Back to Journals » OncoTargets and Therapy » Volume 12
Retinoic acid receptor α facilitates human colorectal cancer progression via Akt and MMP2 signaling
Authors Huang GL, Chen QX, Ma JJ, Sui SY, Wang YN, Shen DY
Received 3 January 2019
Accepted for publication 3 April 2019
Published 23 April 2019 Volume 2019:12 Pages 3087—3098
DOI https://doi.org/10.2147/OTT.S200261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Gui-Li Huang,1 Qing-Xi Chen,2 Jia-Jia Ma,1 Si-Yao Sui,1 Yu-Ning Wang,1 Dong-Yan Shen3
1Agricultural Product Storage and Processing Laboratory, Suzhou Academy of Agricultural Sciences, Suzhou, 215155, People’s Republic of China; 2State Key Laboratory of Cellular Stress Biology, School of Life Sciences, Xiamen University, Xiamen 361102, People’s Republic of China; 3Biobank, The First Affiliated Hospital of Xiamen University, Xiamen 361003, People’s Republic of China
Purpose: Retinoic acid α (RARα) is overexpressed in various tumors and facilitates cancer progression. Although RARα has been shown to facilitate colorectal cancer (CRC) progression, more efforts to characterize mechanisms of RARα in CRC are needed in order to develop better target-based drugs for tumor therapy.
Methods: RARα expression in CRC was assessed by IHC. EdU, QPCR, Western blotting, dual-luciferase reporter assay and ChIP were performed to explore the role of RARα in CRC and the mechanism involoved.
Results: Here, we show an overexpression of RARα in 73.5% (i.e., 25 of 34 human CRC specimens). RARα knockdown decreased cell proliferation, migration, and invasion. Such phenotypic manifestations can be correlated to lowered activation of Akt and expression of PCNA (proliferating cell nuclear antigen) as well as MMP2 (matrix metallopeptidase). Mechanistically, RARα facilitates CRC growth through Akt signaling activation to cause levels of PCNA to be upregulated. Furthermore, RARα promotes migration and invasion of CRC cells by directly recruiting the MMP2 promoter to enhance the expression of MMP2.
Conclusions: These findings demonstrate that CRC carcinogenesis is promoted by RARα via an enhanced Akt signaling and by increasing MMP2 transcription. CRC therapy can examine the use of RARα as a prospective molecular target.
Keywords: colorectal cancer, RARα, proliferation, PCNA, MMP2
Introduction
Colorectal cancer (CRC) is the third most commonly cancer in males and the second in females, the third leading cause of cancer-related mortality all over the world.1 In China, CRC morbidity and mortality increased to become the fifth most common cancer in 2015, and continues to rise as a considerable challenge to the nation’s health.2 Because of difficulty in early diagnosis of CRC,3 the majority of patients are not diagnosed until the late stage.4 This necessitates a rapid understanding of the molecular mechanism of CRC progression to devise new agents for therapy.
Retinoic acid receptors (RARs) are members of the superfamily of steroid/thyroid hormone nuclear receptors.5 To date, three different RAR genes (RARα, RARβ, and RARγ) have been characterized.6 RARα is homologous to other RAR genes and has three major domains. RARα plays diverse roles in many biological processes including carcinogenesis.7 The fusion protein PML/RARα, due to chromosomal translocation t (15;17) hinders the differentiation induced by RARα, impedes the cells at the promyelocytic stage and increases hyperproliferation of blocked promyelocytes, which drives oncogenic alterations in acute promyelocytic leukemia cells.8,9 Survival without relapse for breast cancer patients positive for estrogen receptor alpha (ERα) is less when the levels of intratumoral RARα protein are high.10 All trans-retinoic acid (ATRA) ATRA regulates in part the innate immunity to protect liver via RARα/Akt/Foxo1 pathway.11 The levels of Apolipoprotein CIII secreted by human liver cell lines was inhibited by AM580: an agonist of RARα.12 AM580 causes a significant inhibition on the growth of endometrial cancer and breast tumor,13,14 that indicates RARα as a target in the therapeutic intervention of cancer. Research points out at a crucial involvement of RARα in several cancers including CRC, breast cancer, leukemia, as well as, gastric cancer via several pathways such as ERα, p38α MAPK, G protein alpha Q, Glycogen synthase kinase 3, beta/beta-catenin, nuclear factor kappa B and c-Jun N-terminal kinase.15–17 RARα is highly expressed in CRC tissues,18 however, the possibility of metastasis to be modulated by RARα to affect the progression of CRC is yet not established. The above evidence underscores the importance of the identification of RARα targets, which may lead to strategies for developing improved anticancer drugs.
