Back to Journals » Infection and Drug Resistance » Volume 15
Retrospective Analysis of the Risk Factors of Perioperative Bacterial Infection and Correlation with Clinical Prognosis in Kidney Transplant Recipients
Authors Cheng F, Li Q, Wang J, Wang Z, Zeng F, Zhang Y
Received 10 January 2022
Accepted for publication 15 April 2022
Published 28 April 2022 Volume 2022:15 Pages 2271—2286
DOI https://doi.org/10.2147/IDR.S356543
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Fang Cheng,1,2 Qiang Li,1,2 Jinglin Wang,1,2 Zhendi Wang,3 Fang Zeng,1,2 Yu Zhang1,2
1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, 430022, People’s Republic of China; 3Department of Urology Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
Correspondence: Fang Zeng; Yu Zhang, Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China, Email [email protected]; [email protected]
Background: Infection remains a leading cause of morbidity and mortality in kidney transplant patients. This study aimed to investigate the risk factors of bacterial infection during the perioperative period of transplantation and the effects of infection on long-term clinical outcomes.
Methods: In total, 295 kidney transplantation recipients were included in this retrospective study and assigned to two groups: non-infected and infected. The tacrolimus concentration, pharmacogenomics, laboratory parameters, and clinical outcomes of both groups were evaluated.
Results: A relatively low incidence of urinary tract infection was observed in our cohort, and lung was identified as the most frequent site of infection. Gram-negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae, were the most common infecting strains in kidney transplant recipients. Patients with diabetes showed greater susceptibility to infection. Compared with the non-infected group, tacrolimus concentration was significantly lower on day 7 and 14 in the infected group. White blood cell count, neutrophil count, and C-reactive protein (CRP) in the infected group were markedly higher post-transplantation, while albumin levels were lower relative to the non-infected group. ABCB1 (rs2032582) genotype showed clear associations with infection. Furthermore, the incidence of delayed graft function (DGF) and early acute rejection (AR) before infection was significantly greater in the infected group. Finally, early post-transplant infection was associated with a marked increase in the incidence of AR, post-transplant diabetes mellitus (PTDM), and secondary infection.
Conclusion: Pre-diabetes, longer duration of catheterization, lower albumin, higher CRP, tacrolimus concentration on the day 7 and 14, early AR before infection, and DGF were closely related to postoperative infection in kidney transplantation recipients. Moreover, bacterial infection during the perioperative period was closely associated with AR, PTDM and secondary infection.
Keywords: kidney transplantation, immunotherapy, bacterial infection, risk factors, adverse reactions
Introduction
Kidney transplantation is an effective management option for end-stage renal disease. However, due to poor preoperative basic conditions, operative and related postoperative factors, and use of immunosuppressants, the risk of infection in kidney transplantation recipients is significantly increased relative to the general population.1,2 The reported incidence of infection complications after kidney transplantation is between 49% and 80%.3 Although the prognosis of kidney transplant patients has improved significantly over the past few decades, infection remains a leading cause of morbidity and mortality in this patient population, accounting for approximately 15–20% of overall deaths.4,5
The risk of infection in kidney transplantation patients depends on their net immunosuppression status and epidemiologic exposure.6 A major limitation in clinical care is the inability to measure the degree of immunosuppression of specific immune cells in patients, and therefore the risk of infection cannot be predicted. Previous study suggested that infection is the most common cause of death in the early stages after kidney transplantation, predominantly occurring within the first month.5 Additionally, kidney transplant recipients often develop complications such as diabetes, anemia, and renal failure, which further increase the risk of infection. Patients with diabetes mellitus are commonly considered at high risk of infections, and several studies have validated the link between diabetes and infection risk.7,8 This increased susceptibility to infections may be mediated through defects in host immune defense mechanisms, including impairment of neutrophil function, which plays an essential role in defense, especially against bacterial pathogens.9 Anemia is additionally reported to increase susceptibility to infection by suppressing the immunological response to pathogens.10 Epidemiological studies have confirmed strong associations between severe anemic and invasive bacterial infections, in particular with NTS bacteremia.11 Simultaneous infection with viruses, such as cytomegalovirus and Epstein-Barr virus, is another issue of concern.12 Therefore, early identification, antibiotic treatment for early infection after kidney transplantation and timely individualized adjustment of immunosuppressant agents are necessary to further improve prognosis.
Increased risk of infection is also attributable to the lifelong immunosuppression required to prevent graft rejection. Calcineurin inhibitors (CNIs), such as tacrolimus, are the most commonly used drugs to prevent graft rejection after kidney transplantation.13 However, optimization immunosuppression regimens for kidney transplantation recipients remain a considerable clinical challenge. Data from the 2016 report of the OPTN/SRTR highlight a 1-year post-transplant AR rate of 11.4%–12%, which has remained relatively stable over the last 6 years.14 AR is a risk factor for chronic kidney allograft dysfunction (CKAD), affecting nearly 50% patients within 5 years, and remains the predominant cause of death within the first year after transplantation.15 The immunosuppressive regimen for kidney transplant patients comprises high doses of tacrolimus combined with mycophenolate mofetil and prednisone, which has been linked with increased rates of infection and malignancy owing to effective suppression of adaptive immunity.16 However, few studies have evaluated the effect of reducing or even terminating immunosuppressants treatment during bacterial infection on clinical outcomes (rejection and graft survival). In the current investigation, we aimed to establish the risk factors of bacterial infection in kidney transplantation recipients during the perioperative period as well as the effects of infection on graft function. Changes in blood concentration of tacrolimus during the peri-operation period of kidney transplantation were further determined, with a view to find out the safety range.
