Back to Journals » OncoTargets and Therapy » Volume 11
Retrospective study of survival in human papillomavirus-negative oropharyngeal squamous cell carcinoma treated with primary surgery and associated prognostic factors
Authors Yuan Y , Wang L, Li QX, Zhang JY , Xu ZX, Guo CB
Received 9 November 2017
Accepted for publication 21 February 2018
Published 27 April 2018 Volume 2018:11 Pages 2355—2362
DOI https://doi.org/10.2147/OTT.S156494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Yuan Yuan,1,* Lin Wang,1,* Qing-Xiang Li,1 Jian-Yun Zhang,2 Zhi-Xiu Xu,2 Chuan-Bin Guo1
1Department of Oral and Maxillofacial Surgery, Peking University School and Hospital of Stomatology, Beijing, China; 2Department of Oral Pathology, Peking University School and Hospital of Stomatology, Beijing, China
*These authors contributed equally to this work
Background: Oropharyngeal squamous cell carcinoma (OPSCC) is an aggressive malignancy which has been investigated for decades and reported highly associated with the human papillomavirus (HPV) infection, yet there is no consensus reached on the optimal treatment paradigm. The relatively lower prevalence of HPV in China makes it important to evaluate the outcomes of HPV-negative OPSCC.
Purpose: Our study was carried out in an attempt to evaluate the outcomes of squamous cell carcinoma of the oropharynx treated with primary surgery and identify the associated prognostic factors.
Patients and methods: We retrospectively analyzed the outcomes of the primary surgically treated HPV-negative OPSCC cases at our institution between 2008 and 2013. Overall survival (OS), disease-specific survival (DSS), and disease-free survival (DFS) were determined by Kaplan–Meier analysis. Prognostic factors of outcomes were investigated by uni- and multivariate analyses.
Results: In this study, neck metastasis rate was 61.3%. Level II nodes were the most vulnerable. The 3-year disease-specific survival, overall survival, and disease-free survival rates were 76.7%, 75.6%, and 62.8%, respectively. Forearm free flaps were the most commonly utilized in the reconstructions. A multivariate analysis indicated that N stage and adjuvant radiotherapy were predictive factors for 3-year disease-specific survival.
Conclusion: The outcomes of the surgical treatment of oropharyngeal squamous cell carcinoma were acceptable, and N-stage, adjuvant radiotherapy were identified as prognostic factors.
Keywords: cancer of oropharynx, HPV negative, primary surgery, lymph node metastasis, reconstruction
Introduction
In 2015, 161,000 cases were diagnosed as oro- and laryngopharyngeal carcinoma all over the world, accounting for about 1% (161,000/17,481,000) of total cancer incidence.1 As for China, the National Central Cancer Registry of China estimated the numbers of new lip, oral cavity, pharynx (except nasopharynx) cancer cases and deaths to be 48,100 and 22,100, respectively, in 2015 according to the average incidence rates for 2009–2011.2 Oro- and laryngopharyngeal carcinoma ranked 27th in terms of incidence and 18th in terms of cancer deaths (64,000 cases) in China in 2015.1
Though investigations of oropharyngeal squamous cell carcinoma (OPSCC) have been carried out for decades, its etiologic and clinical characteristics differ substantially among populations, such as the differences in smoking prevalence3,4 and human papillomavirus (HPV) infection rates,5–7 resulting in a lack of consensus regarding the optimal treatment paradigm. The relatively lower HPV infection rate in China5–9 along with the prevalence of tobacco3,10,11 makes the evaluation of treatments for HPV-negative OPSCC an urgent need.
We retrospectively analyzed the outcomes of the primary surgically treated HPV-negative OPSCC cases at Peking University, School and Hospital of Stomatology, Department of Oral and Maxillofacial Surgery. This represents one of the major medical institutions in China. Outcomes from 2008 to 2013 were analyzed in an attempt to identify those factors related to survival status.
Patients and methods
Patients
This research was conducted in compliance with the requirements of the institutional review board of the study hospital and approved by Peking University Institutional Review Board (No.: IRB00001052-16080). One hundred and fourteen patients with histologically confirmed primary HPV-negative OPSCC who were treated with conventional surgery in this hospital with or without adjuvant therapy from January 2008 to December 2013 were included in this study. Patients who had no other synchronous head and neck tumors were eligible.
