Back to Journals » International Journal of Nanomedicine » Volume 14
Resveratrol solid lipid nanoparticles to trigger credible inhibition of doxorubicin cardiotoxicity
Authors Zhang L, Zhu K, Zeng H, Zhang J, Pu Y, Wang Z, Zhang T, Wang B
Received 4 April 2019
Accepted for publication 18 June 2019
Published 31 July 2019 Volume 2019:14 Pages 6061—6071
DOI https://doi.org/10.2147/IJN.S211130
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Lili Zhang,1 Kexin Zhu,1 Hairong Zeng,2 Jiaxin Zhang,1 Yiqiong Pu,3 Zhicheng Wang,4 Tong Zhang,3 Bing Wang1,5
1School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Pharmacy, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 3Experiment Center for Teaching and Learning, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 4Department of Laboratory Medicine, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China; 5Center for Pharmaceutics Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, People’s Republic of China
Background: Doxorubicin (DOX), a broad-spectrum chemotherapy drug, is clinically employed to treat cancers especially for breast cancer and lung cancer. But its clinical applications are limited by the dose-dependent cardiac toxicity. Resveratrol (Res), a polyphenolic antitoxin, has been proved to be capable of improving the cardiomyocyte calcium cycling by up-regulating SIRT-1-mediated deacetylation to inhibit DOX-induced cardiotoxicity.
Purpose: The objective of this study was to develop a solid lipid nanoparticle (SLN) loaded with Res to trigger inhibition of DOX-induced cardiotoxicity.
Methods: Res-SLN was prepared by emulsification-diffusion method followed by sonication and optimized using central composite design/response surface method. The Res-SLN was further evaluated by dynamic light scattering, transmission electron microscopy for morphology and high performance liquid chromatography for drug loading and release profile. And the Res distribution in vivo was determined on rats while the effect of inhibit DOX-induced cardiotoxicity was investigated on mice.
Results: Res-SLN with homogeneous particle size of 271.13 nm was successfully formulated and optimized. The prepared Res-SLN showed stable under storage and sustained release profile, improving the poor solubility of Res. Heart rate, ejection fractions and fractional shortening of Res-SLN treating mice were found higher than those on mice with cardiac toxicity induced by single high-dose intraperitoneal injection of DOX. And the degree of myocardial ultrastructural lesions on mice was also observed.
Conclusion: Res-SLN has a certain therapeutic effect for protecting the myocardium and reducing DOX-induced cardiotoxicity in mice.
Keywords: resveratrol, solid lipid nanoparticles, doxorubicin, heart failure
Introduction
Doxorubicin (DOX) is an anthraquinonoid broad-spectrum chemotherapy drug against lymphoma, breast cancer and other malignant tumors. Actually, DOX is restricted on clinical applications due to its severe cardiac toxicity.1 The certain pathogenesis of DOX-induced cardiotoxicity is still unclear, but intracellular calcium disorders, p53-mediated cardiomyocyte apoptosis, oxidative stress and mitochondrial function disorders are concerned.2–5 Resveratrol (Res) is a polyphenolic antitoxin widely found in natural plants, possessing the properties of anti-inflammatory, anti-tumor, and protecting cardiovascular pharmacological activity.6,7 It is certain that Res can improve the cardiomyocyte calcium cycling by up-regulating silent information regulator-1 (SIRT-1)-mediated deacetylation, thereby inhibiting the production of reactive oxygen species to protect the myocardium and improve DOX-induced cardiotoxicity.8,9
Although Res possesses the effect of attenuating DOX-induced cardiotoxicity, Res is poorly soluble in water, which makes it hard to achieve a satisfactory effect after orally taken. To overcome this obstacle, solid lipid nanoparticle (SLN) is employed. SLN, drew up in the early 1990s, is a drug delivery system made up of skeleton materials and drugs and can improve drug’s solubility and bioavailability.10 The drugs are wrapped or embedded in the lipid nucleus, consisting of physiological compatibility, biodegradable natural or synthetic solid lipid with high-melting point like lecithin or glycerol, and made into particles with the size of about 50–1000 nm.11
The reported Res-SLNs were prepared by the probe ultrasonication method,12 emulsification and solidification method,13,14 solvent injection method15 or homogenization method.16 They were dividedly utilized for transdermal drug delivery, treating breast cancer, anti-inflammatory, enhancing hepatoprotection and brain delivery, but nothing about Res-SLN inhibiting DOX-induced cardiotoxicity was discussed. In this study, we interrogated SLN as a drug delivery carrier of Res for the potential treatment of Dox-induced cardiotoxicity. The Res-SLN was prepared by emulsification and sonication method, followed with optimation of the formulations and pharmacodynamic evaluation. The prepared Res-SLN was able to improve the bioavailability of Res for better protecting the myocardium and inhibiting the DOX-induced cardiac toxicity (Figure 1).
 |
Figure 1 Schematic illustration of the preparation of Res-SLN and its effects against doxorubicin cardiotoxicity. |
Materials and methods
Materials
Res was purchased from ZELANG (Nanjing, China) and Res reference substance (M28F-Q3FG, purity ≥98.513%) was procured from National Institutes for Food and Drug Control of China. PC-98T egg yolk lecithin (AL15018, purity 98%) was obtained from A.V.T. Pharmaceutical Co., Ltd (Shanghai, China). Glycerol monostearate and glycerol tristearate were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China), while poloxamer 188 and sodium dichromate were from Yuanye Bio. (Shanghai, China).
Fifty male Kunming mice (20–25 g), 10 male and female Sprague Dawley (SD) rats (180–220 g) were purchased from Shanghai Slac Laboratory Animal Company and raised in Laboratory Animal Center, Shanghai University of Traditional Chinese Medicine. Experiments were performed in compliance with the requirements of the animal ethics committee of Shanghai University of Traditional Chinese Medicine (Ethical Accreditation No. SZY201507002).