Methods
Patient Selection
This study was a retrospective study, including 295 kidney transplant patients admitted to Union Hospital, Huazhong University of Science and Technology, from January 2015 to May 2019. Enrolment criteria were administration of tacrolimus, mycophenolate mofetil, and corticosteroids after kidney transplantation, patients age of 18–70 years, first kidney transplantation, and availability of complete clinical data. Exclusion criteria were age <18 years or >70 years, combined with other organ transplantations, exposure to cyclosporine or intravenous tacrolimus, co-administration of azole antifungal agents, or missing data. Ultimately, 295 patients receiving kidney transplants from donations after cardiac death (DCD) were recruited for the study. DCD kidneys were regionally distributed within the organ sharing network system of China, which is similar to the United Network for Organ Sharing. All DCD procedures were performed by the Organ Procurement Organization of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, in accordance with the Declaration of Istanbul. The study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) ([2018] S331). Because this investigation did not interfere with patients’ diagnosis or the treatment process, only verbal informed was required from all patients and approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Our research conformed to the ethical standards of the Declaration of Helsinki. Informed consent from the donor or their next of kin was obtained for all transplanted kidneys. We obtain patients’ information, including demographic data, clinical symptoms, underlying comorbidities, laboratory tests and clinical medications administered during the perioperative period, through the electronic medical record system. Adverse reactions and graft function were determined through patient follow-up.
Medication Regimens
All patients received a triple immunosuppressive regimen based on tacrolimus (tacrolimus, mycophenolate mofetil, and corticosteroids). Oral administration of tacrolimus was initiated on the second day after transplantation, starting with an initial dose of 3.0–5.0 mg, twice a day. Mycophenolate mofetil was administered at a dose of 0.5–1.0 g twice a day on the day of transplantation. In all patients who had acute rejection during hospitalization, methylprednisolone pulse therapy was administered.
Anti-Infection Treatment
The perioperative prophylactic antibiotics used were cefoperazone-sulbactam or piperacillin-tazobactam. Antibiotics were discontinued on 7–8 days post-transplant if the patients had no symptoms or normal laboratory parameters of infection were observed. When patients had fever or confirmed bacterial infection in combination with the test indicators, empirical antimicrobial therapy was initiated. In addition, blood, sputum, urine, and abdominal drainage fluid were collected for microbiological analysis. Second-generation and third-generation cephalosporins are commonly used for empirical antimicrobial therapy. For multi-drug-resistant bacteria infection cases, antibiotics were upgraded to tigecycline, vancomycin, meropenem, linezolid, and polymyxin B. When a fungal infection was suspected on chest X-ray, pathogen culture, or (1-3)-β-D-glucan and galactomannan testing (G/GM test), antifungal therapy with caspofungin or micafungin was employed. Patients showing rapid disease progression were intubated in the intensive care unit for continuation of treatment.
Therapeutic Drug Monitoring (TDM)
After kidney transplantation, the tacrolimus concentration was measured three times weekly. If rejection or adverse events are suspected, the frequency is increased. The tacrolimus concentration measured in whole blood with a Roche cobas®e411 electrochemiluminescence (ECL) analyzer ranges from 0.5 to 40 ng/mL. The target trough concentration (C0) of tacrolimus in our hospital in the first month ranged from 8 to 10 ng/mL.
Genotypes
Peripheral blood samples were genotyped using a Capital Biotechnology Precision Medicine Research Array Kit (Thermo Fisher Scientific, Waltham, MA, USA) using the Applied Biosystems Axiom 2.0 platform. We analyzed SNPs shown to affect tacrolimus metabolism in previous studies, including CYP3A4*22 (rs35599367), CYP3A4*1B (rs2740574), CYP3A5*3 (rs776746), ABCB1 3435C>T (rs1045642, and rs2032582), ABCC2 (rs3740066, and rs2273697), PXR (rs6785049) and POR*28 (rs1057868), that could explain residual pharmacokinetic variability.
Clinical Outcomes
Primary outcomes were clinical characteristics of perioperative infection, including incidence, site of infection, causative pathogens, and antibiotics. Secondary outcomes were clinical outcomes after infection in terms of acute rejection and CKAD. Post-transplant diabetes mellitus (PTDM), diarrhea, and liver dysfunction were the most common adverse reactions.