The head and neck squamous cell carcinomas positive for both HPV DNA and p16 immunohistochemistry staining are more likely to show the typical clinical HPV-related behavior.12,13 Therefore, we combined HPV DNA polymerase chain reaction (Assay Kit for HPV 23 types genotyping, PCR-RDB; Triplex International Biosciences Co., Ltd, Beijing, China) and p16 immunohistochemistry13 to identify the clinically relevant HPV infection in our patients. The cases that showed positivity in both p16 staining and PCR detection were identified as active HPV infected and were excluded from the study cohort.
For each patient, we reviewed the medical records (patient’s written informed consent was obtained) to collect information regarding gender, age, history of tobacco and alcohol use, primary tumor subsites (soft palate, base of the tongue, or other oropharyngeal sites), histopathologic factors, treatment modalities, tracheotomy, and nasogastric tube dependency. Clinical staging was performed according to the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system (seventh edition) based on the findings of the physical and radiographic examinations.
Statistical analysis
Kaplan–Meier analysis was performed on the patients’ clinical and pathologic characteristics using the log-rank statistic to compare between strata. Univariate and multivariate analyses were also performed using the Cox proportional hazards model to identify the covariates that best predicted survival rates. A comparison of proportions was performed using χ2, and continuous data were analyzed using a independent t-test. Statistical significance was accepted as p<0.05. Statistical calculations were performed using commercially available SPSS software (IBM SPSS Statistics, Version 20.0; IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics
Clinical characteristics of the 114 patients with primary oropharyngeal carcinoma enrolled in the study are presented in Table 1.
  | Table 1 Clinical characteristics of the 114 patients with HPV-negative primary oropharyngeal carcinoma |
The male:female ratio was 4.2. The median age was 55 years, and the average onset age for male patients was about 5 years lower than that for female patients (mean age of males vs females: 54.95 vs 60.09, p=0.026). Also, 71.9% (82/114) of the patients had a history of heavy tobacco exposure (≥10 pack-years), 60.5% (69/114) had a history of alcohol consumption of >2,500 mL-years, and 58.8% (67/114) had both. Gender was significantly related to tobacco and alcohol exposure (p<0.01). Only 2 female patients reported heavy smoking history and no female drinker was identified, while 80 male patients were smokers and 69 were drinkers. When adjusted by gender, the other clinical characteristics were associated with neither smoking nor drinking status; even the combination of these two risk factors seemed irrelevant (p>0.05).
Surgical treatments
Tumor resection with curative intent and with or without reconstruction was performed in all patients. Among these patients, 53.5% (61/114) went through lip-split approach. Among T1 patients, a transoral approach was applied to 74.2% (23/31), and 6.5% (2/31) underwent bone resection. Ten patients underwent tracheotomy, mostly for preventive purpose because their tumors were located on the base of the tongue or the soft palate. As the tumor size increased, the percentages of patients who underwent the lip-split approach, bone resection, and tracheotomy increased.
Of our patients, 93.0% (106/114) underwent a neck dissection. Eighty-five of them underwent an ipsilateral neck dissection and 21 underwent a bilateral neck dissection. Of all the 127 laterals dissected, 52 modified radical neck dissections, 48 supraomohyoid neck dissections, 15 radical neck dissections, 10 level I–IV neck dissections, and 2 contralateral level I neck dissections were performed.
Overall, 76 patients underwent reconstructive surgeries to correct the defects left after tumor resection. These surgeries involved 77 flaps (one patient had a second reconstructive surgery after the first anterolateral thigh free flap necrosed). Forearm flaps were the most commonly utilized free flap (Table 2).
  | Table 2 Tumor resection and reconstruction procedures according to T stage |
All patients were discharged after the removal of their tracheotomy tubes. The hospital stays after the flap surgeries normally ranged from 7 to 14 days. The nasogastric feeding tubes were removed when the patients were able to return to oral food intake, typically at 1–4 weeks after surgeries.
Pathologic characteristics
Pathologic nodal status, degree of differentiation, infiltration depth, and tumor stage were recorded. The outcomes are presented in Table 3.
  | Table 3 Pathologic characteristics of the 114 patients |
The pathologic characteristics were analyzed according to tobacco smoking and alcohol drinking by gender; even the combination of these two risk factors was not statistically associated with local tumor behavior and nodal metastasis (p>0.05).
