Back to Journals » OncoTargets and Therapy » Volume 13
Resveratrol Suppresses Tumor Progression via Inhibiting STAT3/HIF-1α/VEGF Pathway in an Orthotopic Rat Model of Non-Small-Cell Lung Cancer (NSCLC)
Authors Wang H, Jia R , Lv T, Wang M, He S, Zhang X
Received 20 April 2020
Accepted for publication 5 June 2020
Published 21 July 2020 Volume 2020:13 Pages 7057—7063
DOI https://doi.org/10.2147/OTT.S259016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Huixia Wang,1 Ruzhen Jia,1 Tianle Lv,1 Mei Wang,1 Shiwei He,1 Xia Zhang2
1Respiratory Department, The People’s Hospital of Baoji City, Baoji, Shaanxi 721000, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Central Hospital of Hanzhong City, Hanzhong, Shaanxi 723000, People’s Republic of China
Correspondence: Xia Zhang
Department of Pulmonary and Critical Care Medicine, Central Hospital of Hanzhong City, No. 277, Kangfu Road, Hanzhong, Shaanxi 723000, People’s Republic of China
Tel/ Fax +86 916-2680145
Email [email protected]
Background: The STAT3/HIF-1α/VEGF pathway is associated with the development and progress of various tumors including NSCLC. The aim of the present study was to investigate whether resveratrol (RES) could suppress NSCLC progression via inhibiting the expressions of STAT3, HIF-1α, and VEGF in a nude rat model.
Methods: Twenty-four nude rats were randomly divided into control, NSCLC, and NSCLC+RES groups. An orthotopic rat model of NSCLC was established. The animals in the NSCLC+RES group received the same operation as the NSCLC group and were intragastrically administered RES at 250 mg/kg/day for 12 weeks. Lung tissue samples were harvested for gross tumor burden measurement, histological examinations, RT-PCR, and Western blot assays.
Results: In the NSCLC+RES group, significant decreases in lung weight index, lung tumor burden, STAT3/HIF-1α/VEGF mRNA, and protein levels were observed when compared with the NSCLC group (all P< 0.05). The structural integrity of the lung was less affected and the apoptotic index was significantly higher in the NSCLC+RES group, when compared to the NSCLC group (P< 0.05).
Conclusion: RES suppresses NSCLC partly through inhibiting the expressions of STAT3, HIF-1α, and VEGF. The STAT3/HIF-1α/VEGF pathway might be a candidate drug target for developing new chemotherapy agents derived from RES for the treatment of NSCLC.
Keywords: resveratrol, non-small-cell lung cancer, STAT3, HIF-1α, VEGF, rat
Introduction
Lung cancer is a serious threat to human health. Currently, both the incidence and mortality of lung cancer rank first in all malignant tumors. Lung cancer can be classified into small-cell lung cancer and non-small-cell lung cancer (NSCLC) based on histopathologic characteristics.1 More than 80% of lung cancer is NSCLC, which includes mainly adenocarcinoma and squamous cell carcinoma.2 Though great progress has been made in the past few decades in its diagnosis and treatment, the prognosis of NSCLC is still unsatisfactory, with a 5-year overall survival rate of only 15%.3 Therefore, it is an urgent need to further explore the mechanism of the occurrence and development of NSCLC and seek novel targets for NSCLC therapy.
Signal transduction and transcription activator 3 (STAT3) is a well-characterized oncogene, which regulates various fundamental cellular processes, including proliferation, angiogenesis, and apoptosis.4 Hypoxia inducible factor-1α (HIF-1α), an important endogenous hypoxia marker,5 and its direct target gene vascular endothelial growth factor (VEGF), are two prominent transcription targets for STAT3 and important proangiogenic factors.6 It has been reported that the STAT3/HIF-1α/VEGF pathway was closely associated with tumorigenesis and progress of various tumors, including hepatocellular carcinoma, gastroenteropancreatic neuroendocrine tumor, hemangioma, malignant peripheral nerve sheath tumor, and NSCLC.7,12
Resveratrol (trans-3,4′,5-trihydroxystilbene, RES), a natural polyphenolic compound, is found in a large number of edible plants, especially grapes and peanuts.13 Numerous studies have demonstrated its diverse pharmacological activities, such as immune regulation, neuroprotection, antioxidant and anti-inflammatory properties.14 Its antitumor activity has also been widely studied and the results demonstrated that RES could exert potent antitumor effects via inhibiting cytochrome enzyme and COX-2, regulating cell cycle and NF-κB, inducing apoptosis and autophagy, and suppressing angiogenesis and metastasis.15,16 A recent study demonstrated that RES could inhibit the hepatic expressions of HIF-1α and VEGF in a rat model of liver ischemia-reperfusion injury.17 Another recent research showed that RES downregulated STAT3 expression in primary astrocyte cultures of rats.18 However, if RES could exert its antitumor activity in NSCLC through regulating the STAT3/HIF-1α/VEGF pathway is still unknown.