Perioperative infection was defined as the first bacterial infection during the perioperative period after kidney transplantation. The sites of infection were identified as pulmonary, intra-abdominal, urinary tract, surgical site and blood stream. Diagnosis and treatment of different infections in kidney transplant recipients during the perioperative period were performed according to the “Diagnostic Criteria for Nosocomial Infections” implemented by the Ministry of Health in 2001:17 (1) For pulmonary infection: with corresponding clinical manifestations, such as fever, recent cough and fatigue, chest tightness and shortness of breath, imaging examination were conducted. Chest computed tomography (CT) disclosed patchy high-density shadows. Sputum culture revealed pathogenic bacteria, consistently higher or lower of white blood cells than normal (>10*109/L or <4*109/L), and significantly higher levels of C-reactive protein relative to normal values; (2) Symptoms for intra-abdominal infection were abdominal pain, diarrhea, vomiting, and pathogenic bacteria in stool culture; (3) Urinary tract infection were diagnosed based on fever, frequent urination, and urgency of urination, continuously higher or lower white blood cell counts than normal, presence of pathogenic bacteria in urine culture, and significantly higher C-reactive protein level than the normal value; (4) Surgical site infection was diagnosed based on clinical signs of purulence or fever in addition to one of the symptoms of redness, edema, or pain, with confirmation by the surgeon; (5) Bacteremia or sepsis was diagnosed in cases of body temperature higher than 38.5°C, organ dysfunction, shock, and presence of pathogenic bacteria in blood culture.
AR is defined as an acute deterioration of graft function confirmed by graft biopsies within the first year after transplantation. Cases of acute rejection before infection were classified as early acute rejection. CKAD was defined as a slow change in the function of transplanted kidneys, manifesting as a gradual increase in blood creatinine and urea nitrogen. The individual adverse reactions recorded were PTDM, secondary infections, liver dysfunction and diarrhea. PTDM was defined as one or more of the following factors after transplantation (≥1 month): (1) fasting blood glucose ≥7.0 mmol·L−1 at least twice; (2) random blood glucose ≥11.1 mmol·L−1 at least twice; (3) receiving diet control regimens or hypoglycemic medication. Secondary infection is defined as a second infection event after discharge.18
Statistical Analysis
Categorical variables were calculated as frequency and percentage, and compared with the χ2 test. In case where the values were less than 5, Fisher’s exact test was selected for use. When continuous data were normally distributed, the independent group t test was performed, and data expressed as mean ± standard deviation. Otherwise, the Mann–Whitney test was performed and data expressed as median and interquartile range values. Genotype distribution was evaluated with Hardy-Weinberg equilibrium. Repeated measures analysis of variance was applied to compare differences in the blood concentrations of tacrolimus and results of laboratory tests between the two groups at different times after renal transplantation. Binary logistic regression was performed to identify independent risk factors affecting the infection results. All statistical analyses were performed using SPSS version 24.0 software, with differences considered significant at P value <0.05.
Results
Patient Characteristics
A total of 393 kidney transplantation patients were initially assessed, following which 4 patients with combined organ transplantation, 10 with secondary organ transplantation, 34 received cyclosporine A, 8 received azole antifungal, and 42 lost to follow-up were excluded. In total, 295 kidney transplant recipients were included for the study. All recipients underwent transplantation for the first time, with DCD used as a source of kidneys.
Demographic and clinical characteristics of the recipients and donors are presented in Table 1. Among the 295 recipients, 85 (28.8%) patients experienced infection during the perioperative stage, of whom 54 (63.5%) were male and 31 (36.5%) were female. The median time of infection occurrence was 6 (4–14) days after the operation. In total, 292 (99.0%) recipients received dialysis treatment before the operation, predominantly hemodialysis and peritoneal dialysis. No significant differences in dialysis type and duration were observed between the non-infected and infected groups. The median age of the non-infected group was 42.0 ± 11.04 years; that of the infected group was 43.0 ± 11.80 years. The length of stay for the two groups was as follows: non-infected group, 19 days (range: 18.0–24.0 days); and infected, 22 days (range: 20.0–29.0 days). However, the proportions of patients admitted into ICU for further treatment were significantly different between the non-infected and infected groups (4.8% vs 21.2%). Underlying disease complications were recorded in a number of patients, with hypertension (N = 266 [90.2%]), anemia (N = 119 [40.3%]), diabetes (N = 14 [4.7%]), hepatitis B (N = 15 [5.1%]), arthrolithiasis (N = 8 [2.7%]), and coronary heart disease (N = 7 [2.4%]) identified as the most common pre-existing conditions. Kidney transplant patients with anemia and diabetes were more susceptible to infection. A urethral catheter and D-J stent were placed during kidney transplantation. Duration of catheterization of the two groups, non-infected group, 14 days (range: 14.0–14.0 days); infected group, 16 days (range: 14.0–19.0 days), was significantly different. Postoperative D-J stent placement duration was around 30 days. Preoperative panel-reactive antibody-I (PRA-I) > 10% and PRA-II > 10% were detected in 11(3.7%) and 14(4.7%) patients, respectively. In terms of HLA-A, B, and DR matching of donors and recipients, 2(0.7%) cases of five antigen mismatches, 6 (2.0%) cases of four antigen mismatches, 113(38.3%) cases of three antigen mismatches, 139(47.1%) cases of two antigen mismatches, and 25(8.5%) case of one antigen mismatch, and 10(3.4%) cases of no antigen mismatch were recorded.
 |
Table 1 Demographic Characteristics of Kidney Transplant Recipients and Donors |
DGF occurred in 41 (8.8%) of the 295 patients during the first week after transplantation. The incidence of DGF was significantly higher in the infection group. AR occurred in 26 (8.8%) patients, of whom 9 (3.1%) reached the acute rejection end point first. The incidence of early AR was significant difference. While recipients’ and donors’ sex, age, body mass index, and other parameters were comparable between the two groups.