Moderately differentiated tumors accounted for 71.0% (76/107) of cases. The total neck metastasis rate was 61.3% (65/106). The metastasis rate was 55.1% (70/127) for laterals, 20.5% (107/521) for levels, and 5.4% (178/3,303) for lymph nodes (Table 4). The lymph nodes at ipsilateral level II were the most vulnerable in that they exhibited the highest rate of metastasis in terms of the number of levels and nodes affected. The infiltration depth (p=0.021) and tumor differentiation (p=0.024) were related to metastasis status, while age (p=0.157), gender (p=0.168), the use of both tobacco and alcohol (p=0.215), T stage (T1–2 vs T3–4, p=0.098), tumor location (p=0.744), and growth pattern (p=0.138) seemed irrelevant. In our study, the infiltration depth other than the volume of the tumor seemed to be associated with nodal metastasis status (T1–2/infiltration depth ≤5 mm vs T3–4/infiltration depth ≤5 mm, p=0.117, while T1–2/infiltration depth ≤5 mm vs T1–2/infiltration >5 mm, p=0.004). Among patients who were diagnosed as clinically neck positive and underwent neck dissection, 82.7% (43/52) had pathologically proven neck nodal metastasis. Among all patients, 62 were diagnosed as cN0, 54 underwent neck dissection, and 22 exhibited neck nodal metastasis. Occult neck metastasis was seen in 40.7% of cases.
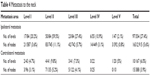  | Table 4 Metastasis to the neck |
Follow-up results
Fifty-eight patients underwent adjuvant radiotherapy (RT), and 11 of these received chemotherapy as well. The follow-up period ranged from 1 to 99 months (median 36 months). The 3-year disease-specific survival (DSS) rate was 76.7% (Figure 1), the overall survival rate was 75.6%, and the disease-free survival rate was 62.8%. A local-regional recurrence or metastasis occurred in 25.4% (29/114) of patients. Among these, 89.7% (26/29) occurred within 24 months after surgery, 96.6% (28/29) occurred within 36 months, and only 1 patient, who refused to give up smoking, exhibited neck metastasis at 50 months after surgery. The 3-year DSS rates of the patients according to their clinicopathologic characteristics are shown in Figure 2. Univariate and multivariate analyses showed that N stage and adjuvant RT were predictive factors for 3-year DSS (Table 5).
  | Figure 1 The 3-year disease-specific survival rate of the 114 patients with HPV-negative oropharyngeal squamous cell carcinoma was 76.7%. |
Osteoradionecrosis (ORN) of the mandible was found in six patients at an average of 34 months (range 9–65 months, median 33 months) after surgery. Two patients had to undergo mandibulectomy and had their defects repaired via fibular flaps. However, one of the flaps necrosed due to ischemia and another pedicle flap (a pectoralis major myocutaneous flap) was utilized.
Discussion
The prevalence of HPV in OPSCC in China6,7 is much lower than in North America,5,8 Europe,5 and Australia.9,14 HPV-associated OPSCC has been reported to have a better prognosis and a lower rate of adverse events,15–18 which may be attributed to the better response to radiation and chemotherapy.19,20 HPV-negative status may explain the relatively reduced DSS and overall survival values in our patients. Studies showed that for HPV-positive OPSCC, the difference between the hazard ratios for primary surgery and primary radiation did not reach statistical significance, while worse outcomes were associated with HPV-negative OPSCC when it was treated with primary radiation as compared to treatment with surgery.15,21 Therefore, evaluation of surgical management for HPV-negative OPSCC is of vital importance.
In this study, 71.9% of the patients were heavy smokers. China is the world’s largest consumer of tobacco. Up to 52.1% of men in China are smokers, and the total number is over 300 million.3,10 Up to one-quarter of the cancer deaths are due to tobacco use.22 Smoking rates during adolescence and young adulthood are increasing10,23 and could initiate up to 80% of lifetime smoking in both high- and low-income countries.24 In China, among adults in their 20s, 15.2% had started to smoke by age 15 years, and 54.7% by age 21 years.25 However, tobacco control policies in industrialized nations have reduced tobacco use.26 In our study, though tobacco and alcohol consumption did not make a significant difference in survival (neither smoker nor drinker vs smoker and drinker in males: 77.8% vs 71.5%, p=0.374; male nonsmoker vs smoker: 80.8% vs 75.2%, p=0.818; female nonsmoker vs smoker: 83.3% vs 50%, p=0.372; male nondrinker vs drinker: 85.7% vs 72.1%, p=0.225), it did result in a decline in outcomes. Our results may have been affected by the disability of measuring the second-hand smoking status in the female patients, which could lead to the harm of tobacco being underestimated.
