lyndon A. Cumulative quantitative assessment of blood loss. CMQCC Obstet Hemorrhage Toolkit Vers. 2015;2:80–85.
23. Zhang WH, Deneux-Tharaux C, Brocklehurst P, Juszczak E, Joslin M, Alexander S. Effect of a collector bag for measurement of postpartum blood loss after vaginal delivery: cluster randomised trial in 13 European countries. BMJ. 2010;340:c293. doi:10.1136/bmj.c293
24. Hancock A, Weeks AD, Lavender DT. Is accurate and reliable blood loss estimation the ‘crucial step’ in early detection of postpartum haemorrhage: an integrative review of the literature. BMC Pregnancy Childbirth. 2015;15(1):230. doi:10.1186/s12884-015-0653-6
25. Hamm RF, Wang E, Romanos A, O’rourke SK, Srinivas S. SrinivasImplementation of quantification of blood loss does not improve prediction of hemoglobin drop in deliveries with average blood loss. Am J Perinatol. 2018;35(2):134–139. doi:10.1055/s-0037-1606275
26. Konig G, Holmes AA, Garcia R, et al. In vitro evaluation of a novel system for monitoring surgical hemoglobin loss. Anesth Analg. 2014;119(3):595. doi:10.1213/ANE.0000000000000198
27. Holmes AA, Konig G, Ting V, et al. Clinical evaluation of a novel system for monitoring surgical hemoglobin loss. Anesth Analg. 2014;119(3):588. doi:10.1213/ANE.0000000000000181
28. American College of Surgeons Trauma Committee. Advanced Trauma Life Support for Doctors.
29. Guly HR, Bouamra O, Little R, et al. Testing the validity of the ATLS classification of hypovolaemic shock. Resuscitation. 2010;81(9):1142–1147. doi:10.1016/j.resuscitation.2010.04.007
30. Pacagnella RC, Souza JP, Durocher J, et al. A systematic review of the relationship between blood loss and clinical signs. PLoS One. 2013;8(3):e57594. doi:10.1371/journal.pone.0057594
31. Mhyre JM, D’Oria R, Hameed AB, et al. The maternal early warning criteria: a proposal from the national partnership for maternal safety. J Obstet Gynecol Neonatal Nurs. 2014;43(6):771–779. doi:10.1111/1552-6909.12504
32. Nathan HL, El Ayadi A, Hezelgrave NL, et al. Shock index: an effective predictor of outcome in postpartum haemorrhage? BJOG Int J Obstet Gynaecol. 2015;122(2):268–275. doi:10.1111/1471-0528.13206
33. El Ayadi AM, Nathan HL, Seed PT, et al. Vital sign prediction of adverse maternal outcomes in women with hypovolemic shock: the role of shock index. PLoS One. 2016;11(2):e0148729. doi:10.1371/journal.pone.0148729
34. Andrikopoulou M, D’Alton ME. Postpartum hemorrhage: early identification challenges. Semin Perinatol. 2019;43(1):11–17. PMID: 30503400. doi:10.1053/j.semperi.2018.11.003
35. Varatharajan L, Chandraharan E, Sutton J, Lowe V, Arulkumaran S. Outcome of the management of massive postpartum hemorrhage using the algorithm “HEMOSTASIS”. Int J Gynaecol Obstet. 2011;113(2):152–154. doi:10.1016/j.ijgo.2010.11.021
36. Shields LE, Smalarz K, Reffigee L, Mugg S, Burdumy TJ, Propst M. Comprehensive maternal hemorrhage protocols improve patient safety and reduce utilization of blood products. Am J Obstet Gynecol. 2011;205(4):368. e1–8. doi:10.1016/j.ajog.2011.06.084
37. Burgansky A, Montalvo D, Siddiqui NA. The safe motherhood initiative: the development and implementation of standardized obstetric care bundles in New York. Semin Perinatol. 2016;40(2):124–131. doi:10.1053/j.semperi.2015.11.019
38. Mukherjee S, Arulkumaran S. Post-partum haemorrhage obstetrics. Obstetrics Gynaecol Reprod Med. 2009;19(5):121–126. doi:10.1016/j.ogrm.2009.01.005
39. Escobar MF, Nassar AH, Theron G, et al.; FIGO Safe Motherhood and Newborn Health Committee. FIGO recommendations on the management of postpartum hemorrhage 2022. Int J Gynaecol Obstet. 2022;157(Suppl1):3–50. PMID: 35297039; PMCID: PMC9313855. doi:10.1002/ijgo.14116
40. Pacheco LD, Saade GR, Costantine MM, Clark SL, Hankins GD. An update on the use of massive transfusion protocols in obstetrics. Am J Obstet Gynecol. 2016;214:340–344. doi:10.1016/j.ajog.2015.08.068
