Back to Journals » Medical Devices: Evidence and Research » Volume 15
Retrospective Comparison of Clinical and Economic Outcomes of Non-Donor Patients Undergoing Radical Nephrectomy Using One of Two Different Linear Stapler Technologies for Transection of the Renal Vessels: Fixed-Height Gripping Surface Reloads vs Variable-Height Reloads
Authors Johnston SS, Johnson BH , Chakke D, Roy S, Grange P, Pollack E
Received 11 May 2022
Accepted for publication 5 August 2022
Published 2 September 2022 Volume 2022:15 Pages 317—328
DOI https://doi.org/10.2147/MDER.S372629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Stephen S Johnston,1 Barbara H Johnson,1 Divya Chakke,2 Sanjoy Roy,3 Philippe Grange,4 Esther Pollack3
1MedTech Epidemiology and Real-World Data Sciences, Johnson & Johnson, New Brunswick, NJ, USA; 2Analytics, Mu Sigma, Bangalore, India; 3Franchise Health Economics and Market Access, Johnson & Johnson, Raritan, NJ, USA; 4Medical, Johnson & Johnson, Cincinnati, OH, USA
Correspondence: Stephen S Johnston, Real-World Data Analytics and Research, MedTech Epidemiology and Real-World Data Sciences, Johnson & Johnson, 410 George Street, New Brunswick, NJ, USA, Tel +1-443-254-2222, Email [email protected]
Purpose: To compare outcomes of non-donor patients undergoing radical nephrectomy using fixed-height gripping surface (FHGS) vs variable-height Tri-Staple™ (VHTS) reloads for transection of the renal vessels.
Patients and Methods: Using the Premier Healthcare Database of US hospital discharge records, we selected non-donor patients undergoing inpatient radical nephrectomy with dates of admission between 1 October 2015, and 31 December 2020 (first=index admission). The primary outcome was in-hospital hemostasis-related complications (hemorrhage, acute posthemorrhagic anemia, and/or procedure to control bleeding) during the index admission. Secondary outcomes included index admission intraoperative injury, blood transfusion, conversion from minimally invasive to open surgery, total hospital costs, length of stay (LOS), discharge status, and mortality as well as 30-day all-cause inpatient readmission. We used stable balancing weights to balance the FHGS and VHTS groups on numerous patient, procedure, and hospital/provider characteristics, allowing a maximum post-weighting standardized mean difference ≤ 0.01 for all covariates; we also exactly matched the groups on laterality (right vs left kidney) and intended surgical approach (open, laparoscopic, robotic). We used bivariate multilevel mixed-effects generalized linear models accounting for hospital-level clustering to compare the study outcomes between the FHGS and VHTS groups.
Results: After weighting, the FHGS and VHTS groups comprised 2952 and 795 patients, respectively. The observed incidence proportion of the primary outcome of hemostasis-related complications during the index admission was similar between the groups (8.6% for FHGS vs 9.0% for VHTS, difference 0.4% [95% CI − 3.2% to 2.5%], P=0.808). Differences between the FHGS and VHTS groups were not statistically significant for any of the secondary outcomes.
Conclusion: Endoscopic surgical staplers have become common for transection of the renal vessels during radical nephrectomy, with FHGS and VHTS being the predominant reload types. In this retrospective study of 3747 non-donor patients undergoing radical nephrectomy, use of FHGS vs VHTS reloads was associated with similar clinical and economic outcomes.
Keywords: radical nephrectomy, hemostasis-related complications, endoscopic surgical staplers, healthcare utilization
Introduction
The adoption of minimally invasive surgical techniques for radical nephrectomy has grown substantially over the past two decades, beginning with the introduction of laparoscopic assistance, followed by robotic-assisted laparoscopic techniques, and most recently, robotically stapled nephrectomy.1,2 Minimally invasive radical nephrectomy, whether laparoscopic or robotic, has been associated with lower morbidity than open radical nephrectomy, while the laparoscopic and robotic approaches have demonstrated similar surgical outcomes.3–5 As a result of these trends in surgical approach, endoscopic surgical staplers have become common for transection of the renal vessels during minimally invasive radical nephrectomy; furthermore, they may also be used in certain circumstances for this task in open radical nephrectomy.