Therefore, our present study is aimed at investigating whether RES could suppress NSCLC progression via inhibiting the STAT3/HIF-1α/VEGF pathway in a nude rat model.
Materials and Methods
Cell Line and Reagents
The human NSCLC cell line A549 was purchased from Shanghai Cell Biology Medical Research Institute (Shanghai, China). Cells were cultured in RPMI 1640 containing 10% FBS at 37°C in a 5% CO2 incubator. RES was obtained from Xi’an Jinheng Chemical Co., Ltd (Xi’an, China) and dissolved in sterile saline to a final concentration of 5 mg/mL. Anti-STAT3 (ab76315), anti-HIF-1α (ab2185), anti-VEGFA (ab46154), anti-β-actin (ab8227), and HRP Goat Anti-Rabbit (IgG) secondary antibody (ab6721) were all obtained from Abcam (Cambridge, MA, USA).
Animals
Male Rowett nude rats (rnu/rnu) (age, 8 weeks old; weight, 240–260 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Experimental rats were kept under specific-pathogen-free (SPF) conditions at 22±2°C, 45–60% humidity, with 12-hour light/dark cycles and free access to autoclaved deionized water and irradiated pelleted food, except for an overnight fast before surgery. All experiments were conducted in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Ethics Committee of Xi’an Jiaotong University Health Science Centre.
Model of NSCLC
An orthotopic rat NSCLC model described previously19 was adopted. Briefly, rats were anesthetized using 64% N2O-32% O2-4% isoflurane and a total of 20×106 A549 cells were inoculated into the rat lung by intranasal drip. For the control group, animals underwent the same surgical procedure and received the same amount of solvent only (0.5 mL RPMI-1640 medium containing 10% FBS). Animals were monitored constantly until fully recovery from anesthesia.
Experimental Design
In total, 24 experimental nude rats were randomized into three groups (n=8 in each group): 1) control; 2) NSCLC; and 3) NSCLC+RES group. Animals in the NSCLC+RES group received the same operative procedure as NSCLC rats, and were intragastrically administered RES at 250 mg/kg/day for 12 weeks. Twelve weeks after inoculation of A549 cells, rats were euthanized by administering an overdose of intravenous pentobarbital sodium (150 mg/kg), and the lungs were harvested for subsequent experiments.
Lung Weight Index and Gross Tumor Burden Measurement
After the rats were euthanized, the body weights and the wet weights of the lungs were measured and recorded to calculate the lung weight index using the formula: lung weight (mg) divided by body weight (g). The gross tumor burden was determined using an arbitrary scoring system from 1 to 4, based on the surface tumors and palpable parenchymal tumors (grade 1: minimal; grade 2: mild; grade 3: moderate; and grade 4: marked tumor burden, respectively).20
H-E Staining and TUNEL Assay
Immediately after weighing, the lung tissue was fixed in 10% formalin (fixation time 24 hours, room temperature) and embedded in paraffin. Then the tissue blocks were cut into 5 μm-thick sections and routinely stained with hematoxylin (15 minutes)-eosin (5 minutes) at room temperature. These sections were examined under light microscopy with 400x magnification (Olympus, Olympus LX70, Japan).
For TUNEL assay, the sections were dewaxed and rehydrated, and the apoptosis rate in lung tissue was evaluated using a commercial TUNEL assay kit (Shanghai Ruisai Biotechnology Co., Ltd., China) in accordance to manufacturer’s instructions. Ten fields were selected randomly from each tumor or lung tissue slide and the rate of positive stained cells was calculated under light microscopy with 400x magnification (Olympus).