41. Wang H, Chen MB, Zheng XW, Zheng QH. Effectiveness and safety of hypotensive resuscitation in traumatic hemorrhagic shock: a protocol for meta‐analysis. Medicine. 2019;98:e18145. doi:10.1097/MD.0000000000018145
42. Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma. Crit Care. 2019;23:98. doi:10.1186/s13054-019-2347-3
43. Owattanapanich N, Chittawatanarat K, Benyakorn T, Sirikun J. Risks and benefits of hypotensive resuscitation in patients with traumatic hemorrhagic shock: a meta‐analysis. Scand J Trauma Resusc Emerg Med. 2018;26:107. doi:10.1186/s13049-018-0572-4
44. Henriquez DDCA, Bloemenkamp KWM, Loeff RM, et al. Fluid resuscitation during persistent postpartum haemorrhage and maternal outcome: a nationwide cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;235:49–56. doi:10.1016/j.ejogrb.2019.01.027
45. Gillissen A, van den Akker T, Caram‐Deelder C, et al. Association between fluid management and dilutional coagulopathy in severe postpartum haemorrhage: a nationwide retrospective cohort study. BMC Pregnancy Childbirth. 2018;18:398. doi:10.1186/s12884-018-2021-9
46. Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–S96. doi:10.1097/01.ta.0000199549.80731.e6
47. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs. a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi:10.1001/jama.2015.12
48. Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi:10.1097/TA.0b013e3180324124
49. Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi:10.1097/01.ta.0000250497.08101.8b
50. Del Junco DJ, Holcomb JB, Fox EE, et al. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S24–30. doi:10.1097/TA.0b013e31828fa3b9
51. Tanaka H, Matsunaga S, Yamashita T, et al. A systematic review of massive transfusion protocol in obstetrics. Taiwan J Obstet Gynecol. 2017;56:715–718. doi:10.1016/j.tjog.2017.10.001
52. Kogutt BK, Vaught AJ. Postpartum hemorrhage: blood product management and massive transfusion. Semin Perinatol. 2019;43(1):44–50. PMID: 30527516; PMCID: PMC8015778. doi:10.1053/j.semperi.2018.11.008
53. Englehart MS, Schreiber MA. Measurement of acid-base resuscitation endpoints: lactate, base deficit, bicarbonate or what? Curr Opin Crit Care. 2006;12:569–574. doi:10.1097/MCC.0b013e328010ba4f
54. Lewis CT, Naumann DN, Crombie N, Midwinter MJ. Prehospital point-of-care lactate following trauma: a systematic review. J Trauma Acute Care Surg. 2016;81:748–755. doi:10.1097/TA.0000000000001192
55. Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137:209–220. doi:10.1378/chest.09-0252
56. Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256(3):476–486. doi:10.1097/SLA.0b013e3182658180
57. Haas T, Spielmann N, Speer O, et al. Comparison of Thromboelastometry (ROTEM®) with standard plasmatic coagulation testing in paediatric surgery. Br J Anaesth. 2012;108(1):36–41. doi:10.1093/bja/aer342
58. Hunt H, Stanworth S, Curry N, et al. Thromboelastography (TEG) and Rotational Thromboelastometry (ROTEM®) for trauma induced coagulopathy in adult trauma patients with bleeding (review.). Cochrane Library. 2015;2. doi:10.1002/14651858.CD010438.pub2
59. Afshari A, Wikkelso A, Brok J, Moller AM, Wetterslev J. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM®) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Library. 2011;3. doi:10.1002/14651858.CD007871.pub2
60. Da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NKJ. Effect to Thromboelastography (TEG) and Rotational Thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance, and mortality in trauma: a descriptive systematic review. Critical Care. 2014;18:518. doi:10.1186/s13054-014-0518-9
61. Snegovskikh D, Souza D, Walton Z, et al. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J Clin Anesth. 2018;44:50–56. PMID: 29121548. doi:10.1016/j.jclinane.2017.10.003