The current work showed overexpression of RARα in human CRC specimens, that augmented the capability of these tumor cells to proliferate, invade and migrate through an activation of Akt and matrix metallopeptidase MMP2 signaling pathways.
Materials and methods
Reagents
Wnt3a was from R&D systems (Minneapolis, MN, USA). LY294002 was obtained from Sigma (St. Louis, MO, USA). 5-Ethynyl-2-deoxyuridine (EdU) was sourced from RiboBio (Guangzhou, China). Abcam Ltd. (Cambridge, United Kingdom) was the source of polyclonal antibodies against RARα, Akt, as well as its phosphorylated form. Polyclonal antibody against β-actin was purchased from Cell Signaling Technology (Danvers, MA, USA). Monoclonal antibodies PCNA was procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Lipofectamine 2000, Goat anti-rabbit and anti-mouse secondary antibodies were sourced from Invitrogen (Carlsbad, CA, USA). Millipore (Billerica, MA, USA) provided the PVDF membrane. The EliVision Plus kit was obtained from Maixin Bio (Fuzhou, China).
Tumor samples
A total of 34 patients with colorectal cancer subjected to resection at the First Affiliated Hospital of Xiamen University were the source of colorectal tissues that were paired tumorous and paracancerous. Informed consent was obtained from each patient. The protocol used was in accordance with the Institute Research Ethics Committee of the First Affiliated Hospital of Xiamen University in line with the 1975 Declaration of Helsinki.
Details of plasmids
The pIRES2-EGFP vector was used to clone human RARα (coding regions) via the Xho I/Bam HI sites to generate constructs for expression of RARα. Construction of RNA interference vector targeting RARα was described previously.18 Lipofectamine 2000 was used to transfect 293T cells with pll3.7-shRARα or control vector and lentivirus packaging plasmids (PMDL/VSVG/REV) in accordance with the instructions of the manufacturer. Following a 48 hr culture of the transfected cells at 37 °C, infection was done using a medium containing the virus. The promoter region of MMP2 was constructed by inserting fragments into Xho I/Kpn I sites of the pGL3-basic vector. The MMP2 promoter mutants were generated by site-directed mutagenesis.
Culture of cells
The human CRC cell lines HT29, RKO and HCT116 were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). RPMI1640 medium (Hyclone, Logan, UT, USA) was used to grow HT29, while Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, USA) was used for RKO and HCT116 culture. Supplements in both cases were 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) and penicillin-streptomycin (100 U/mL). Culture conditions were a 5% CO2 in a humidified incubator at 37 °C. Following transfection with control or RARα, screening of monoclonal stable cell line was done to establish RKO and HCT116 transfections.
Immunohistochemical studies (IHC)
IHC was done according to an earlier protocol.19 A 1:200 dilution of anti-RARα antibody was added to the CRC tissue sections that were embedded in paraffin. Following incubation at 4 °C overnight, corresponding secondary antibody was added and incubated at room temperature for 40 min. EliVision Plus Kit (Maixin Bio, Fuzhou, China) was used to detect the slides in accordance with instructions of the manufacturer. Expression of RARα protein was classified into four levels according to staining intensity of CRC tissues. While ± and + are as low expression, and ++ and +++ are considered as high expression.