Characteristics of Perioperative Infection in Kidney Recipients
The distributions of infection episodes according to infectious sites are shown in Table 2. Pulmonary infection (45.9%) was the most frequent perioperative infection, followed by intra-abdominal (21.2%) and urinary tract infection (17.6%). Notably, the incidence of urinary tract infection at our center was relatively low compared with previous literature reports. Causative agents and treatment choices for infection is shown in Table 3. A total of 37 cases of bacterial strain were isolated from recipients. Among the 12 cases (32.4%) of gram-positive bacteria, Staphylococcus aureus was the predominant species of gram-positive bacteria; 25 cases (67.6%) of gram-negative bacteria, and Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa were predominant. Among the 85 infected recipients, 15 were treated with at least two antibiotics. Cefoperazone-sulbactam (37.6%) was the most commonly prescribed antibiotic regimen, followed by piperacillin-tazobactam (30.6%), carbapenems (28.2%), linezolid (10.6%), vancomycin (7.1%), and polymyxin B (3.5%).
 |
Table 2 Incidence of Infection at Different Sites During the Perioperative Period of Renal Transplantation |
 |
Table 3 Causative Agents of Infection and Treatment Choices |
Effect of Immunosuppressive Drugs on Perioperative Infection
Immunosuppressive drugs and tacrolimus concentration are shown in Table 4. In total, 260 (88.1%) patients received induction therapy with antithymocyte globulin, whereas basiliximab was used in 35(11.9%) patients. Mycophenolate mofetil was prescribed for 283 (95.9%) patients as an antiproliferative agent. We observed no significant differences between the immunosuppressive drug groups in terms of infection burden. In addition, the early tacrolimus concentration at days 7 and 14 post-transplantation in the infected group was significantly lower than that in the non-infected group.
 |
Table 4 Effects of Immunosuppressive Drugs on Infection After Kidney Transplant |
Effect of Laboratory Findings on Perioperative Infection
Repeated measures analysis of variance was applied to compare laboratory findings, including blood routine, kidney function, CRP and PCT between the two groups (Figures 1–4). Notably, significant differences in kidney function indexes (Cre, BUN and uric acid) were observed from day 3 post-transplantation. Furthermore, we focused on the associations between relevant laboratory indexes (baseline and first day after transplantation) and infection (Table 5). The results disclosed no significant difference in the baseline values of laboratory findings between the two groups before kidney transplantation. However, some indexes were significantly different following transplantation. In terms of blood indexes between the two groups, WBC and Neu in the infected group were significantly higher relative to those in the non-infected group post-transplantation. In addition, we observed significant difference in albumin (ALB) level between the two groups. CRP and PCT in the non-infected group were significantly higher than those in the infected group following transplantation.
 |
Table 5 Effects of Laboratory Parameters on Infection After Kidney Transplant |
Pharmacogenetic Analysis
We further investigated whether polymorphisms affected infection risk in kidney transplantation recipients. All polymorphisms met the conditions of Hardy‐Weinberg equilibrium (P > 0.05, Table S1). Notably, no mutations in the CYP3A4*1B allele were detected, and only two of the 295 patients carried the minor allele of CYP3A4*22 in the 295 patients. Differences in these polymorphisms were compared between the two groups (Table 6). ABCB1 (rs2032582) genotype displayed a clear association with infection risk (P = 0.032), where the frequency of the TT/CC genotype was significantly higher in the infected (63.5%) than the non-infected group (45.7%). No significant differences were observed for the other alleles.
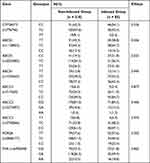 |
Table 6 Pharmacogenetics Analysis of Non-Infected and Infected Groups |
Prognostic Factors of Infection in Kidney Transplantation Recipients
Anemia, diabetes, length of stay, ICU treatment, duration of catheterization, tacrolimus concentration on days 7 and 14, WBC, Neu, ALB, CRP, PCT on the first day post-transplantation, early AR, DGF and ABCB1 (rs2032582) were factors influencing the incidence of infection. These 15 factors were selected for subsequent logistic regression analyses. Diabetes (OR: 1.430, 95CI%: 1.211–2.476), duration of catheterization (OR: 1.687, 95CI%: 1.044–2.724), tacrolimus concentration on day 7 (OR: 0.869, 95% CI: 0.753–0.974), tacrolimus concentration on day 14 (OR: 0.963, 95% CI: 0.872–0.981), albumin (OR: 0.886, 95% CI: 0.797–0.986), CRP (OR: 1.489, 95% CI: 1.307–1.890), early AR (OR: 3.004, 95% CI: 2.865–4.352), and DGF (OR: 3.763, 95% CI: 2.168–8.365) were the most useful independent risk factors for early post-transplant infection (Table 7).