Very early analyses of the US Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database spanning 1992–2007 investigated early trends in the use of such staplers in nephrectomy.6,7 Subsequently, a recently published study presented an analysis of the MAUDE database examining stapling during hilar ligation in minimally invasive radical nephrectomy from January 1, 2009 to August 1, 2019.8 Though these prior studies provide useful insights into various aspects of stapling, they were inherently unable to make conclusions about comparative outcomes between different stapling devices: the MAUDE database is a passive surveillance system of medical device reports and the FDA itself warns that for a wide variety of reasons, these data “cannot be used to establish rates of events, evaluate a change in event rates over time or compare event rates between devices.”9
Currently, endoscopic surgical staplers are predominantly produced by two manufacturers: Medtronic, and Ethicon. Between these two manufacturers, key differentiating features of the staplers are their corresponding reloads. Whereas Medtronic staplers currently use reloads with variable-height Tri-Staple (VHTS) cartridge faces that are designed to deliver graduated compression, Ethicon staplers have adopted fixed-height gripping surface reloads (FHGS) with proprietary pocket extensions to stabilize and hold in place tissue for the deployment of staples with uniform height (“Gripping Surface Technology”). However, to-date, no study has examined whether different reload formations may be associated with differences in clinical or economic outcomes in radical nephrectomy.
Therefore, we conducted this retrospective study using a large hospital research database to compare outcomes of non-donor patients undergoing radical nephrectomy using VHTS vs FHGS reloads for transection of the renal vessels.
Materials and Methods
Data Source
We extracted the study data from the Premier Healthcare Database® (PHD), which is a population-based hospital research database that contains administrative records routinely contributed by over 700 US hospitals that are members of the Premier healthcare performance improvement alliance, representing approximately 25% of annual US inpatient discharges.10 This database includes discharge-level information on patient demographics, diagnoses, procedures, medical supplies, costs, and hospital and provider characteristics. This information is provided in the form of standardized administrative fields, hospital charge master data, and International Classification of Diseases, 10th Revision, Clinical Modification and Procedure Classification System (ICD-10-CM/ICD-10-PCS) diagnosis and procedure codes (Supplemental Appendix 1). The PHD has been widely used for epidemiologic and economic research, forming the basis of over 600 peer-reviewed publications since 2006.
We conducted this study under an exemption from Institutional Review Board oversight for US-based studies using de-identified healthcare records, as dictated by Title 45 Code of Federal Regulations (45 CFR 46.101(b)(4)).
Patient Selection
Using the PHD, we selected non-donor patients undergoing inpatient radical nephrectomy with dates of admission between October 1, 2015 and December 31, 2020. We designated the first observed inpatient admission meeting these criteria as the “index admission”, required patients to be at least 18 years of age as of the day of index admission, and required patients to have at least one hospital charge master record indicating the use of VHTS or FHGS reloads on the day of the radical nephrectomy. We excluded patients from the study if they met any of the following criteria during the index admission: primary or secondary diagnosis code for donor nephrectomy; use of the Ethicon Powered Vascular Stapler (to reduce misclassification of tasks in which staplers were used); use of both VHTS and FHGS reloads; point of origin was from another institution; major concomitant colorectal resection or procedure involving the vena cava; zero or negative total hospital costs, room and board, or supply costs.
Classification of Study Groups
We classified patients as having either VHTS or FHGS reloads used on the day of the radical nephrectomy based on records in each hospital’s charge master, which is a comprehensive administrative record of billable procedures, equipment fees, supplies, devices, drugs, imaging services, and room and board, among other items. We identified reloads based on model numbers specific to each reload (eg, EGIA60AVM; GST60W) or text descriptions (eg, STAPLES ENDO GIA TRISTAPLE 60MM; STAPLER RELOAD GST 60MM). The list of charge master descriptions identified by the initial search was reviewed by two separate authors to ensure accuracy. Robotic staplers were not included within this analysis. Ultimately, two mutually exclusive groups were established: the VHTS group and the FHGS group.
