Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 12
Retrospective Comparative Analysis of Opioid Use and Intra-Articular Corticosteroid Injections Before and After Initial Treatment of Knee Osteoarthritis with Hylan G-F 20
Authors Khangulov V, Zhang X, Munson SH, Peyerl F, Rey F
Received 13 January 2020
Accepted for publication 14 May 2020
Published 3 June 2020 Volume 2020:12 Pages 79—85
DOI https://doi.org/10.2147/OARRR.S245766
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Victor Khangulov,1 Xuan Zhang,1 Sibyl H Munson,1 Fred Peyerl,1 Francoise Rey2
1Department of Health Economics and Outcomes Research, Boston Strategic Partners, Inc., Boston, MA, USA; 2Sanofi, Paris, France
Correspondence: Victor Khangulov 15 Graham Street #2, Jersey City, NJ 07307, USA
Tel +1-646-397-9420
Email [email protected]
Background: Knee osteoarthritis (OA) is a painful condition affecting > 250 million people worldwide and is a leading cause of disability. Intra-articular (IA) corticosteroids and/or oral opioids are often recommended for the management of knee OA pain. There are, however, concerns regarding their safety and tolerability.
Study Question: Do patients diagnosed with knee OA show a decrease in opioids or IA corticosteroid injections prescribed/administered in hospitals following hylan G-F 20 treatment?
Study Design: This case-crossover, retrospective study using Health Facts®, a de-identified electronic health records database, enrolled patients ≥ 18 years with knee OA treated with hylan G-F 20 between January 1, 2000 and March 31, 2016, with data within 6 months before/after treatment.
Measures and Outcomes: Primary endpoints compared days on opioids, amounts of opioids, and number of IA corticosteroid injections before/after hylan G-F 20 treatment via paired t-tests.
Results: A total of 513 patients were qualified for analysis. In the opioid cohort, the average total number of days on opioids (N = 50; 5.0 vs 13.5 days; P = 0.007) and average total amount of opioids (N = 44; 165.4 morphine mg equivalents [MME] vs 493.7 MME; P = 0.013) were lower 6 months after hylan G-F 20 treatment than 6 months before treatment. In the IA corticosteroid cohort, the average number of IA corticosteroid injections decreased after hylan G-F 20 treatment (N = 36; 0.56 in the 6-month follow-up vs 1.39 before treatment; P < 0.0001). Additional time frames of 1– 5 months before and after treatment were examined; similar conclusions were drawn for patients with > 2 months of data.
Conclusion: Patients with knee OA previously treated with opioids or IA corticosteroid injections who received hylan G-F 20 demonstrated statistically significant decreases in these medications > 2 months following hylan G-F 20 treatment versus > 2 months before treatment.
Keywords: corticosteroids, opioids, osteoarthritis, viscosupplementation, hyaluronic acid, hylan G-F 20
Introduction
Osteoarthritis (OA) of the knee is a painful condition affecting more than 250 million people worldwide and is a leading cause of disability.1 In a systematic analysis of 1160 sequelae of 289 diseases and injuries from 1990 to 2010, musculoskeletal disorders were the second largest contributor to years living with disability (YLDs) globally and in nearly all regions, causing nearly 21.3% of all YLDs. OA specifically contributed to 17.1 million YLDs, with OA of the knee, in particular, accounting for nearly 83% of total OA burden.1
Viscosupplementation, the intra-articular (IA) injection of a hyaluronic acid (HA) such as hylan G-F 20, may be used for the treatment of pain associated with knee OA in patients with inadequate response to initial therapies,2 which include exercise, weight reduction intervention in overweight individuals, simple analgesics (eg, acetaminophen), and non-steroidal anti-inflammatory drugs (NSAIDs).2–5 Hyaluronic acid has been shown to be safe and effective in managing the pain of knee OA.6 Evidence from surveys and trials shows that HA reduces pain and increases knee function, with benefits lasting longer than with IA corticosteroids;6,7 repeated injections demonstrated improvement in pain and function for up to 40 months.6 In addition, studies have shown that HA is associated with a low incidence of mild-to-moderate adverse events such as pain and swelling at the injection site6,7 and a lower risk of deep knee infections compared with IA corticosteroid injection.8
In the absence of disease-modifying treatments,7 a multimodal treatment approach, which may include IA corticosteroids and/or oral opioids2–4 as well as HA, is often recommended for the management of the pain associated with knee OA.9 However, opioids and IA corticosteroids are associated with concerns regarding safety and tolerability. Opioids, for example, are associated with a wide range of adverse events including nausea, vomiting, constipation, urinary retention, and respiratory depression, which can lead to death.10 In addition, opioid use may lead to dependence.11 Similarly, in addition to the increased risk for deep knee infections compared with IA HA,8 IA corticosteroids have been associated with gross cartilage damage and chondrocyte toxicity at higher doses (>3 mg/dose or 18–24 mg/cumulative total dose in vivo).12
Growing recognition of the issues with opioids and IA corticosteroid injections indicates that reducing the use of these agents might be beneficial to knee OA patients. Treatment with hylan G-F 20, a high molecular weight, cross-linked, injectable HA product may help to reduce the use of other pain medications such as opioids and corticosteroids in knee OA.13 The current retrospective study used electronic health records (EHRs) from a large US database to determine whether the days on opioids, the amount of opioids, or the number of IA corticosteroid injections changed following initial treatment with hylan G-F 20 in adult patients with knee OA.