 |
Table 7 Logistic Regression Analysis of Risk Factors Associated with Infection in Kidney Transplant Recipients |
Effect of Perioperative Infection on Clinical Outcomes of Kidney Transplantation
All adverse reactions are listed in Table 8. AR occurred in a total of 26 (8.8%) patients, and 17 (5.8%) patients displayed rejection after infection. While 9 (10.6%) of the recipients were in the infected group, 8 (3.8%) of the recipients were in the non-infected group (P = 0.024). As for CKAD, 11 (12.9%) in the infected group and 17 (8.1%) in the non-infected group. Secondary infections and PTDM were the most frequent adverse reactions in all patients. The differences in rates of secondary infections (31.8% vs 9.5%, p < 0.001) and PTDM (11.8% vs 1.9%, p < 0.001) were significant between the groups. In contrast, no significant differences were observed in the incidence of liver dysfunction (4.0% vs 2.4%, p = 0.582) and diarrhea (5.9% vs 3.3%, p = 0.316) between the groups.
 |
Table 8 Comparison of Adverse Reactions Between Non-Infected and Infected Groups |
Discussion
Bacterial infections constitute the majority of symptomatic infections (accounting for 70%) after transplantation.19,20 However, efficient diagnosis of bacterial infections and related complications after kidney transplantation remains a considerable challenge. Early regulation of the factors influencing postoperative infections and formulation of preventive measures is particularly important. Here, we focused on the risk factors of bacterial infection in kidney transplantation recipients during the perioperative period and the effects of infection on clinical outcomes, taking into consideration relevant factors, such as recipients’ and donor’ characteristics, laboratory parameters, genotypes, and clinical outcomes.
Urinary tract infections are reported to be more common in female recipients,21,22 potentially due to the female urethra being shorter and closer to the anus, and therefore more easily affected by intestinal flora, such as E. coli, leading to retrograde infection.23 However, a number of studies have suggested that there is no difference between genders.24,25 In our center, a relationship between infection and gender was not observed, which could be attributed to the low incidence of urinary tract infection in the patient population. Placement of the D-J tube during kidney transplantation could be used to protect against vesicoureteral anastomosis, which may be a potential risk factor for early urinary tract infection after kidney transplantation.26 In previous studies, shortening the placement time of D-J tube led to reduced incidence of urinary tract infection and did not increase the occurrence of urinary leakage or ureteral obstruction.27 Urinary catheter is another reported risk factor for urinary tract infection, and the risk of urinary tract infection increases by 3% to 7% for patients with urinary catheter indwelling for more than 1 day after kidney transplantation.28 Consistently, in our experiments, catheter indwelling time was an independent risk factor for perioperative infection after kidney transplantation. Patients with diabetes mellitus are generally considered at high risk of infection, as supported by evidence from animal models demonstrating pathophysiological interactions between hyperglycemia and the development of infection.8,29 We additionally identified pre-diabetes as a factor related to infection in kidney transplantation recipients.
C-reactive protein (CRP) is a non-specific acute-phase reaction protein for tissue injury.30 When the body is in a state of inflammation or injury, the CRP concentration increases within 6–8 hours, and reaches peak levels within 24–48 hours. The CRP value not affected by gender, age, anemia or hyperglobulinemia.31 Albumin is mainly used as a surrogate biomarker for diagnosing malnutrition, and also serves as a marker of illness severity based on significantly decreased levels during states of inflammation and disease.32 Albumin provides protection from inflammatory processes and associated damage to microcirculation and tissues.33 Hypoalbuminemia, defined as serum albumin concentration <3.5 g/dL, is associated with risk of infection in the general population, including patients with chronic kidney disease or ESRD, and those requiring dialysis.34,35 Moreover, low pre- and post-transplant serum albumin levels are significantly linked with delayed graft function, increased graft failure, and all-cause mortality in kidney transplantation recipients.36,37 Our results supported the potential utility of CRP, and albumin as potential indicators of infection.
DGF is reported to increase the risk of urinary tract infections after kidney transplantation.38 Patients with DGF require dialysis treatment and adjustment of the immunosuppressive regimen, and longer hospital stay are associated with an increased risk of urinary tract infection.39 Recipients with AR are more likely to develop infection, which may be attributed to greater immunosuppression intensity, resulting in immune trauma and low immune response of the host to pathogens.40 Early S. aureus (mainly respiratory) infection has been associated with a higher incidence of acute rejection in lung transplantation.41 In our study, DGF and early AR were identified as independent risk factors of infection during the perioperative period.
The ABCB1 (rs1045642) polymorphism has been shown to be correlated with H. pylori infection.42 In contrast, our results suggested that the TC, mutant genotype of ABCB1 2677A/T SNP, was associated with protection against infection; however, we observed no significant difference in multivariate analysis. Given the potential utility of the ABCB1 genotype in evaluating post-transplant infection risk, further larger-scale studies of this association are warranted.