Measurement of Outcomes
This study’s primary outcome was in-hospital hemostasis-related complications, defined as a composite of diagnosis related to intraoperative and postprocedural hemorrhage, hematoma, or seroma of a genitourinary system organ or structure, acute posthemorrhagic anemia, or a procedure code for control of bleeding in the genitourinary tract recorded during the index admission. Secondary outcomes measured during the index admission included diagnosis of intraoperative injury, receipt of blood product transfusion, conversion from minimally invasive to open surgery, total hospital costs from the hospital perspective, length of stay (LOS), discharge status, and mortality, as well as 30-day all-cause inpatient readmission to the same hospital in which the index admission occurred. Information on mortality occurring outside of the hospital is not available in the PHD. See Supplemental Appendix 1 for specific ICD-10-CM/PCS diagnosis and procedure codes used to measure the study outcomes, where applicable.
Total hospital costs were standardized to 2020 US Dollars based on the Medical Care component of the Consumer Price Index. In the PHD, hospital costs are reported directly by the hospitals from which data are sourced in this database. Costs are determined based on each hospital’s own charge master. Analyses of all-cause hospital readmissions were limited to patients in Institutions that continued to contribute data to the Premier Healthcare Database through or beyond 30 days after the index admission.
Measurement of Covariates
We measured study covariates using records from the index admission. Patient demographics included age, sex, marital status, race, payer type, and year of index admission. Patient clinical characteristics included the Charlson Comorbidity Index Score,11 Elixhauser’s comorbidities,12 and other selected comorbidities, encompassing individual flags for the following: adhesions, congestive heart failure, cardiac dysrhythmias, valvular disease, pulmonary circulation disorders, peripheral vascular disorders, hypertension uncomplicated, hypertension complicated, other neurological disorders, chronic pulmonary disease, diabetes uncomplicated, diabetes complicated, hypothyroidism, renal failure, liver disease, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis/collagen, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, nutritional anemia, deficiency anemia, nutritional and metabolic disorders, alcohol abuse and disorders, drug abuse, psychoses, and depression. Diagnoses for comorbidities could not have a Present on Admission indicator of “No”.
Procedural characteristics included surgical indication (renal cell carcinoma, other cancer, or non-cancer primary diagnosis for the index admission), intended surgical approach (laparoscopic, robotic, open), laterality (left, right, bilateral), elective vs non-elective procedure, and individual flags for the following concomitant secondary procedures that were observed with >1% frequency in the study patients: ureter/bladder resection, ureter/bladder excision, procedures related to the spleen, procedures related to the lymph nodes, hepatopancreatobiliary excision, gastrointestinal excision, procedures related to the adrenal gland, and procedures related to adhesions.
Hospital/provider characteristics included urban vs rural hospital, teaching vs non-teaching hospital, hospital bed size category, annual radical nephrectomy volume, and surgical specialty of the physician performing the procedure.
Statistical Analyses
We used stable balancing weights (SBW), an optimization-based analogue to inverse propensity score weighting, to balance all study covariates listed above.13 SBW directly targets covariate balance by treating weighting as an optimization problem, whereby an objective function is maximized subject to constraints: the objective function is to find the mathematically guaranteed weights of minimum variance (leading to the largest weighted sample size) subject to researcher-specified covariate balance constraints (ie, a prespecified maximum standardized mean difference [SMD] for each covariate between the study groups).
SBW weighting procedure is performed using R statistical software (www.r-project.org) and the -sbw- and -quadprog- packages. To find the optimal SMD threshold, we used a “grid search”, whereby the smallest overall SMD threshold is chosen relative to resultant sample size loss. An SMD value ≤0.10 is indicative of good balance.14 The resultant SMD threshold was 0.01. We also matched patients exactly on intended surgical approach and laterality. We applied the SBWs to the VHTS group, thereby re-weighting the VHTS group to appear nearly identical to the FHGS group.
We compared outcomes between the VHTS and FHGS groups using bivariate multilevel mixed-effects generalized linear models with robust standard errors, wherein patients were treated as nested within hospitals and the weights from the SBW procedure were incorporated as probability weights. A logit link and binomial error distribution were used when comparing binary outcomes, a log link and negative binomial error distribution was used when comparing length of stay. P<0.05 was set as the threshold for statistical significance.
Results
Patient and Hospital/Provider Characteristics
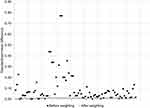
Before weighting, there were 2952 patients in the FHGS group and 1032 in the VHTS group, with substantial differences on several weighting covariates (Figure 1). After weighting, all 2952 patients in the FHGS group and 795 patients in the VHTS group were retained for analysis, coming from a total of 165 individual hospitals. All post-weighting standardized mean differences were ≤|0.01|, indicating excellent covariate balance between the study comparison groups.