Quantitative Real-Time PCR
The tumor or lung tissue was taken to extract the total RNA by using TriPure Reagent Isolation Reagent (Roche Diagnostic, Penzberg, Germany). After determining the RNA concentration using a spectrophotometer, 500 nanograms of total RNA were reversely transcribed using the PrimeScript RT Reagent Kit (Takara, Japan). Then real-time qPCR was conducted with the SYBR@remix Dimer Eraser™ kit (Takara) in accordance to the manufacturer’s protocol. The expression levels of mRNA were standardized by that of β-actin. STAT3 primer: sense 5-′AGAGGCGGCAGCAGATAGC-3′ and anti-sense 5-′TTGTTGGCGGGTCTGAAGTT-3′; VEGF primer: sense 5-′CCATGCAGATCATGCGGATCA-3′ and anti-sense 5-′CCTTGGCTTGTCACATCTGCAA-3′; HIF-1α primer: sense 5-′ACCTATGACCTGCTTGGTGCTGAT-3′ and anti-sense 5-′CAGTTTCTGTGTCGTTGCTGCCAA-3′; and β-actin primer: sense 5-′ATCGTGGGCCGCCCTAGGCA-3′ and antisense 5-′ TGGCCTTAGGGTTCAGAGGGG-3′.
Western Blot Analysis
Total protein was extracted from the tumor or lung tissue using T-PER™ tissue protein extraction buffer (Pierce; Thermo Fisher Scientific, Inc.) containing protease inhibitors. Equal amounts of proteins (50 µg/well) from each sample were separated by 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE), and transferred onto nitrocellulose membranes (GE Healthcare Life Sciences). After being blocked with 5% bovine serum albumin, the membranes were incubated with primary antibodies at 37°C for 2 hours, followed by the addition of HRP-conjugated secondary antibodies at 37°C for 1 hour. The primary and secondary antibodies used were listed in Cell line and reagents. Then the signal was detected by enhanced chemiluminescence method. Protein expression was quantified by densitometry with β-actin as the loading control.
Statistical Analysis
Data were presented as mean±SD. Comparisons among groups were analyzed using one-way ANOVA followed by Tukey’s post hoc test using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). A P-value<0.05 was considered statistically significant.
Results
Lung Weight Index and Lung Tumor Burden Measurement
Compared to the NSCLC group, the lung weight index in rats treated with RES was significantly decreased (11.0±1.2 vs. 15.1±1.4, P<0.05, Table 1). Besides, our results indicated that RES significantly reduced tumor burden in lungs when compared to the NSCLC group (1.88±0.85 vs. 2.88±0.90, P<0.05) (Table 1).
 |
Table 1 Effect of RES on the Lung Weight Index and Gross Tumor Burden in the Lung |
H-E Staining and TUNEL Assay in Lung Tissue
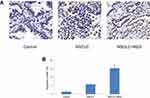
The histological changes of lung tissues were examined by H-E staining (Figure 1). In the NSCLC group, tumor lesions could be found in the lung, and the tumor cells were large, irregular in shape, squeezed, and deformed, and the ratio of nucleus to cytoplasm increased, showing the characteristics of NSCLC. Compared to the NSCLC group, the structural integrity of the lung was less affected in the NSCLC+RES group. TUNEL staining was used to determine cell apoptosis in rat lung tissue (Figure 2). Compared to NSCLC animals, the apoptotic index was significantly elevated in the NSCLC+RES group (P<0.05).
 |
Figure 1 H-E staining of lung tissues in different groups. |
The Expression of STAT3, HIF-1α, and VEGF in Lung Tissue
The expressions of STAT3, HIF-1α, and VEGF in lung tissues of animals were examined by RT-qPCR and Western blot assay. Compared to control animals, the mRNA/protein levels of STAT3, HIF-1α, and VEGF were all markedly increased in NSCLC group rats (all P<0.05). However, compared to the NSCLC group, treatment with RES led to a significant decrease in these parameters in the NSCLC+RES group (all P<0.05) (Figure 3).