Currently, individualized adjustment of immunosuppressive agents for kidney transplant recipients with bacterial infection is a research hotspot. While the improvement of immunosuppressive programs has significantly increased the survival rates of transplant patients, this strategy is associated with a higher risk of opportunistic infections. Several earlier studies investigated the interactions between immunosuppressants and antimicrobial agents in transplant recipients.43,44 For example, some antibiotics promote the metabolism of tacrolimus, which suppresses the levels of specific doses of immunosuppressants, thus increasing the risk of rejection.45 However, down-regulation of metabolism can lead to increased blood levels of immunosuppressants at a given dose, potentially resulting in nephrotoxicity, excessive immunosuppression, and increased risk of life-threatening infection. Overuse of immunosuppressive agents may trigger adverse events such as kidney injury, metabolic syndrome and acute infection.46 The issue of whether to increase or decrease the doses of immunosuppressive agents in kidney transplant recipients is therefore a topic of controversy.47 Currently, the mainstream view is that it may be safer to reduce the use of immunosuppressants in kidney transplant recipients with infection. The Chinese Society of Organ Transplantation of the Chinese Medical Association (CSOT) highlighted that for the infected recipients, the immunosuppressants should be adjusted in time, and combination drug administration reduced. When the infection develops seriously, the immunosuppressant can be temporarily discontinued. Reducing the dose of immunosuppressant is associated with a better prognosis for infected recipients. In our hospital, oral tacrolimus treatment is usually initiated on day 2 post-transplantation. The tacrolimus blood concentration should be limited to 8–10 ng/mL in the first month, and the dose reduced in cases where the recipient has tested positive for infection. Our results showed that tacrolimus concentration was significantly lower in the infected group on day 7 and 14 post-transplantation compared with the non-infected group, which requires further validation.
Post-transplant infection is clearly associated with poor clinical prognosis, such as increased mortality and higher risk of graft loss.48,49 Our finding suggested that early infection increased the risks of acute rejection, PTDM, and secondary infection. Some studies have reported that infections trigger rejection in different transplant settings.50,51 Moreover, patients with diabetes mellitus may be at higher risk of infection-related mortality than the general population.52,53 Therefore, regular monitoring of the kidney function of patients who have experienced infection is essential to prevent adverse clinical events. The mechanisms underlying the relationship between infection and poor clinical outcomes of kidney transplant recipients are currently unclear but may be related to increased allogeneic and proinflammatory responses induced by infection, which requires further study.
The main limitation of our analysis was that long-term outcomes, such as graft failure and death were not considered, which should be followed up for evaluation. Moreover, our study was a single-center design involving only a small number of patients. Larger-scale studies over the longer term are warranted to further confirm the validity of our results. In addition, treatment information for patients in the ICU, such as intubation time, was unavailable and thus excluded from our analyses.
Conclusion
A relatively low incidence of UTI was recorded in our cohort, and the most common site of infection was the lung. Furthermore, pre-diabetes, longer duration of catheterization, lower albumin, higher CRP, tacrolimus concentration on day 7 and 14, early AR before infection, and DGF were independent risk factors for postoperative infection in kidney transplantation recipients. Finally, early post-transplant infection significantly increased the incidence of acute rejection, PTDM, and secondary infection. The collective results provide valuable insights into the factors contributing to early infection in kidney transplant recipients. We strongly recommend that individual transplant centers should explore their own infection risk factors and causative agents for optimal management of their patients.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author Yu Zhang ([email protected]) on reasonable request.
Ethical Approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional ethics board of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) ([2018] S331).