Immunofluorescence (IF)
IF was performed as previously described.20 After seeding cells on a glass slide and overnight culture, 4% paraformaldehyde was used to fix cells for 15 min. Triton X-100 (0.5%) was used for permeabilization of cells for 20 min. The blocked cells were incubated with 1:100 dilution of RARα -primary antibody. This was followed by 1:100 of secondary antibodies conjugated to Alexa Fluor 647. Nuclei were stained with 1 μg/mL DAPI. Leica TCS SP5 II laser confocal microscope (Leica, Barcelona, Spain) was used to record images.
Labeling with 5-Ethynyl-2-deoxyuridine (EdU)
The proliferation of cells was studied using EdU assay in accordance with our previous work.18
Assay for wound healing
A 10 μL micropipette tip was used to scratch monolayer cells that were 80–90% confluent. After a fixed interval, double washing with PBS was done following which, 4% paraformaldehyde was used to fix cells for 20 min. Crystal violet was used to stain cells for half an hour. Images were captured using a Leica microscope.
Transwell assay
For cell invasion assay, we used a 24-well transwell containing 8-mm pore size inserts coated with Matrigel (BD Biosciences, Franklin Lakes, New Jersey). 700 μL medium was used to fill each well while the insert chamber was seeded with 2×104 cells in 300 μL medium. The invading cells were stained and visualized with a Leica microscope. ImageJ software was used to quantify the number of cells.
Quantitative real-time PCR
RNeasy kit (Tiangen, Beijing, China) was used for extracting total in accordance with the protocol of the manufacturer. ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) was utilized for the assay. While RARα and GAPDH primers have been earlier published,18 the sequences of MMP1, MMP2, MMP3 and MMP9 can be issued on request.
Dual-luciferase reporter assays
RT-PCR was used to amplify the promoter region of MMP2 (nucleotides −1800 to +211 base pair) was from genomic DNA of a human followed by cloning into the pGL3-basic vector (Promega, Madison, Wisconsin). The assays were done in accordance with a procedure discussed earlier.18
Gel zymography assay
The enzymatic activity of MMP2 was performed by gel zymography as previously described.21
Western blotting
For an 8 hr exposure to LY294002 (20 μM), cells were lysed with ice-cold RIPA buffer. Protein lysates in equal quantities were resolved on SDS-PAGE (10%) followed by transfer to PVDF membranes (Millipore, Temecula, CA, USA). Specific primary antibodies were used to incubate the membrane overnight at 4 °C, following which secondary antibodies conjugated to horseradish peroxidase were added. An enhanced chemiluminescence (ECL) system (Pierce, Rockford, IL, USA) was used to detect the signals.
Chromatin immunoprecipitation assay (chip)
This was done in accordance with the protocol of Millipore (Billerica, MA, USA). The mean length of DNA was reduced as follows: 1% formaldehyde was added to RKO cells for 10 min at 37 °C followed by lysis and sonication (15 rounds of 9 seconds each). Protein A-agarose/salmon sperm DNA was applied as a pretreatment at 4 °C for 60 min. Next, incubation of negative control and tests was done with anti-mouse IgG and anti-RARα, respectively at 4 °C, overnight. A 2 hr application of protein A-agarose was carried out to precipitate the complexes. Following washes, PCR was used to amplify the MMP2 genomic region that flanked the potential binding areas of RARα.
Studies using animals
The protocols used were performed in accordance with animal protocols approved by the Laboratory Animal Center of Xiamen University. Twelve male BALB/c nude mice (4 weeks of age) were randomly assigned into 2 groups of 6 each that received either controls or shRARα-RKO cells. Following sacrifice of mice 24 days after inoculation, tumors were subjected to photography and western blot processing. The volume of tumors was quantified with the equation:1/2 × length × width.2
Statistical analysis
GraphPad Prism 6 (San Diego, CA, USA) was utilized for analysis with data of minimum of three independent experiments expressed as means ± SEM of minimum 3 independent experiments. For comparing the 2 groups, One-way analysis of variance (ANOVA) was applied with statistical significance at P* ≤0.05.