Discussion
STAT3 is the most commonly activated member of the STAT family in human malignant tumors.21 It can be activated by multiple proinflammatory factors and growth factors and be implicated in the proliferation, differentiation, invasion, metastasis, angiogenesis, and apoptosis of various tumor cells.22 STAT3 can induce the expression of factors that contribute to cellular proliferation and survival, such as Bcl-2; invasiveness and metastasis, such as E-cadherin and MMP-9; immunosuppression, such as TGFβ and IL-10, and angiogenesis, such as HIF-1α and VEGF.23,25 HIF-1α and VEGF are two prominent transcriptional targets for STAT3.7,26 Besides, STAT3 was proposed to be required for the function of the HIF-1α complex.27
The rapid growth of solid tumors may cause hypoxia of tumor microenvironment and HIF-1α is one of the main factors for promoting tumor cells’ adaptation to the low oxygen tension.28 In a hypoxic environment, accumulated HIF-1α entered the nucleus to form a HIF-1 complex by binding to HIF-1β. Then the complex bound with the hypoxia responsive elements of HIF target genes and activates the expression of these genes, such as VEGF.29 The expression of VEGF was closely related to both developmental and pathological angiogenesis,30 which can promote the proliferation of endothelial cells, increase vascular endothelial permeability, and stimulate the formation of new blood vessels.31 Numerous studies had demonstrated the role of STAT3, HIF-1α, as well as VEGF in the occurrence and development of various cancers.32,34 More importantly, some recent studies have revealed that the STAT3/HIF-1α/VEGF pathway was activated in NSCLC.11,12,35
The relationship between STAT3 expression and apoptosis had been reported in many studies. Generally, while the expression, production, phosphorylation, or activation of STAT3 was found in various cancer cells, the apoptosis process in these cells was inhibited. Evidences showed that STAT3 regulates multiple genes that could suppress the cellular apoptosis pathway and contribute to oncogenesis.36
In recent years, studies on resveratrol as a potential antitumor agent have been paid more and more attention, and a variety of cellular pathways have been reported to be involved in the anti-tumor effects of RES.37,39 Sun et al40 reported that RES significantly inhibited the progression of xenografted lung cancer in mice, and this effect was associated with decreased expression of p-STAT3 in a tumor.Another study revealed that RES could efficiently inhibit breast cancer lung metastasis in mice and the inactivation of STAT3 by RES is one of the underlying mechanisms.41 Zhang et al42 showed that RES obviously suppressed both basal and hypoxia-induced expressions of HIF-1α, as well as the expression of VEGF, in human oral squamous cell carcinoma line SCC-9 and human hepatocellular carcinoma cell line HepG2. Cao et al43 However, so far there is no experimental study that has determined whether RES could exert its antitumor activity in NSCLC through regulating the STAT3/HIF-1α/VEGF pathway.
As expected, in the present study we found that, compared to the NSCLC group, treatment with RES (intragastrically administered at 250 mg/kg/day for 12 weeks) could lead to significant decreases in lung weight index and lung tumor burden, as well as a less affected lung structural integrity in the NSCLC+RES group. These findings affirmed the results of previous studies showing the antitumor effects of RES in NSCLC. The morphology of the lung in the NSCLC+RES group was different as compared with control and NSCLC groups. We speculated that there are two reasons: the first is due to the sampling position; the second is related to the antiapoptotic activity of RES on human pulmonary alveolar epithelial cells.44 Further studies on the precise role of RES in this phenomenon are needed in the future.
We further found that compared to the NSCLC group, the apoptotic index was significantly higher, while the STAT3/HIF-1α/VEGF mRNA and protein levels were all significantly decreased in the NSCLC+RES group. These findings were consistent with that of previously published research demonstrating the anti-tumor effects of RES in NSCLC through promoting cell apoptosis.45,47 More importantly, we suggested for the first time that RES might exert its antitumor activity in NSCLC, at least partly, through inhibiting the STAT3/HIF-1α/VEGF pathway.
In conclusion, our current study has provided the first evidence that RES exerts its anti-tumor effects, at least in part, through inhibiting the expressions of STAT3, HIF-1a, and VEGF in a rat model of NSCLC. The STAT3/HIF-1α/VEGF pathway might be a candidate drug target for developing new chemotherapy agents derived from RES for the treatment of NSCLC. However, the deeper mechanism underlying the involvement of RES in suppressing NSCLC are still unknown and further studies, including gene knockout and rescue experiments, as well as PDX experiment, need to be performed in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Novello S, Scagliotti G, de Castro G
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
3. Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi:10.1038/nrc3775
4. Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30(2):88–106. doi:10.3109/08977194.2012.660936
5. Zhang J, Zhang Q, Lou Y, et al. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67(5):1872–1889. doi:10.1002/hep.29681
6. Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer - more than a “gut” feeling? Cell Div. 2010;17(5):14. doi:10.1186/1747-1028-5-14
7. Liu P, Atkinson SJ, Akbareian SE, et al. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1α/VEGF signalling. Sci Rep. 2017;7(1):12651. doi:10.1038/s41598-017-12855-w
8. Lopez-Aguiar AG, Postlewait LM, Ethun CG, et al. STAT3 inhibition for gastroenteropancreatic neuroendocrine tumors: potential for a new therapeutic target? J Gastrointest Surg. 2019;1–11.