Materials and Methods
Data Source
Data over a 15-year period (January 1, 2000 to March 31, 2016) were obtained from Health Facts® (Cerner Corp., North Kansas City, MO, USA), a Health Insurance Portability and Accountability Act (HIPAA)-compliant de-identified EHR database covering >60 million patients that is sourced from >600 participating facilities throughout the United States. The EHRs contained coded administrative records (eg, diagnostic, procedural, demographic, payer, and billing), data on drug administration while in a hospital or a clinic setting, as well as parameters from pharmacy, laboratory, microbiology, and hospital (including medical, surgical, and emergency department) systems. Patient records were linked through time as well as across facilities, allowing for longitudinal tracking of individual patients across all of the facilities covered by the database.
Study Population
Patients aged ≥18 years with data in the Health Facts database who were diagnosed with knee OA and had been treated with hylan G-F 20 (SYNVISC®; Genzyme Biosurgery, Cambridge, MA, USA) or hylan G-F 20 single (SYNVISC®; Genzyme Biosurgery, Cambridge, MA, USA) were included. Patients were excluded if they died within 6 months after the initial hylan G-F 20 treatment; had no recorded visits in the database within 6 months before or 6 months after the initial treatment (to ensure the patient was treated within facilities covered by the database); were treated with a viscosupplement other than hylan G-F 20; had a major surgical procedure prior to initial hylan G-F 20 treatment; had received an antidepressant; or had a prior or current diagnosis of fibromyalgia, rheumatoid arthritis, ankylosing spondylitis, osteonecrosis, other arthritis or collagen disease, or OA in other regions of the body. Exclusion of patients with these potentially confounding painful conditions and OA in other regions of the body was performed to reduce the potential of opioid and corticosteroid utilization for the treatment of conditions outside of knee OA.
Study Design
Patients’ index dates were determined at the initial hylan G-F 20 treatment, defined as either 1–3 injections of hylan G-F 20 (2 mL) over the course of 21 days or a single injection of hylan G-F 20 (6 mL). Availability of patient data was calculated as the period between the earliest patient visit in the 6 months before hylan G-F 20 injection and the first injection of hylan G-F 20 for the before-treatment period, and as the period from the third (or last if <3 injections of hylan G-F 20) injection within a 21-day period following the initial injection (hylan G-F 20), or the time between the initial treatment (hylan G-F 20 single) and either up to 6 months after, or patient dropout due to a major procedure, during the after-treatment period (Supplementary Figure 1).
Assessments
Primary endpoints measured the days on opioids (count of days that opioids were recorded as received, or covered by supply [outpatient] in the baseline or follow-up period), total amount of opioids used (in morphine mg equivalents [MME]), and total number of IA corticosteroid injections during the baseline (6 months before hylan G-F 20) and follow-up periods (6 months after hylan G-F 20). Six months’ time was considered since hylan G-F 20 is effective for at least 6 months. Opioid formulations with intravenous and oral routes of administration were included. There was no restriction on the amount of opioids; amounts for any given day were converted to MME and summed over each of the baseline and follow-up periods. Comparisons were also made for patients with data for ±1 through ±5 months before and after hylan G-F 20 treatment (Supplementary Figure 2). For each time frame, patients with records covering the specified time frame (eg, ± 1 month, ± 2 months, etc.) on either side of the index treatment date were analyzed.
Statistical Analysis
Primary endpoints included days on opioids, MME, and the number of IA corticosteroid injections during baseline (6 months prior) and following (6 months after) hylan G-F 20 treatment. Endpoints were compared via paired t-tests for 6 months pre- and post-treatment, as well as for other equivalent time periods pre- and post-treatment (eg, comparing 3 months before hylan G-F 20 with 3 months after), based on data availability (Supplementary Figure 2). A two-tailed P value < 0.05 was considered statistically significant. The paired t-tests were used for the differences in days on opioids, amount of opioids (MME), and the number of corticosteroid injections before and after index hylan G-F 20 treatment. The distribution of the differences between baseline and follow-up values was plotted to verify the assumptions of the paired t-test, and were found to be relatively symmetric.