Results
Overexpression of RARα human CRC samples
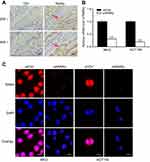
To determine the role of RARα in CRC, IHC analysis was used to determine the protein levels of RARα in a set of 34 pairs of human CRC specimens and the surrounding non-tumorous tissues (Figure 1A). RARα was localized both in cytoplasm and in the nucleus of CRC. In comparison to the surrounding non-tumorous tissues that surround 34 human CRC specimens, the study showed an overexpression of RARα protein in 25/34 samples ie 73.5% of tissues. The results are in line with our previous study that involved a different platform for analysis and patient cohort.18 This is suggestive of a putative role of RARα in CRC carcinogenesis.
Knockdown of RARα causes suppression of proliferation of CRC cells
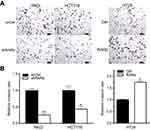
To assess RARα’s role in the growth of CRC cells, stably RARα-knockdown CRC cells were established. The expression of RARα in CRC cells were shown in our previous studies.18 RKO and HCT116 cells were stably transfected with pll3.7 vector (shCtrl) or RARα-knockdown vector pll3.7-shRARα, in which elevated RARα protein level was observed, whereas HT29 cells that express relatively less RARα protein were transfected with pIRES2-EGFP vector (Ctrl) or RARα-expression vector pIRES2-EGFP-RARα (RARα). The mRNA and protein levels of RARα in RARα-knockdown cells were much lower than those in their respective control cells (Figure 1B and C), indicating that RARα was effectively knockdown. Knockdown of RARα significantly decreased cell proliferation in both RKO and HCT116 cells, along with a concomitant reduction of PCNA. Contrastingly, overexpression of RARα in HT29 cells caused a significant increase in cell proliferation compared to those with Ctrl, with an increase of PCNA (Figures 2 and 3A). The observations corroborate the role of RARα in the progress of CRC.
Research has shown the activation of PI3K/Akt signaling by RARα by interacting with p85α of PI3K.22 Our next step was to measure the levels of phosphorylated Akt to see the effect of RARα on the pathway. Consistent with previous studies, we found that RARα knockdown suppressed the phosphorylated Akt at S473 (Figure 3A). PCNA was downregulated in the shRARα cells, as well as in cells treated with LY294002, specific inhibitors of Akt (Figure 3A and B). Meanwhile, PCNA expression was remarkably enhanced by RARα overexpression and was abolished by the specific inhibitor of Akt (Figure 3C). These results confer support to the notion that RARα promotes CRC cell proliferation through enhancing Akt signaling.
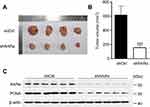
The xenograft tumor in nude mice was used to investigate the functional role of RARα in regulating the growth of CRC in vivo. Nude mice received subcutaneous injections of RARα-knockdown and control cells. Our results show that tumors formed by RARα knockdown cells grow much slower than those formed by the control cells (Figure 4A). The mean volume of RARα-knockdown tumors was about fourfold smaller than that from control tumors (Figure 4B). Moreover, these knockdown tumors also displayed a significantly decreased level of PCNA (Figure 4C). Together, these results demonstrate that RARα is a potent promoter of cancer cell growth.
RARα knockdown hinders invasion and migration of CRC cell
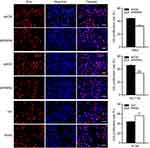
It has been reported that RARα is involved in metastasis of several types of cancers23–26 This led us to hypothesis that RARα might involve in metastasis of CRC cells. In order to assess this, the ability of cells with RARα-knockdown to migrate and invade was examined. Knockdown of RARα inhibited the migration and invasion of RKO cells and HCT116 cells, whereas RARα overexpression promoted HT29 cells migration and invasion (Figures 5 and 6A and B). These results indicate that the migrative and invasive potential of CRC cells is significantly reduced by RARα knockdown and markedly increased by RARα overexpression.