Funding
This work was supported by the Key R&D Program of Hubei Province (No.2020BCA060).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Port FK, Merion RM, Roys EC, Wolfe RA. Trends in organ donation and transplantation in the United States, 1997–2006. Am J Transpl. 2008;8(4 Pt 2):911–921. doi:10.1111/j.1600-6143.2008.02170.x
2. Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transpl. 2015;15(12):3024–3040. doi:10.1111/ajt.13486
3. Fishman JA. Infection in organ transplantation. Am J Transpl. 2017;17(4):856–879. doi:10.1111/ajt.14208
4. Howard RJ, Patton PR, Reed AI, et al. The changing causes of graft loss and death after kidney transplantation. Transplantation. 2002;73(12):1923–1928. doi:10.1097/00007890-200206270-00013
5. Kinnunen S, Karhapää P, Juutilainen A, Finne P, Helanterä I. Secular trends in infection-related mortality after kidney transplantation. Clin J Am Soc Nephrol. 2018;13(5):755–762. doi:10.2215/CJN.11511017
6. Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients–an analysis of USRDS data. Am J Transpl. 2007;7(3):653–661. doi:10.1111/j.1600-6143.2006.01674.x
7. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513–521. doi:10.2337/dc17-2131
8. Hayer MK, Farrugia D, Begaj I, Ray D, Sharif A. Infection-related mortality is higher for kidney allograft recipients with pretransplant diabetes mellitus. Diabetologia. 2014;57(3):554–561. doi:10.1007/s00125-013-3124-5
9. Simonsen JR, Järvinen A, Harjutsalo V, Forsblom C, Groop PH, Lehto M. The association between bacterial infections and the risk of coronary heart disease in type 1 diabetes. J Intern Med. 2020;288(6):711–724. doi:10.1111/joim.13138
10. Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338(6108):768–772. doi:10.1126/science.1224577
11. Mooney JP, Galloway LJ, Riley EM. Malaria, anemia, and invasive bacterial disease: a neutrophil problem?. J Leukoc Biol. 2019;105(4):645–655. doi:10.1002/JLB.3RI1018-400R
12. Helanterä I, Schachtner T, Hinrichs C, et al. Current characteristics and outcome of cytomegalovirus infections after kidney transplantation. Transpl Infect Dis. 2014;16(4):568–577. doi:10.1111/tid.12247
13. Jouve T, Noble J, Rostaing L, Malvezzi P. An update on the safety of tacrolimus in kidney transplant recipients, with a focus on tacrolimus minimization. Expert Opin Drug Saf. 2019;18(4):285–294. doi:10.1080/14740338.2019.1599858
14. Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 annual data report: kidney. Am J Transpl. 2018;18(Suppl1):18–113. doi:10.1111/ajt.14557
15. Pallardó Mateu LM, Sancho Calabuig A, Capdevila Plaza L, Franco Esteve A. Acute rejection and late renal transplant failure: risk factors and prognosis. Nephrol Dial Transpl. 2004;19(Suppl 3):iii38–iii42. doi:10.1093/ndt/gfh1013
16. Song GG, Lee YH. Comparison of treatment response and serious infection using tacrolimus, tacrolimus with mycophenolate mofetil, in comparison to cyclophosphamide as induction treatment for lupus nephritis. Int J Clin Pharmacol Ther. 2020;58(10):550–556. doi:10.5414/CP203736
17. Ministry of Health, PRC. Diagnostic criteria for nosocomial infection (Trial). Chin Med J. 2001;05(61–67):10–12.
18. Hjelmesaeth J, Jenssen T, Hartmann A. Diagnosing PTDM. Transplantation. 2003;75(10):1761. doi:10.1097/01.TP.0000064543.07931.F6
19. Illesy L, Szabo-Pap M, Toth F, et al. Bacterial infections after kidney transplantation: a single-center experience. Transpl Proc. 2016;48(7):2540–2543. doi:10.1016/j.transproceed.2016.07.011
20. Pourmand G, Salem S, Mehrsai A, Taherimahmoudi M, Ebrahimi R, Pourmand MR. Infectious complications after kidney transplantation: a single-center experience. Transpl Infect Dis. 2007;9(4):302–309. doi:10.1111/j.1399-3062.2007.00229.x
21. Goldman JD, Julian K. Urinary tract infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl. 2019;33(9):e13507. doi:10.1111/ctr.13507
22. Ariza-Heredia EJ, Beam EN, Lesnick TG, Kremers WK, Cosio FG, Razonable RR. Urinary tract infections in kidney transplant recipients: role of gender, urologic abnormalities, and antimicrobial prophylaxis. Ann Transpl. 2013;18:195–204. doi:10.12659/AOT.883901
23. Dharnidharka VR, Agodoa LY, Abbott KC. Effects of urinary tract infection on outcomes after renal transplantation in children. Clin J Am Soc Nephrol. 2007;2(1):100–106. doi:10.2215/CJN.01820506
24. Greissman S, Mattiazzi A, Mendoza M, et al. Antimicrobial resistance and recurrent bacterial urinary tract infections in hospitalized patients following kidney transplantation: a single-center experience. Transpl Infect Dis. 2020;22(4):e13337. doi:10.1111/tid.13337
25. Bodro M, Sanclemente G, Lipperheide I, et al. Impact of urinary tract infections on short-term kidney graft outcome. Clin Microbiol Infect. 2015;21(12):
26. Soylu L, Aydin OU, Atli M, et al. Does early removal of double J stents reduce urinary infection in living donor renal transplantation?. Arch Med Sci. 2019;15(2):402–407. doi:10.5114/aoms.2018.73524
27. Visser IJ, van der Staaij JPT, Muthusamy A, Willicombe M, Lafranca JA, Dor F. Timing of ureteric stent removal and occurrence of urological complications after kidney transplantation: a systematic review and meta-analysis. J Clin Med. 2019;8:5. doi:10.3390/jcm8050689