Results
Disposition
The initial database analysis identified 2644 patients who had received either hylan G-F 20 or hylan G-F 20 single between January 1, 2000 and March 31, 2016; N = 1659 of these patients were diagnosed with knee OA. After applying inclusion and exclusion criteria, 513 patients were available for analysis (Patient attrition, Supplementary Figure 3; Patient demographics, Table 1). Of these 513 patients, 395 (77.0%) patients had >2 months of baseline data available and, on average, had between 5 and 6 hospital/clinic visits within 6 months prior to hylan G-F 20 treatment.
 |
Table 1 Patient Demographics, Comorbidities, and Concomitant Medication Use at Baseline |
Patient Demographics and Characteristics
The majority of patients were female (64.7%) and white (72.3%); average age was 61.7 years. The most common comorbid conditions were hypertension, diabetes, and obesity (Table 1). Of the 513 patients in the cohort, 72 (14.0%) had received opioids and 169 (32.9%) had received ≥1 IA corticosteroid injection within the 6 months before treatment with hylan G-F 20.
Opioid Use Following Hylan G-F 20 Treatment
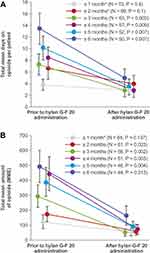
Among the 72 patients who received opioids within the 6 months before treatment with hylan G-F 20, 50 patients had 6 months each of baseline and follow-up data available. Among these patients, the mean total number of days on opioids 6 months after hylan G-F 20 treatment was statistically significantly lower than 6 months prior to hylan G-F 20 treatment (N = 50; 5.0 vs 13.5; P = 0.007). Forty-four of the 50 patients’ records included opioid amounts. Of these patients, the total amount of opioids per patient during the 6 months after index treatment with hylan G-F 20 was significantly lower than the 6 months prior to treatment (N = 44; 165.4 vs 493.7 MME; P = 0.013). Data for patients with <6 months of data before and after index treatment also showed significantly fewer days on opioids per patient after treatment with hylan G-F 20, with the exception of the group with data for 1 month before and after treatment with hylan G-F 20 (Figure 1A; subgroups are labeled based on the number of months of data available for analysis during each of the baseline and follow-up periods). Similar to the number of days on opioids per patient, significant differences were seen for reduced total amounts of opioids after versus before hylan G-F 20 treatment for patients with data for ≥2 months before and after treatment (Figure 1B).
IA Corticosteroid Injections Following Hylan G-F 20 Treatment
Among the 169 patients who received a corticosteroid injection prior to treatment with hylan G-F 20, 36 patients had 6 months of data both prior to and following index treatment. The mean number of IA corticosteroid injections per patient was significantly lower 6 months after hylan G-F 20 treatment than 6 months before among patients who received IA corticosteroid injections prior to hylan G-F 20 treatment (N = 36; 0.56 vs 1.39; P < 0.0001). Similar statistically significant results were found for all patient groups with data <6 months before and after treatment (Figure 2; subgroups are labeled based on the number of months of data available for analysis during each of the baseline and follow-up periods).
Discussion
In this analysis, adult patients with knee OA who were treated with opioids or IA corticosteroid injections before receiving hylan G-F 20 demonstrated significant decreases in the use of these medications after hylan G-F 20 treatment. These results are consistent with those of a retrospective review that found a reduction in oral pain medication for up to 6 months following treatment with hylan G-F 20.14 A recent study comparing the efficacy of HA with glucocorticoid injection in knee OA also demonstrated a significant reduction in Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scores and in concomitant therapy with analgesics/anti-inflammatory drug use, compared with baseline.15 In addition, a systematic review of 34 studies on long-term safety implications of common treatments for osteoarthritis of the knee has concluded that intra-articular HA and platelet-rich-plasma injections may provide additional relief of symptoms in these patients with no safety issues.16
Given that opioids and IA corticosteroids are often prescribed for patients with knee OA when initial therapies such as exercise and NSAIDs do not adequately alleviate pain, these results are particularly relevant. Opioids, for example, are not only associated with adverse events such as respiratory depression,10 but can lead to dependence,11 which is of particular concern given a recent report of an upward trend in opioid prescriptions for knee OA.15 Specifically, an analysis of Medicare Beneficiary Service data demonstrated a trend toward an increase in opioid prescriptions for knee OA from 2003 to 2009,17 suggesting that as the population ages and the number of people affected by symptomatic OA increases, the number of opioid prescriptions and the issues associated with them will continue to grow. Reducing the use of corticosteroids may also be advantageous. Some corticosteroids can elevate glucose levels in patients with diabetes,18 which is particularly relevant for this analysis because >12% of the patients included had comorbid diabetes. Furthermore, IA corticosteroids are less effective than IA HA in the long term (4–26 weeks),7 potentially increasing the risk of developing injection site infections8 and cartilage toxicity12 because of the need for more frequent injections.