OPSCC is an aggressive malignancy that is usually diagnosed in its advance stages and has a high rate of lymphatic metastasis.16,27 This is in accordance with our study, in which 61.3% (65/106) of the patients were histologically proven to have positive necks. Also, 70.7% (75/106) of our patients were in stage III or IV. Advanced stages usually indicate poor prognoses, regardless of the treatment modalities selected, while better prognoses are associated with early-stage OPSCCs regardless of whether they are treated with primary surgery or RT.28
According to our study, nodes in ipsilateral level II were the most vulnerable, followed by nodes in level III. The infiltration depth (p=0.021) and tumor differentiation (p=0.024) were related to metastasis status, while age (p=0.157), gender (p=0.168), the use of both tobacco and alcohol (0.215), T stage (T1–2 vs T3–4, p=0.098), tumor location (p=0.744), and growth pattern (p=0.138) seemed to be irrelevant. Infiltration depth other than the volume of the tumor should be considered.
Previously, due to the lack of sufficient examinations and the limits of the techniques used, a diagnosis of negative neck was relatively unreliable. However, studies of squamous cell carcinoma of the oral cavity conducted by the same hospital during a similar period illustrated that the occult metastasis was lower than in OPSCC,29,30 which also indicated the aggressiveness of OPSCC. Currently, with the help of multiple examination techniques, the sensitivity and specificity of cervical nodal metastasis identification have sharply increased; yet, occult metastases still occur, indicating the necessity of thorough examinations and care in neck dissection decisions, regardless of tumor stage.
As shown in our study, adjuvant RT improved the survival rate of patients with pTNM3–4 tumors, but not of those with pTNM1–2 tumors. Margins and node status influenced the decision to administer adjuvant RT.31 We recommend that patients with pTNM3–4 tumors receive adjuvant RT. For pTNM I–II patients, taking the toxic effects of RT and the potential for a lack of response into consideration, we hold a conservative opinion regarding adjuvant RT.
The 3-year DSS rate was 76.7%, and uni- and multivariable analysis identified N stage and adjuvant RT as prognostic factors. Metastasis in even one single node led to a decrease in survival as compared with N0, though this difference did not reach statistical significance when adjuvant RT was applied.
ORN of the mandible is a complication of RT that is characterized by nonhealing mucosal and bone injury that occurs spontaneously or after trauma and seriously impacts the quality of life of patients.32 The rate of ORN of the mandible increased by years after therapy, and the highest incidence of ORN is reported to occur between 6 months and 2 years after RT.33 Among our patients, 10.3% (6/58) suffered from complications at a median time period of 33 months after therapy. Along with ORN, sequelae such as pharyngeal fibrosis and xerostomia may hamper the oral food intake process, though RT may offer better organ preservation.28 Since the sequelae other than ORN were less quantified in our records, we failed to perform analysis concerning this issue.
Conclusion
We studied the clinical and pathologic characteristics and the oncologic outcomes of patients with HPV-negative oropharyngeal carcinoma who took surgery as first-line therapy in northern China, as well as the prognostic factors associated with their survival. We identified having node metastasis and not receiving adjuvant RT as factors that influenced prognoses. Tumor differentiation and infiltration depth were related to metastasis status. Tobacco and alcohol use decreased the survival rate, though not by a significant degree. We recommend thorough examinations and care in choosing not to perform neck dissections in patients with OPSCC, even in its early clinical stages. Adjuvant RT is recommended if there are positive lymph nodes or an advanced tumor stage. For early-stage, HPV-negative OPSCC, taking the toxic effects and the potential for a lack of response into consideration, we hold a conservative opinion regarding adjuvant RT.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No. 81470707), the Peking University School and Hospital of Stomatology Youth Research Fund (No. PKUSS20160108), as well as King’s College London – Peking University Health Science Center Joint Institute for Medical Research Program (No. PKU2017ZC001-9).
Disclosure
The authors report no conflicts of interest in this work.