28. Fiorentino M, Pesce F, Schena A, Simone S, Castellano G, Gesualdo L. Updates on urinary tract infections in kidney transplantation. J Nephrol. 2019;32(5):751–761. doi:10.1007/s40620-019-00585-3
29. Rich J, Lee JC. The pathogenesis of Staphylococcus aureus infection in the diabetic NOD mouse. Diabetes. 2005;54(10):2904–2910. doi:10.2337/diabetes.54.10.2904
30. Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279(47):48487–48490. doi:10.1074/jbc.R400025200
31. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi:10.1172/JCI200318921
32. Vincent JL, Russell JA, Jacob M, et al. Albumin administration in the acutely ill: what is new and where next? Crit Care. 2014;18(4):231. doi:10.1186/cc13991
33. Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M. Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. 2016;33:62–70. doi:10.1016/j.jcrc.2015.12.019
34. Minatoguchi S, Nomura A, Imaizumi T, et al. Low serum albumin as a risk factor for infection-related in-hospital death among hemodialysis patients hospitalized on suspicion of infectious disease: a Japanese multicenter retrospective cohort study. Ren Replace Ther. 2018;4(1):30. doi:10.1186/s41100-018-0173-8
35. Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, Furth S. Serum albumin level and risk for mortality and hospitalization in adolescents on hemodialysis. Clin J Am Soc Nephrol. 2008;3(3):759–767. doi:10.2215/CJN.02720707
36. Becker BN, Becker YT, Heisey DM, et al. The impact of hypoalbuminemia in kidney-pancreas transplant recipients. Transplantation. 1999;68(1):72–75. doi:10.1097/00007890-199907150-00014
37. Haller C. Hypoalbuminemia in renal failure: pathogenesis and therapeutic considerations. Kidney Blood Press Res. 2005;28(5–6):307–310. doi:10.1159/000090185
38. Ma ZZ, Li L, Han YX, Duan YD, Wang WZ, Niu ME. Analysis of risk factors for early urinary tract infection after kidney transplantation. Transl Androl Urol. 2020;9(5):2211–2217. doi:10.21037/tau-20-1248
39. Mazzeffi M, Jonna S, Blanco N, et al. Intraoperative red blood cell transfusion, delayed graft function, and infection after kidney transplant: an observational cohort study. J Anesth. 2018;32(3):368–374. doi:10.1007/s00540-018-2484-x
40. Flabouris K, Chadban S, Ladhani M, Cervelli M, Clayton P. Body mass index, weight-adjusted immunosuppression and the risk of acute rejection and infection after kidney transplantation: a cohort study. Nephrol Dial Transpl. 2019;34(12):2132–2143. doi:10.1093/ndt/gfz095
41. Shields RK, Clancy CJ, Minces LR, et al. Staphylococcus aureus infections in the early period after lung transplantation: epidemiology, risk factors, and outcomes. J Heart Lung Transpl. 2012;31(11):1199–1206. doi:10.1016/j.healun.2012.08.012
42. BaniHani MN, Khabour OF, Alzoubi KH, et al. The association between ABCB1 C1236T/C3435T SNPs and H. pylori infection among Jordanians. Genes. 2020;11:1. doi:10.3390/genes11010063
43. Van Matre ET, Satyanarayana G. Pharmacokinetic drug-drug interactions between immunosuppressant and anti-infective agents: antimetabolites and corticosteroids. Ann Transpl. 2018;23:66–74. doi:10.12659/AOT.906164
44. Berdaguer S, Bautista J, Brunet M, Cisneros JM. Antimicrobial and immunosuppressive drug interactions in solid organ transplant recipients. Enferm Infecc Microbiol Clin. 2012;30(Suppl 2):86–92. doi:10.1016/S0213-005X(12)70087-3
45. Paterson DL, Singh N. Interactions between tacrolimus and antimicrobial agents. Clin Infect Dis. 1997;25(6):1430–1440. doi:10.1086/516138
46. Campagne O, Mager DE, Brazeau D, Venuto RC, Tornatore KM. The impact of tacrolimus exposure on extrarenal adverse effects in adult renal transplant recipients. Br J Clin Pharmacol. 2019;85(3):516–529. doi:10.1111/bcp.13811
47. Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transpl. 2010;10(12):2615–2623. doi:10.1111/j.1600-6143.2010.03310.x
48. Kim JS, Jeong KH, Lee DW, et al. Epidemiology, risk factors, and clinical impact of early post-transplant infection in older kidney transplant recipients: the Korean organ transplantation registry study. BMC Geriatr. 2020;20(1):519. doi:10.1186/s12877-020-01859-3
49. Møller DL, Sørensen SS, Wareham NE, et al. Bacterial and fungal bloodstream infections in pediatric liver and kidney transplant recipients. BMC Infect Dis. 2021;21(1):541. doi:10.1186/s12879-021-06224-2
50. Lee JR, Bang H, Dadhania D, et al. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation. 2013;96(8):732–738. doi:10.1097/TP.0b013e3182a04997
51. Erdbruegger U, Scheffner I, Mengel M, et al. Impact of CMV infection on acute rejection and long-term renal allograft function: a systematic analysis in patients with protocol biopsies and indicated biopsies. Nephrol Dial Transpl. 2012;27(1):435–443. doi:10.1093/ndt/gfr306
52. Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. 2016;37(1):37–61. doi:10.1210/er.2015-1084
53. Ahmed SH, Biddle K, Augustine T, Azmi S. Post-transplantation diabetes mellitus. Diabetes Ther. 2020;11(4):779–801. doi:10.1007/s13300-020-00790-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