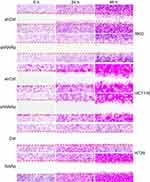 | Figure 5 Knockdown of RARα reduces CRC cells migration. The representative images of wound healing: the migration of cells at 24 and 48 h are shown. Assays were independently repeated in triplicate. |
RARα increases the transcription of MMP2
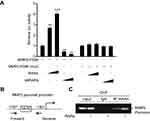
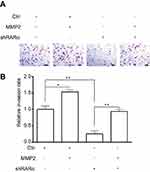
To explore the mechanisms by which knockdown of RARα inhibited metastasis of CRC cells, QPCR was performed to test metastasis-associated gene expression. The mRNA expression of MMP2, but not MMP1, MMP3 or MMP9, was decreased by knockdown and increased by overexpression of RARα (Figure 7A). Furthermore, knockdown of RARα reduced the enzyme activity of MMP2, while RARα overexpression induced the enzyme activity (Figure 7B), a matrix metalloproteinase which plays an important role in the migration and invasion of a variety of tumor cells. Rescue of MMP2 expression in RARα-knockdown RKO cells restored cell invasion (Figure S1). A search for the human MMP2 gene promoter was the next step to study the role of RARα on MMP2 mRNA expression. A plasmid pGL3-basic with luciferase reporter was used to clone the sequence of the gene spanning 1.8 kb upstream to the ATG site at 211 bp downstream of the transcription start site. The activity of MMP2 promoter increased significantly when RARα was transfected into 293T cells while RARα-targeted shRNA notably impaired the activity as determined by dual-luciferase reporter assays (Figure 7C). A truncated fragment with P598 allowed for reporter activity dependent on equivalent RARα (Figure 7D), that highlights the involvement of these positions in the activity of luciferase.
Analysis of P598 revealed a proximal RARα-binding site (RARE) in the promoter region of MMP2.The proximal site was mutated and subjected to analysis to see if it was involved in RARα associated activation of MMP2. Mutation of proximal RARE site removed the inhibitory effect of RARα in RKO cells (Figure 8A), suggesting that this site might be responsible for RARα binding in vivo. ChIP assay was conducted to see if RARα could be recruited to the RARE site. The results showed that RARα was recruited to the RARE in the promoter region of MMP2, and the recruitment was increased in the presence of exogenous RARα (Figure 8B and C). Taken together, our findings establish that RARα activates MMP2 expression via direct binding to the MMP2 promoter, which provides compelling evidence that MMP2 is a direct target gene of RARα.
Discussion
Accumulating evidence indicates that aberrant RARα expression is a common phenomenon in multitude cancers, including leukemia, breast cancer, lung cancer, and gastric carcinoma.10,27–29 Despite the reports of the promotional role of RARα in carcinogenesis of CRC or a potential marker of prognosis in CRC,18 the exact role and expression are yet to be ascertained in CRC progression. This work confirmed an overexpression of RARα on human CRC samples while knockdown of the protein significantly suppressed the proliferation of CRC cells and their migration and invasion. These findings demonstrate that RARα plays an important role in CRC carcinogenesis.
A basic feature in cancer cells is the sustenance of proliferative signaling.30 PCNA, often overexpressed in cancer cells, is a multifunctional protein essential for DNA replication and repair.31 In the present study, we observed that the expression of PCNA was significantly reduced by RARα knockdown and increased by RARα overexpression, indicating that RARα promotes CRC growth by positively regulating the expression of PCNA. Since PCNA is the target gene of Akt signaling, which is frequently activated in cancer,32,33 we were prompted to determine whether RARα promotes CRC growth by activation of Akt signaling. Our present results demonstrated that RARα promotes the growth of CRC cells through enhancing the expression of Akt-mediated expression of PCNA to accelerate proliferation.
It is increasingly apparent that research into the capability of cancer cells to migrate and invade has gained momentum dramatically over the past decade. MMPs,as critical regulatory genes, play a crucial function in aiding invasion by tumors by destroying the basement membrane and extracellular matrix. MMP2 is considered an important enzyme in cancer cells to invade and metastasize,34 with proof of these functions in cancers such as CRC.35 A finding of noteworthy significance in this work is that knockdown of RARα suppresses the expression of MMP2 rather than other MMPs. However, how the expression of MMP2 is controlled by RARα has not yet been determined. Our study showed that RARα was recruited to the promoter of MMP2 to facilitate the expression of MMP2, and eventually promoted CRC invasion and metastasis.