Tables 1–3, and 4 display information on post-weighting patient demographics, patient clinical characteristics, procedure characteristics, and hospital/provider characteristics, respectively. Overall, the mean patient age was 63 years, 41% were female, and most (80%) were of white race (Table 1). The five most common comorbidities were hypertension (69%), diabetes (28%), obesity (22%), renal failure (22%), and chronic pulmonary disease (14%) (Table 2). Most (57%) patients’ surgical indication was for renal cell carcinoma, with robotics being used in 39% of the procedures, and the majority (91%) of admissions being elective (Table 3). Patients were predominantly treated in urban hospitals (96%) and nearly all (96%) underwent surgery by a urologist (Table 4).
 |
Table 1 Patient Demographic Characteristics After Weighting |
 |
Table 2 Patient Clinical Characteristics After Weighting |
 |
Table 3 Procedural Characteristics After Weighting |
 |
Table 4 Hospital/Provider Characteristics After Weighting |
Analyses of Primary Outcome
Figure 2 shows the results to the analyses of the primary outcome of in-hospital hemostasis-related complications during the index admission. The incidence proportion of the primary outcome of hemostasis-related complications during the index admission was similar between the groups (8.6% for FHGS vs 9.0% for VHTS; the risk difference between the FHGS group and the VHTS group was −0.4% (95% confidence interval −3.2% to 2.5%), P=0.808. Although hemostasis-related complications represented a composite outcome, events were primarily driven by diagnosis of acute posthemorrhagic anemia: of the patients with a hemostasis-related complication, 98.8% had a diagnosis of acute posthemorrhagic anemia, whereas only 4.5% (0.4% of the patients overall) had a diagnosis related to intraoperative or postprocedural hemorrhage, hematoma, or seroma of a genitourinary system organ or structure; there was one case of procedure for control of bleeding in the genitourinary tract, within the FHGS group (P=0.318 vs VHTS). Results of analyses of the primary outcome before weighting (ie, unadjusted analyses) are shown in Supplemental Table 1.
Analyses of Secondary Outcomes
Table 5 shows the results of the analyses of secondary outcomes. Differences between the FHGS and VHTS groups were not statistically significant for any secondary outcome: intraoperative injury (no events in either group); blood transfusion (6.0% for FHGS vs 8.1% for VHTS, P=0.335); conversion from minimally invasive to open surgery (1.7% for FHGS vs 1.5% for VHTS, P=0.794); index admission mean total hospital costs ($13,730 for FHGS vs $14,470 for VHTS, P=0.705); index admission mean length of stay (3.7 days for FHGS vs 4.1 days for VHTS, P=0.153); discharge to home (94.4% for FHGS vs 94.8% for VHTS, P=0.735); mortality (0.58% for FHGS vs 0.53% for VHTS, P=0.905); and, 30-day all-cause inpatient readmission to the same hospital in which the index admission occurred (6.2% for FHGS vs 6.0% for VHTS, P=0.866). Results of analyses of the secondary outcomes before weighting (ie, unadjusted analyses) are shown in Supplemental Table 1.
 |
Table 5 Analyses of Secondary Outcomes |
Discussion
This study reports the first comparative effectiveness assessment between FHGS and VHTS reloads for transection of the renal vessels among non-donor patients undergoing radical nephrectomy. We found that use of FHGS vs VHTS reloads for transection of the renal vessels was not associated with significantly different clinical or economic outcomes.
Hemostasis-related complications were driven primarily by diagnoses of acute post-hemorrhagic anemia, with <0.5% of the patients having a diagnosis for hemorrhage, hematoma, or seroma of a genitourinary system organ or structure. The rate of blood transfusion (6.0% for FHGS and 8.1% for VHTS) fell well within the range reported in prior literature, which is as low as 4% to as high as 24% for radical nephrectomy.15,16
The present study’s findings of statistical parity in outcomes between these reload technologies is based on an epidemiologically appropriate database and statistical methodology for comparative effectiveness research. A prior MAUDE database analysis reported that Ethicon staplers (agnostic to reload type) were associated with ostensibly more complications when not considering a population denominator.5 However, because differences in the real-world number (denominator) of staplers used were unknown, the complication rate could not be calculated. Indeed, whereas our query of the database yielded 2952 patients in the FHGS group, it yielded only 1032 in the VHTS group; therefore, under the circumstances of no differences in outcomes between these technologies, the crude number of events would be expected to be approximately 2.9 times higher in the FHGS vs VHTS group, a difference that is larger than what was reported in the MAUDE data. Thus, it is very plausible that the prior findings were primarily a reflection of differences in the volume of staplers used across manufacturers.