9. Fu X, Zhai S, Yuan J. Interleukin-6 (IL-6) triggers the malignancy of hemangioma cells via activation of HIF-1α/VEGFA signals. Eur J Pharmacol. 2018;841:82–89. doi:10.1016/j.ejphar.2018.10.022
10. Rad E, Dodd K, Thomas L, Upadhyaya M, Tee A. STAT3 and HIF1α signaling drives oncogenic cellular phenotypes in malignant peripheral nerve sheath tumors. Mol Cancer Res. 2015;13(7):1149–1160. doi:10.1158/1541-7786.MCR-14-0182
11. Xue D, Yang Y, Liu Y, et al. MicroRNA-206 attenuates the growth and angiogenesis in non-small cell lung cancer cells by blocking the 14-3-3ζ/STAT3/HIF-1α/VEGF signaling. Oncotarget. 2016;7(48):79805–79813. doi:10.18632/oncotarget.12972
12. Wang M, Wang W, Ding J, Wang J, Zhang J. Downregulation of Rab17 promotes cell proliferation and invasion in non-small cell lung cancer through STAT3/HIF-1α/VEGF signaling. Thorac Cancer. 2020;11(2):379–388. doi:10.1111/1759-7714.13278
13. Huang XT, Li X, Xie ML, et al. Resveratrol: review on its discovery, anti-leukemia effects and pharmacokinetics. Chem Biol Interact. 2019;306:29–38. doi:10.1016/j.cbi.2019.04.001
14. Rauf A, Imran M, Suleria HAR, Ahmad B, Peters DG, Mubarak MS. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017;8(12):4284–4305. doi:10.1039/C7FO01300K
15. Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr. 2018;58(9):1428–1447. doi:10.1080/10408398.2016.1263597
16. Han Y, Jo H, Cho JH, Dhanasekaran DN, Song YS. Resveratrol as a tumor-suppressive nutraceutical modulating tumor microenvironment and malignant behaviors of cancer. Int J Mol Sci. 2019;20(4):E925. doi:10.3390/ijms20040925
17. Zhang M, Li W, Yu L, Wu S, Pant AB. The suppressive effect of resveratrol on HIF-1α and VEGF expression after warm ischemia and reperfusion in rat liver. PLoS One. 2014;9(10):e109589. doi:10.1371/journal.pone.0109589
18. Wu M, Wang L, Li F, et al. Resveratrol downregulates STAT3 expression and astrocyte activation in primary astrocyte cultures of rat. Neurochem Res. 2020;45(2):455–464. doi:10.1007/s11064-019-02936-9
19. March TH, Marron-Terada PG, Belinsky SA. Refinement of an orthotopic lung cancer model in the nude rat. Vet Pathol. 2001;38(5):483–490. doi:10.1354/vp.38-5-483
20. Song JM, Molla K, Anandharaj A, et al. Triptolide suppresses the in vitro and in vivo growth of lung cancer cells by targeting hyaluronan-CD44/RHAMM signaling. Oncotarget. 2017;8(16):26927–26940. doi:10.18632/oncotarget.15879
21. Galoczova M, Coates P, Vojtesek B. STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett. 2018;23(1):12. doi:10.1186/s11658-018-0078-0
22. Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845(2):136–154. doi:10.1016/j.bbcan.2013.12.005
23. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi:10.1038/nrclinonc.2018.8
24. Yan Q, Jiang L, Liu M, et al. ANGPTL1 interacts with integrin α1β1 to suppress HCC angiogenesis and metastasis by inhibiting JAK2/STAT3 signaling. Cancer Res. 2017;77(21):5831–5845. doi:10.1158/0008-5472.CAN-17-0579
25. Behera R, Kumar V, Lohite K, Karnik S, Kundu GC. Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis. 2010;31(2):192–200. doi:10.1093/carcin/bgp289
26. Wu X, Liu S, Hu Z, Zhu G, Zheng G, Wang G. Enriched housing promotes post-stroke neurogenesis through calpain 1-STAT3/HIF-1α/VEGF signaling. Brain Res Bull. 2018;139:133–143. doi:10.1016/j.brainresbull.2018.02.018
27. Qian T, Hong J, Wang L, et al. Regulation of CD11b by HIF-1α and the STAT3 signaling pathway contributes to the immunosuppressive function of B cells in inflammatory bowel disease. Mol Immunol. 2019;111:162–171. doi:10.1016/j.molimm.2019.04.005
28. Vaupel P, Multhoff G. Hypoxia-/HIF-1α-driven factors of the tumor microenvironment impeding antitumor immune responses and promoting malignant progression. Adv Exp Med Biol. 2018;1072:171–175.