These results collectively demonstrate that our study highlights the crucial role of RARα in promoting the carcinogenesis of human CRC by enhancing cell proliferation and invasiveness, demonstrating that pharmacological targeting of RARα is a promising approach for CRC treatment.
Acknowledgments
This work was supported by Grants from Subsidized Project of Suzhou Academy of Agricultural Sciences (Grant Nos. KJ (18)101, 8111701), Agricultural Science and Technology Independent Innovation Funds of Jiangsu Province (Grant No. CX (17) 3029) and the National Nature Science Foundation of China (Grant No. 81572394).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. El Bairi K, Tariq K, Himri I, et al. Decoding colorectal cancer epigenomics. Cancer Genet. 2018;220:49–76. doi:10.1016/j.cancergen.2017.11.001
4. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi:10.3322/caac.21395
5. Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3(11):950–964. doi:10.1038/nrd1551
6. Schenk T, Stengel S, Zelent A. Unlocking the potential of retinoic acid in anticancer therapy. Br J Cancer. 2014;111(11):2039–2045. doi:10.1038/bjc.2014.412
7. Di Masi A, Leboffe L, De ME, et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. doi:10.1016/j.mam.2014.12.003
8. Ablain J, de The H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. 2011;117(22):5795–5802. doi:10.1182/blood-2011-02-329367
9. Masciarelli S, Capuano E, Ottone T, et al. Retinoic acid and arsenic trioxide sensitize acute promyelocytic leukemia cells to ER stress. Leukemia. 2018;32(2):285–294. doi:10.1038/leu.2017.231
10. Johansson HJ, Sanchez BC, Mundt F, et al. Retinoic acid receptor alpha is associated with tamoxifen resistance in breast cancer. Nat Commun. 2013;4:2175. doi:10.1038/ncomms3175
11. Zhong C, Pu L, Fang M, Rao J, Wang X. ATRA regulates innate immunity in liver ischemia/Reperfusion injury via RARalpha/Akt/Foxo1 signaling. Biol Pharm Bull. 2018;41(4):530–535. doi:10.1248/bpb.b17-00832
12. Lee SJ, Mahankali M, Bitar A, et al. A novel role for RARalpha agonists as apolipoprotein CIII inhibitors identified from high throughput screening. Sci Rep. 2017;7(1):5824. doi:10.1038/s41598-017-05163-w
13. Bosch A, Bertran SP, Lu Y, et al. Reversal by RARalpha agonist Am580 of c-Myc-induced imbalance in RARalpha/RARgamma expression during MMTV-Myc tumorigenesis. Breast Cancer Research. 2012;14(4):R121. doi:10.1186/bcr3169
14. Cheng YH, Utsunomiya H, Pavone ME, Yin P, Bulun SE. Retinoic acid inhibits endometrial cancer cell growth via multiple genomic mechanisms. J Mol Endocrinol. 2011;46(2):139–153. doi:10.1530/JME-10-0064
15. Chattopadhyay A, Abecassis I, Redner RL. NPM-RAR binding to TRADD selectively inhibits caspase activation, while allowing activation of NFkappaB and JNK. Leuk Lymphoma. 2015;56(12):3401–3406. doi:10.3109/10428194.2015.1023799
16. Ross-Innes CS, Stark R, Holmes KA, et al. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 2010;24(2):171–182. doi:10.1101/gad.552910
17. Donini CF, Di Zazzo E, Zuchegna C, et al. The p85alpha regulatory subunit of PI3K mediates cAMP-PKA and retinoic acid biological effects on MCF7 cell growth and migration. Int J Oncol. 2012;40(5):1627–1635. doi:10.3892/ijo.2012.1383
18. Huang GL, Zhang W, Ren HY, et al. Oncogenic retinoic acid receptor alpha promotes human colorectal cancer growth through simultaneously regulating p21 transcription and GSK3beta/beta-catenin signaling. Cancer Lett. 2017;388:118–129. doi:10.1016/j.canlet.2016.11.038