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and National Cancer Incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Li Q, Hsia J, Yang G, et al. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364(25):2469–2470. | ||
Pierce JP, Messer K, White MM, Cowling DW, Thomas DP. Prevalence of heavy smoking in California and the United States, 1965–2007. JAMA. 2011;305(11):1106–1112. | ||
Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35(5):747–755. | ||
Wang F, Zhang H, Xue Y, et al. A systematic investigation of the association between HPV and the clinicopathological parameters and prognosis of oral and oropharyngeal squamous cell carcinomas. Cancer Med. 2017;6(5):910–917. | ||
Wang Z, Xia RH, Ye DX, Li J. Human papillomavirus 16 infection and TP53 mutation: two distinct pathogeneses for oropharyngeal squamous cell carcinoma in an Eastern Chinese population. PLoS One. 2016;11(10):e0164491. | ||
Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. | ||
Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748–2754. | ||
Xiao L, Feng GZ, Jiang Y, Zhang JR, Liu LX. 中国初中学生烟草使用及其影响因素研究 [Tobacco use rate and associated factors in middle school students in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(5):567–571. Chinese. | ||
Chen ZM, Peto R, Iona A, et al. Emerging tobacco-related cancer risks in China: a nationwide, prospective study of 0.5 million adults. Cancer. 2015;121 (Suppl 17):3097–3106. | ||
Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus – associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–747. | ||
Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121(11):2465–2472. | ||
Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;28(19):3269–3272. | ||
Wang MB, Liu IY, Gornbein JA, Nguyen CT. HPV-positive oropharyngeal carcinoma: a systematic review of treatment and prognosis. Otolaryngol Head Neck Surg. 2015;153(5):758–769. | ||
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. | ||
Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. | ||
Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15. | ||
Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50(15):2636–2648. | ||
Gillison ML, Restighini C. Anticipation of the impact of human papillomavirus on clinical decision making for the head and neck cancer patient. Hematol Oncol Clin North Am. 2015;29(6):1045–1060. | ||
Lacau St Guily J, Rousseau A, Baujat B, et al; Papillophar Group. Oropharyngeal cancer prognosis by tumour HPV status in France: the multicentric Papillophar study. Oral Oncol. 2017;67:29–36. | ||
Chen ZM, Peto R, Iona A, et al; China Kadoorie Biobank Collaborative Group. Emerging tobacco-related cancer risks in China: a nationwide, prospective study of 0.5 million adults. Cancer. 2015;121 (Suppl 17):3097–3106. | ||
Zhang J, Ou JX, Bai CX. Tobacco smoking in China: prevalence, disease burden, challenges and future strategies. Respirology. 2011;16(8):1165–1172. | ||
Giovino GA, Mirza SA, Samet JM, et al; GATS Collaborative Group. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380(9842):668–679. | ||
Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5(7):e141. | ||
WHO urges more countries to require large, graphic health warnings on tobacco packaging: the WHO report on the global tobacco epidemic, 2011 examines anti-tobacco mass-media campaigns. Cent Eur J Public Health. 2011;19(3):133–151. | ||
Pitchers M, Martin C. Delay in referral of oropharyngeal squamous cell carcinoma to secondary care correlates with a more advanced stage at presentation, and is associated with poorer survival. Br J Cancer. 2006;94(7):955–958. | ||
Diaz-Molina JP, Rodrigo JP, Alvarez-Marcos C, et al. Resultados oncológicos y funcionales del tratamiento no quirúrgico comparado con el quirúrgico en los carcinomas epidermoides de orofaringe [Functional and oncological results of non-surgical vs surgical treatment in squamous cell carcinomas of the oropharynx]. Acta Otorrinolaringol Esp. 2012;63(5):348–354. Spanish. | ||
Niu LX, Feng Z, Li JN, Li CZ, Peng X, Guo CB. Prognostic factors of squamous cell carcinoma of the buccal mucosa: a retrospective study of 168 cases in North China. J Oral Maxillofac Surg. 2014;72(11):2344–2350. | ||
Niu LX, Feng ZE, Wang DC, Zhang JY, Sun ZP, Guo CB. Prognostic factors in mandibular gingival squamous cell carcinoma: a 10-year retrospective study. Int J Oral Maxillofac Surg. 2017;46(2):137–143. | ||
Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967–2980. | ||
Rogers SN, D’Souza JJ, Lowe D, Kanatas A. Longitudinal evaluation of health-related quality of life after osteoradionecrosis of the mandible. Br J Oral Maxillofac Surg. 2015;53(9):854–857. | ||
Caparrotti F, Huang SH, Lu L, et al. Osteoradionecrosis of the mandible in patients with oropharyngeal carcinoma treated with intensity-modulated radiotherapy. Cancer. 2017;123(19):3691–3700. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