Limitations
The limitations of this study include the fact that the database used for this analysis captures only prescriptions filled at hospital pharmacies, thus the amount of medication received may be underestimated. However, we believe that patients likely did not change the pharmacy locations where they filled prescriptions before and after hylan G-F 20 treatment. In addition, patient visits that took place outside the set of institutions serviced by Cerner were not captured in the analysis.
Conclusion
Data from this retrospective analysis showed that the use of opioids and IA corticosteroid injections decreased in the months following injection of hylan G-F 20 for the management of pain for knee OA among those who utilized these pain medications prior to treatment.
Data Sharing Statement
The data that support the findings of this study are available from the Cerner Health Facts® database (https://sc-ctsi.org/resources/cerner-health-facts), although institutional restrictions apply to the availability of these data, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Cerner Corp.
Acknowledgments
The authors would like to acknowledge the contributions of Wilson Ngai (Sanofi) for his review and valuable feedback on the manuscript draft. This study was sponsored by Sanofi. Assistance with the writing and development of the manuscript was provided by Deborah Stull of Evidence Scientific Solutions, Philadelphia, PA, and was funded by Sanofi.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
XZ, SM, and FP are consultants for Boston Strategic Partners, Inc., and received fees from Sanofi for conducting this research. VK was an employee of Boston Strategic Partners, Inc. at the time of the study. FR is a full-time employee and shareholder of Sanofi, Inc. The authors report no other conflicts of interest in this work.
References
1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
2. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–474. doi:10.1002/acr.21596
3. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571–576. doi:10.5435/JAAOS-21-09-571
4. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi:10.1016/j.joca.2014.01.003
5. National Institute for Health and Care Excellence (NICE). Osteoarthritis: care and management. Available from: https://www.nice.org.uk/guidance/cg177.
6. Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4):S28–33. doi:10.1016/j.semarthrit.2015.11.008
7. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–1711. doi:10.1002/art.24925
8. Xu C, Peng H, Li R, et al. Risk factors and clinical characteristics of deep knee infection in patients with intra-articular injections: a matched retrospective cohort analysis. Semin Arthritis Rheum. 2018;47(6):911–916. doi:10.1016/j.semarthrit.2017.10.013
9. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15(9):981–1000. doi:10.1016/j.joca.2007.06.014
10. Kane-Gill SL, Rubin EC, Smithburger PL, Buckley MS, Dasta JF. The cost of opioid-related adverse drug events. J Pain Palliat Care Pharmacother. 2014;28(3):282–293. doi:10.3109/15360288.2014.938889
11. Campbell G, Nielsen S, Larance B, et al. Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the Pain and Opioids IN Treatment (POINT) cohort. Pain Med. 2015;16(9):1745–1758. doi:10.1111/pme.12773
12. Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3:2325967115581163.
13. McIntyre LF, Beach W, Bhattacharyya S, Yadalam S, Bisson B, Kim Y. Impact of hyaluronic acid injections on pain management medications utilization. Am J Pharm Benefits. 2017;9:195–199.
14. Waddell DD, Bricker DC. Clinical experience with the effectiveness and tolerability of hylan G-F 20 in 1047 patients with osteoarthritis of the knee. J Knee Surg. 2006;19(01):19–27. doi:10.1055/s-0030-1248072
15. Parisi S, Ditto MC, Priora M, et al. Ultrasound-guided intra-articular injection: efficacy of hyaluronic acid compared to glucocorticoid in the treatment of knee osteoarthritis. Minerva Med. 2019;110(6):515–523. doi:10.23736/S0026-4806.19.06190-1
16. Charlesworth J, Fitzpatrick J, Perera NKP, Orchard J. Osteoarthritis – a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet Disord. 2019;20(1):151. doi:10.1186/s12891-019-2525-0
17. Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014;66(10):1489–1495. doi:10.1002/acr.22360
18. Aleem AW, Syed UAM, Nicholson T, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the subacromial space of the shoulder. Arch Bone Jt Surg. 2017;5(5):315–321.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.