62. Deppe AC, Weber C, Zimmermann J, et al. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J Surg Res. 2016;203(2):424–433. doi:10.1016/j.jss.2016.03.008
63. Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2017;72(4):519–531. doi:10.1111/anae.13765
64. Nakayama Y, Nakajima Y, Tanaka KA, et al. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth. 2015;114(1):91–102. doi:10.1093/bja/aeu339
65. Amgalan A, Allen T, Othman M, Ahmadzia HK. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women’s SSC of the ISTH. J Thromb Haemost. 2020;18(8):1813–1838. PMID: 32356929. doi:10.1111/jth.14882
66. McNamara H, Kenyon C, Smith R, Mallaiah S, Barclay P. Four years’ experience of a ROTEM® -guided algorithm for treatment of coagulopathy in obstetric haemorrhage. Anaesthesia. 2019;74(8):984–991. PMID: 30950521. doi:10.1111/anae.14628
67. Lee J, Wyssusek KH, Kimble RMN, et al. Baseline parameters for rotational thromboelastometry (ROTEM®) in healthy pregnant Australian women: a comparison of labouring and non-labouring women at term. Int J Obstet Anesth. 2020;41:7–13. PMID: 31831279. doi:10.1016/j.ijoa.2019.10.003
68. Ducloy-Bouthors AS, Duhamel A, Kipnis E. Postpartum haemorrhage related early increase in D-dimers is inhibited by tranexamic acid: haemostasis parameters of a randomized controlled open labelled trial. Br J Anaesth. 2016;116(5):641–648. doi:10.1093/bja/aew021
69. Shakur H, Roberts I, Fawole B, WOMAN trial collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084):2105–2116. doi:10.1016/S0140-6736(17)30638-4
70. World Health Organization. WHO Recommendation on Tranexamic Acid for the Treatment of Postpartum Haemorrhage. Geneva, Switzerland: WHO; 2017.
71. Cortet M, Deneux-Tharaux C, Dupont C. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108(6):984–989. doi:10.1093/bja/aes096
72. McQuilten ZK, Wood EM, Bailey M, Cameron PA, Cooper DJ. Fibrinogen is an independent predictor of mortality in major trauma patients: a five-year statewide cohort study. Injury. 2017;48:1074–1081. doi:10.1016/j.injury.2016.11.021
73. Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus. 2012;10:23–27. doi:10.2450/2011.0015-11
74. Matsunaga S, Takai Y, Nakamura E. The clinical efficacy of fibrinogen concentrate in massive obstetric haemorrhage with hypofibrinogenaemia. Sci Rep. 2017;7:46749. doi:10.1038/srep46749
75. Pacheco LD, Saade GR, Hankins GDV. Medical management of postpartum hemorrhage: an update. Semin Perinatol. 2019;43(1):22–26. PMID: 30503399. doi:10.1053/j.semperi.2018.11.005
76. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive Summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):7s–47s. doi:10.1378/chest.1412S3
77. Younis M, Ray-Zack M, Haddad NN. Prothrombin complex concentrate reversal of coagulopathy in emergency general surgery patients. World J Surg. 2018;42. doi:10.1007/s00268-018-4520-2
78. Murakami M, Kobayashi T, Kubo T, Hata T, Takeda S, Masuzaki H. Experience with recombinant activated factor VII for severe post-partum hemorrhage in Japan, investigated by Perinatology Committee, Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2015;41(8):1161–1168. PMID: 26013425. doi:10.1111/jog.12712
79. Phillips LE, McLintock C, Pollock W, et al.; Australian and New Zealand Haemostasis Registry. Recombinant activated factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009;109(6):1908–1915. PMID: 19923520. doi:10.1213/ANE.0b013e3181c039e6
80. Lavigne-Lissalde G, Aya AG, Mercier FJ, et al. Recombinant human FVIIa for reducing the need for invasive second-line therapies in severe refractory postpartum hemorrhage: a multicenter, randomized, open controlled trial. J Thromb Haemost. 2015;13(4):520–529. PMID: 25594352. doi:10.1111/jth.12844
81. Ghadimi K, Welsby IJ. Pro: factor concentrates are essential for hemostasis in complex cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):558–564. doi:10.1053/j.jvca.2017.05.042