29. Zhang D, Lv FL, Wang GH. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci. 2018;22(16):5071–5076. doi:10.26355/eurrev_201808_15699
30. Mao Z, Xu B, He L, Zhang G. PVT1 promotes angiogenesis by regulating miR-29c/Vascular Endothelial Growth Factor (VEGF) Signaling Pathway in Non-Small-Cell Lung Cancer (NSCLC). Med Sci Monit. 2019;25:5418–5425. doi:10.12659/MSM.917601
31. Melincovici CS, Boşca AB, Şuşman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–467.
32. Wang Y, Shen Y, Wang S, Shen Q, Zhou X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018;415:117–128. doi:10.1016/j.canlet.2017.12.003
33. Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. A review. Curr Mol Med. 2018;18(6):343–351. doi:10.2174/1566524018666181109121849
34. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi:10.1007/s10456-017-9562-9
35. Wu X, Deng Y, Zu Y, Yin J. Histone demethylase KDM4C activates HIF1α/VEGFA signaling through the costimulatory factor STAT3 in NSCLC. Am J Cancer Res. 2020;10(2):491–506.
36. Fathi N, Rashidi G, Khodadadi A, Shahi S, Sharifi S. STAT3 and apoptosis challenges in cancer. Int J Biol Macromol. 2018;117:993–1001. doi:10.1016/j.ijbiomac.2018.05.121
37. Han G, Xia J, Gao J, Inagaki Y, Tang W, Kokudo N. Anti-tumor effects and cellular mechanisms of resveratrol. Drug Discov Ther. 2015;9(1):1–12. doi:10.5582/ddt.2015.01007
38. Feng M, Zhong LX, Zhan ZY, Huang ZH, Xiong JP. Enhanced antitumor efficacy of resveratrol-loaded nanocapsules in colon cancer cells: physicochemical and biological characterization. Eur Rev Med Pharmacol Sci. 2017;21(2):375–382.
39. Sinha D, Sarkar N, Biswas J, Bishayee A. Resveratrol for breast cancer prevention and therapy: preclinical evidence and molecular mechanisms. Semin Cancer Biol. 2016;40–41:209–232. doi:10.1016/j.semcancer.2015.11.001
40. Sun L, Chen B, Jiang R, Li J, Wang B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell Immunol. 2017;311:86–93. doi:10.1016/j.cellimm.2016.11.002
41. Lee-Chang C, Bodogai M, Martin-Montalvo A, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191(8):4141–4151. doi:10.4049/jimmunol.1300606
42. Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther. 2005;4(10):1465–1474. doi:10.1158/1535-7163.MCT-05-0198
43. Cao Z, Fang J, Xia C, Shi X, Jiang BH. trans-3,4,5’-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10(15):5253–5263. doi:10.1158/1078-0432.CCR-03-0588
44. Zhang C, Qingping L, Kang L, et al. Resveratrol inhibits hyperxia-induced cell apoptosis through up-regulating SIRT1 expression in HPAECs. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(5):590–595.
45. Wang J, Li J, Cao N, Li Z, Han J, Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018;11:7777–7786. doi:10.2147/OTT.S159095
46. Rasheduzzaman M, Jeong JK, Park SY. Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent and suppression of Akt/NF-κB signaling. Life Sci. 2018;208:208–220. doi:10.1016/j.lfs.2018.07.035
47. Li X, Wang D, Zhao QC, Shi T, Chen J. Resveratrol inhibited non-small cell lung cancer through inhibiting STAT-3 signaling. Am J Med Sci. 2016;352(5):524–530. doi:10.1016/j.amjms.2016.08.027
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.