To our knowledge, only one prior study by Rawlins and colleagues had previously compared clinical and economic outcomes between FHGS and VHTS reloads, specifically among patients undergoing laparoscopic sleeve gastrectomy using powered staplers.17 They found that the ECHELON FLEX™ GST system (with FHGS) was associated with a lower rate of hemostasis-related complications as compared with the Signia™ Stapling System (with VHTS reloads). In the present study, the incidence proportion of hemostasis-related complications was numerically lower for FHGS vs VHTS reloads (8.6% vs 9.0%), but this difference did not reach statistical significance. A potential driver of the differences between the present and prior study is that radical nephrectomy is a more complex procedure than laparoscopic sleeve gastrectomy, performed for multiple different potential surgical indications and often involving concomitant procedures on adjacent anatomy, thereby increasing the potential for unmeasured variability to cloud the extent to which individual differences in the outcomes associated with devices can be identified in retrospective observational data.
The primary strengths of this study are that it is based on a large cohort of 3747 patients, coming from 165 hospitals, using a data source that allows for epidemiologic inferences about comparative outcomes, and using a statistical methodology that resulted in extremely well-balanced cohorts. However, this study also has important limitations. First, although we used a rigorous statistical methodology to balance the cohorts on measurable characteristics, there is no single data source (aside from a randomized controlled trial) that can rule out the potential for residual confounding from unmeasured factors such as the skill of the surgeon and their supporting clinical team, the complexity of a patient’s anatomy, the extent of a patient’s disease, use of concomitant medications that may influence hemostasis-related bleeding risk, or other potential factors. For example, there may be a wide level of variability in surgeon preference regarding the concomitant use of energy devices or other forms of vascular pedicle ligation, such as surgical ligation; furthermore, information on whether the renal hilar vessels were stapled separately or enbloc is unavailable in the PHD. Additionally, the ICD-10-PCS diagnosis coding system delineates between metastatic and non-metastatic cancer, on which the two groups were well balanced, but does not provide specific information on cancer stage, tumor size, and other detailed oncological factors, which could have influenced outcomes such as blood loss. Second, the identification of FHGS and VHTS reloads was based upon the hospital charge master, which may be subject to misclassification if the wrong product was entered in the charge master. Third, identification of hemostasis-related complications using ICD-10-CM/PCS coding is also subject to misclassification resulting from potential administrative coding or documentation errors. Finally, due to severe sample size constraints, we were unable to examine donor nephrectomies and nephrectomies that were robotically stapled; future studies are needed to confirm and extend the present study’s findings to these patient groups and technologies.
Conclusion
Endoscopic surgical staplers have become common for transection of the renal vessels during radical nephrectomy, with FHGS and VHTS being the predominant reload types. In this retrospective study of 3747 non-donor patients undergoing radical nephrectomy, use of FHGS vs VHTS reloads was associated with similar clinical and economic outcomes.
Abbreviations
FDA, US Food and Drug Administration; FHGS, fixed-height gripping surface reload; ICD-10-CM/ICD-10-PCS, International Classification of Diseases, 10th Revision, Clinical Modification and Procedure Classification System diagnosis and procedure codes; LOS, length of stay; MAUDE, Manufacturer and User Facility Device Experience; PHD, Premier Healthcare Database®; SMD, standardized mean difference; SBW, stable balancing weights; VHTS, variable-height Tri-Staple reload.
Data Sharing Statement
The Premier Healthcare Database is available for commercial license from Premier Inc.
Ethics Approval and Informed Consent
We conducted this study under an exemption from Institutional Review Board oversight for US-based studies using de-identified healthcare records, as dictated by Title 45 Code of Federal Regulations (45 CFR 46.101(b)(4)).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Johnson & Johnson.
Disclosure
Stephen Johnston, Barbara Johnson, Philippe Grange, Sanjoy Roy, and Esther Pollack are employees and stockholders of Johnson & Johnson. Divya Chakke is employed by Mu Sigma, which was paid by Johnson & Johnson to conduct data programming and statistical analyses. The authors report no other conflicts of interest in this work.