82. World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization; 2012.
83. Gilmandyar D, Thornburg LL. Surgical management of postpartum hemorrhage. Semin Perinatol. 2019;43(1):27–34. PMID: 30578144. doi:10.1053/j.semperi.2018.11.006
84. Cheung WM, Hawkes A, Ibish S, et al. The retained placenta: historical and geographical rate variations. J Obstet Gynaecol. 2011;31:37–42. doi:10.3109/01443615.2010.531301
85. Althabe F, Therrien MNS, Pingray V, et al. Postpartum hemorrhage care bundles to improve adherence to guidelines: a WHO technical consultation. Int J Gynecol Obstet. 2020;148:290–299. doi:10.1002/ijgo.13028
86. Motherhood FS; Newborn Health Committee. Non‐pneumatic anti‐shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynecol Obstet. 2015;128:194–195. doi:10.1016/j.ijgo.2014.10.014
87. Mourad‐Youssif M, Ojengbede OA, Meyer CD, et al. Can the Non‐pneumatic Anti‐Shock Garment (NASG) reduce adverse maternal outcomes from postpartum hemorrhage? Evidence from Egypt and Nigeria. Reprod Health. 2010;7:24. doi:10.1186/1742-4755-7-24
88. Pileggi‐Castro C, Nogueira‐Pileggi V, Tunçalp Ö, Oladapo OT, Vogel JP, Souza JP. Non‐pneumatic anti‐shock garment for improving maternal survival following severe postpartum haemorrhage: a systematic review. Reprod Health. 2015;12:28. doi:10.1186/s12978-015-0012-0
89. Revert M, Rozenberg P, Cottenet J, Quantin C. Intrauterine balloon tamponade for severe postpartum hemorrhage. Obstet Gynecol. 2018;131(1):143–149. doi:10.1097/AOG.0000000000002405
90. Barton JR, Sibai BM. Evaluation and Management of Postpartum Hemorrhage. Management of Acute Obstetric Emergencies: Female Pelvic Surgery Video Atlas Series, 1e (Female Pelvic Video Surgery Atlas Series). Philadelphia, PA: Elsevier; 2011.
91. Burke TF, Danso-Bamfo S, Guha M, Oguttu M, Tarimo V, Nelson BD. Shock progression and survival after use of a condom uterine balloon tamponade package in women with uncontrolled postpartum hemorrhage. Int J Gynecol Obstet. 2017;139:34–38. doi:10.1002/ijgo.12251
92. Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222(4):293.e1–293.e52. PMID: 31917139. doi:10.1016/j.ajog.2019.11.1287
93. Dumont A, Bodin C, Hounkpatin B, et al. Uterine balloon tamponade as an adjunct to misoprostol for the treatment of uncontrolled postpartum haemorrhage: a randomised controlled trial in Benin and Mali. BMJ Open. 2017;7:e016590. doi:10.1136/bmjopen-2017-016590
94. Natarajan A, Alaska Pendleton A, Nelson BD, et al. Provider experiences with improvised uterine balloon tamponade for the management of uncontrolled postpartum hemorrhage in Kenya. Int J Gynecol Obstet. 2016;135:210–213. doi:10.1016/j.ijgo.2016.05.006
95. Einerson BD, Son MS, Schneider P, Fields I, Miller ES. Association between intrauterine balloon tamponade duration and postpartum hemorrhage outcomes. Am J Obstet Gynecol. 2017;216(3):300. doi:10.1016/j.ajog.2016.10.040
96. D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136(5):882–891. PMID: 32909970; PMCID: PMC7575019. doi:10.1097/AOG.0000000000004138
97. Haslinger C, Weber K, Zimmermann R. Vacuum-induced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138(3):361–365. PMID: 34352848; PMCID: PMC8366764. doi:10.1097/AOG.0000000000004510
98. Doumouchtsis SK, Nikolopoulos K, Talaulikar VS, Krishna A, Arulkumaran S. Menstrual and fertility outcomes following the surgical management of postpartum haemorrhage: a systematic review. BJOG. 2014;121(4):382. doi:10.1111/1471-0528.12546
99. Vanwinkel S, Claes L, Van den Bosch T. Obstetrical outcome after B‐Lynch sutures and ligation of uterine arteries: a case report. Case Rep Womens Health. 2021;30:e00303. doi:10.1016/j.crwh.2021.e00303
100. Smith KL, Baskett TF. Uterine compression sutures as an alternative to hysterectomy for severe postpartum hemorrhage. J Obstet Gynaecol Can. 2003;25(3):197. doi:10.1016/S1701-2163(16)30106-2
101. Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62:540–547. doi:10.1097/01.ogx.0000271137.81361.93