19. Huang GL, Song W, Zhou P, et al. Oncogenic retinoic acid receptor gamma knockdown reverses multi-drug resistance of human colorectal cancer via Wnt/beta-catenin pathway. Cell Cycle. 2017;16(7):685–692. doi:10.1080/15384101.2017.1295180
20. Huang GL, Zhang W, Ren HY, Shen XY, Chen QX, Shen DY. Retinoid X receptor alpha enhances human cholangiocarcinoma growth through simultaneous activation of Wnt/beta-catenin and nuclear factor-kappaB pathways. Cancer Sci. 2015;106(11):1515–1523. doi:10.1111/cas.12802
21. Huang GL, Luo Q, Rui G, et al. Oncogenic activity of retinoic acid receptor gamma is exhibited through activation of the Akt/NF-kappaB and Wnt/beta-catenin pathways in cholangiocarcinoma. Mol Cell Biol. 2013;33(17):3416–3425. doi:10.1128/MCB.00384-13
22. Rm D, Yh L, Am P, Suzuki YJ. Retinoic acid inhibits airway smooth muscle cell migration. Am J Respir Cell Mol Biol. 2006;34(6):695–703. doi:10.1165/rcmb.2005-0306OC
23. Dutta A, Sen T, Banerji A, Das S, Chatterjee A. Studies on multifunctional effect of All-Trans Retinoic Acid (ATRA) on Matrix Metalloproteinase-2 (MMP-2) and its regulatory molecules in human breast cancer cells (MCF-7). J Oncol. 2009;2009:627840. doi:10.1155/2009/627840
24. Kou Y, Li L, Li H, et al. Berberine suppressed epithelial mesenchymal transition through cross-talk regulation of PI3K/AKT and RARalpha/RARbeta in melanoma cells. Biochem Biophys Res Commun. 2016;479(2):290–296. doi:10.1016/j.bbrc.2016.09.061
25. Doi A, Ishikawa K, Shibata N, et al. Enhanced expression of retinoic acid receptor alpha (RARA) induces epithelial-to-mesenchymal transition and disruption of mammary acinar structures. Mol Oncol. 2015;9(2):355–364. doi:10.1016/j.molonc.2014.09.005
26. Zaragoza R, Gimeno A, Miralles VJ, et al. Retinoids induce MMP-9 expression through RARalpha during mammary gland remodeling. Am J Physiol Endocrinol Metab. 2007;292(4):E1140–1148. doi:10.1152/ajpendo.00463.2006
27. Mistry AR, Pedersen EW, Solomon E, Grimwade D. The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease. Blood Rev. 2003;17(2):71–97.
28. Srinivas H, Xia D, Moore NL, et al. Akt phosphorylates and suppresses the transactivation of retinoic acid receptor alpha. Biochem J. 2006;395(3):653–662. doi:10.1042/BJ20051794
29. Ren HY, Liu F, Huang GL, et al. Positive feedback loop of IL-1beta/Akt/RARalpha/Akt signaling mediates oncogenic property of RARalpha in gastric carcinoma. Oncotarget. 2017;8(4):6718–6729. doi:10.18632/oncotarget.14267
30. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
31. Indiani C, O‘Donnell M. The replication clamp-loading machine at work in the three domains of life. Nat Rev Mol Cell Biol. 2006;7(10):751–761. doi:10.1038/nrm2022
32. Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol. 2016;82(4):943–956. doi:10.1111/bcp.13021
33. Steelman LS, Chappell WH, Abrams SL, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging. 2011;3(3):192–222. doi:10.18632/aging.100296
34. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200(4):448–464. doi:10.1002/path.1400
35. Tang W, Zhu Y, Gao J, et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer. 2014;110(2):450–458. doi:10.1038/bjc.2013.724
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.