References
1. Jeong IG, Khandwala YS, Kim JH, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA. 2017;318(16):1561–1568. doi:10.1001/jama.2017.14586. PMID: 29067427; PMCID: PMC5818800.
2. Giffen Z, Ezzone A, Ekwenna O. Robotic stapler use: is it safe?-FDA database analysis across multiple surgical specialties. PLoS One. 2021;16(6):e0253548. doi:10.1371/journal.pone.0253548. PMID: 34166443; PMCID: PMC8224848.
3. Crocerossa F, Carbonara U, Cantiello F, et al. Robot-assisted radical nephrectomy: a systematic review and meta-analysis of comparative studies. Eur Urol. 2021;80(4):428–439. doi:10.1016/j.eururo.2020.10.034. Epub 2020 Nov 18. PMID: 33218826.
4. You C, Du Y, Wang H, et al. Laparoscopic versus open partial nephrectomy: a systemic review and meta-analysis of surgical, oncological, and functional outcomes. Front Oncol. 2020;10:583979. doi:10.3389/fonc.2020.583979. PMID: 33194725; PMCID: PMC7658533.
5. Li J, Peng L, Cao D, et al. Comparison of perioperative outcomes of robot-assisted vs. laparoscopic radical nephrectomy: a systematic review and meta-analysis. Front Oncol. 2020;10:551052. doi:10.3389/fonc.2020.551052. PMID: 33072578; PMCID: PMC7531174.
6. Hsi RS, Saint-Elie DT, Zimmerman GJ, Baldwin DD. Mechanisms of hemostatic failure during laparoscopic nephrectomy: review of Food and Drug Administration database. Urology. 2007;70(5):888–892. doi:10.1016/j.urology.2007.06.1116. Epub 2007 Oct 24. PMID: 17919695.
7. Hsi RS, Ojogho ON, Baldwin DD. Analysis of techniques to secure the renal hilum during laparoscopic donor nephrectomy: review of the FDA database. Urology. 2009;74(1):142–147. doi:10.1016/j.urology.2008.11.010. Epub 2009 May 5. PMID: 19406458.
8. Gopal N, Long B, Phillips J, Eshghi M. Endovascular stapler complications during minimally invasive nephrectomy: an updated review of the FDA MAUDE database From 2009-2019. Urology. 2021;153:181–184. doi:10.1016/j.urology.2021.02.010. Epub 2021 Feb 15. PMID: 33600834.
9. U.S. Food & Drug Administration. MAUDE - Manufacturer and User Facility Device Experience. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/textsearch.cfm.
10. Premier Applied Sciences®, Premier Inc. Premier Healthcare Database White Paper: data that informs and performs, November 4, 2019. Available from: https://learn.premierinc.com/white-papers/premier-healthcaredatabase-whitepaper.
11. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619.
12. Elixhauser Comorbidity Software for ICD-10-CM (beta version) Healthcare Cost and Utilization Project (HCUP); 2018. Agency for Healthcare Research and Quality, Rockville, MD. Available from: www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp.
13. Zubizarreta JR. Stable weights that balance covariates for estimation with incomplete outcome data. J Am Stat Assoc. 2015;110(511):910–922.
14. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi:10.1002/sim.3697
15. Anele UA, Marchioni M, Yang B, et al. Robotic versus laparoscopic radical nephrectomy: a large multi-institutional analysis (ROSULA Collaborative Group). World J Urol. 2019;37(11):2439–2450. doi:10.1007/s00345-019-02657-2. Epub 2019 Feb 7. PMID: 30734072.
16. Nazemi T, Galich A, Sterrett S, Klingler D, Smith L, Balaji KC. Radical nephrectomy performed by open, laparoscopy with or without hand-assistance or robotic methods by the same surgeon produces comparable perioperative results. Int Braz J Urol. 2006;32(1):15–22. doi:10.1590/s1677-55382006000100003. PMID: 16519823.
17. Rawlins L, Johnson BH, Johnston SS, et al. Comparative effectiveness assessment of two powered surgical stapling platforms in laparoscopic sleeve gastrectomy: a retrospective matched study. Med Devices. 2020;13:195–204. doi:10.2147/MDER.S256237
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.