102. Ganguli S, Stecker MS, Pyne D, Baum RA, Fan CM. Uterine artery embolization in the treatment of postpartum uterine hemorrhage. J Vasc Interv Radiol. 2011;22(2):169–176. doi:10.1016/j.jvir.2010.09.031
103. Lee HY, Shin JH, Kim J, et al. Primary postpartum hemorrhage: outcome of pelvic arterial embolization in 251 patients at a single institution. Radiology. 2012;264:903–909. doi:10.1148/radiol.12111383
104. Liu Z, Wang Y, Yan J, et al. Uterine artery embolization versus hysterectomy in the treatment of refractory postpartum hemorrhage: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;33(4):693–705. PMID: 30354858. doi:10.1080/14767058.2018.1497599
105. Capmas P, Picone O, Musset D, Frydman R, Fernandez H. Fertilité et grossesses après prise en charge invasive des hémorragies du postpartum[Fertility and pregnancy outcome following invasive management of severe postpartum hemorrhage]. J Gynecol Obstet Biol Reprod. 2012. 41(3):298–306. French. French. doi:10.1016/j.jgyn.2012.01.001
106. Jitsumori M, Matsuzaki S, Endo M, et al. Obstetric outcomes of pregnancy after uterine artery embolization. Int J Womens Health. 2020;12:151–158. PMID: 32184677; PMCID: PMC7064279. doi:10.2147/IJWH.S236443
107. Jiang L, Wang X. A new non-invasive procedure for refractory PPH after vaginal delivery and caesarean section. J Obstet Gynaecol. 2021;41(5):791–796. PMID: 33143495. doi:10.1080/01443615.2020.1803237
108. Shi X, Wu H, Liu C, Zhu X. Circular suture of the uterine serosa and myometrium layer around placental attachment site for refractory postpartum hemorrhage. J Obstet Gynaecol Res. 2021;47(5):1735–1742. PMID: 33590569. doi:10.1111/jog.14695
109. Carvajal JA, Ramos I, Kusanovic JP, Escobar MF. Damage‐control resuscitation in obstetrics. J Matern Fetal Neonatal Med. 2022;35:785–798. doi:10.1080/14767058.2020.1730800
110. Ordoñez CA, Manzano-Nunez R, Parra MW, et al. Prophylactic use of resuscitative endovascular balloon occlusion of the aorta in women with abnormal placentation: a systematic review, meta-analysis, and case series. J Trauma Acute Care Surg. 2018;84(5):809–818. doi:10.1097/TA.0000000000001821
111. Morrison JJ, Galgon RE, Jansen JO, et al. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80(2):324–334. doi:10.1097/TA.0000000000000913
112. Gungor T, Simsek A, Ozdemir AO, Pektas M, Danisman N, Mollamahmutoglu L. Surgical treatment of intractable postpartum hemorrhage and changing trends in modern obstetric perspective. Arch Gynecol Obstet. 2009;280:351–355. doi:10.1007/s00404-008-0914-y
113. Pacheco LD, Lozada MJ, Saade GR, Hankins GDV. Damage‐control surgery for obstetric hemorrhage. Obstet Gynecol. 2018;132:423–427. doi:10.1097/AOG.0000000000002743
114. Ordóñez CA, Nieto AJ, Carvajal JA, et al. Damage control surgery for the management of major obstetric hemorrhage: experience from the Fundación Valle del Lili, Cali, Colombia. Panam J Trauma Crit Care Emerg Surg. 2017;6:1–7. doi:10.5005/jp-journals-10030-1164
115. West N, Dawes R. Trauma resuscitation and the damage control approach. Surgery. 2018;36:409–416.
116. Scheich B. Implementation and outcomes of the AWHONN postpartum hemorrhage project. J Obstet Gynecol Neonatal Nurs. 2018;47(5):684–687. PMID: 30055125. doi:10.1016/j.jogn.2018.06.003
117. Obstetric Hemorrhage Checklist.
118. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–273. doi:10.1111/j.1538-7836.2007.02297.x
119. Morrison GA, Koch J, Royds M, et al. Fibrinogen concentrate vs. fresh frozen plasma for the management of coagulopathy during thoraco-abdominal aortic aneurysm surgery: a pilot randomised controlled trial. Anaesthesia. 2019;74(2):180–189. PMID: 30467829. doi:10.1111/anae.14495
120. Macones G. Puerperal uterine inversion. UpToDate; 2022. Available from: https://www.uptodate.com/contents/puerperal-uterine-inversion.
121. The Jada System. US Jada Brochure. Organon; 2022. Available from: https://www.thejadasystem.com/static/pdf/US_JDA_110085_Jada_Brochure2.0.pdf.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.