Preparation of Res solid lipid nanoparticles
Res-loaded SLNs were prepared using emulsification-diffusion method followed by sonication.17 Briefly, a certain amount of egg yolk lecithin and Res were dissolved completely in 30 mL absolute alcohol and then mixed with 120 mg molten glycerol monostearate (GMS) to form an oil phase. The organic solvent was removed by vacuum rotary evaporation (Yarong, Shanghai, China) to obtain a layer of lipid film. The water phase containing poloxamer 188 was added to the lipid film in 40 mins through a needle under sustained ultrasound. The Res-SLN was collected after intermittently sonicated by a probe sonicator (Xinzhi, Ningbo, China) at 400 W for 12 mins. The amount of egg yolk lecithin, Res and the volume of the water phase were optimized using central composite design/response surface method (Table 1).
 |
Table 1 Facts and levels for central composite design-response surface method |
Morphological characterization of Res-SLN
The Res-SLN was diluted by pure water. Particle size distribution and ζ-potential of Res-SLN were determined by Nano ZS 90 Dynamic Light Scattering (Malvern Instruments, Malvern, UK). The mean diameter of Res-SLN stored hermetically at different temperatures on the 5th and 10th day was also determined to evaluate its stability. After that, the Res-SLN was imaged under transmission electron microscopy (TEM) to investigate its morphology by the approach of a reported procedure.18 Res-SLN was diluted at 0.5 mg/mL and placed on a carbon-coated copper grid, followed by being stained with phosphotungstic acid solution (1%, w/v) for 1 min. The prepared samples were imaged under TEM (FEI, Columbus, OH, USA).
Determination of drug loading and entrapment efficiency
To calculate the entrapment efficiency and drug loading of Res-SLN, the unencapsulated Res was isolated from Res-SLN through 10 KD ultrafiltration centrifuge tube (Millipore, Billerica, MA, USA) by centrifugation at 15,000 rpm for 30 mins (Anting, Shanghai, China) and detected by HPLC. The HPLC system (Agilent, Santa Clara, CA, USA) was under the following condition: Topsil C18 column (4.6×150 mm, 5 μm, Welch, Shanghai, China); injection temperature, 25°C; mobile phase, acetonitrile–water (25:75, v/v, 1.0 mL/min); detection wavelength, 305 nm; sample size, 10 μL.
Stability of Res-SLN
The effect of storage temperature on the stability of Res-SLN was studied. Optimized Res-SLNs were prepared repeatedly and stored at 4°C or 25°C. Samples were taken to determine the amount of Res by HPLC on the 1st, 5th and 10th day.
In vitro release of Res-SLN
In vitro release study of Res from Res-SLN was performed by the dialysis method; 30 mL Res-SLN injected into a dialysis bag (8000–14,000 Da, molecular weight cut-off) was sunk into 400 mL simulated gastric fluid, stirred at the speed of 50 rpm and maintained at 37°C. At regular intervals, 2 mL Res containing simulated gastric fluid was taken out and replaced by 2 mL fresh-simulated gastric fluid. The aliquots were filtrated with 0.45 μm Millipore filtration before injected into the HPLC system.
In vivo pharmacokinetic evaluation
Pharmacodynamic study was assessed in SD rats. Ten rats were randomly divided into two groups and received intragastric administration (10 mL/kg weight) of Res-SLN or Res-lipid physical mixture at 10.2 mg/mL doses. At regular intervals, 0.5 mL blood was collected through orbit at 0, 5, 10, 20, 30, 60, 90, 120, 180, 360, 720 and 1440 mins after administration into centrifuge tubes containing heparin sodium. The blood samples were subsequently centrifuged at 3000 rpm for 10 min to obtain plasma.
For determination of Res content in plasma, 50 μL genistein as internal standard (IS) was added into 100 μL plasma before mixed with 850 μL. The mixture was vortexed for 5 mins and centrifuged at 12,000 rpm for 10 mins. The supernatant was collected and quantified by ultra-HPLC (UPLC) in tandem with mass spectrometry (MS).
The Ultimate 3000 UPLC system (Thermo, Waltham, MA, USA) was applied and chromatographic separation was achieved on a Syncronis C18 column (2.1×50 mm, 1.7 μm; Thermo). The LC gradient elution was programmed as 70% water:30% methanol (0 min), 5% water:95% methanol (3 mins), and 70% water:30% methanol (8 mins). The flow rate was 0.3 mL/min. Injection volume was 10 μL. The MS condition for Res and IS quantification of TSQ triple quadrupole mass spectrometry (Thermo) with electrospray ionization is shown in Table 2.
 |
Table 2 The MS condition for Res and IS quantification |
Pharmacodynamic study of Res-SLN
Animal experiments
Male KM mice (20–25 g) were kept in an experimental animal barrier environment for 1 week adaptive feeding. Fifty mice were randomly divided into 5 groups: control group (normal saline), model group (normal saline), Res group (20 mg/kg free Res solution), Res-SLN group (1 g/kg Res-SLN lyophilized powder suspension, equivalent to 20 mg/kg free Res), and positive control group (60 mg/kg Digoxin solution). After 3 days of administration, except the control group, all mice were treated with single intraperitoneal injection of 20 mg/kg DOX solution.19 The control group was injected with the same volume of normal saline. Thereafter, mice in each group received the corresponding drug for another 5 days according to the above-mentioned dose.
Measurement of heart functions
Echocardiography was applied to the measurement of heart functions. Every mouse was treated with isoflurane for anesthesia and placed in a supine position with abdominal hair removed on a Philips Sonos 5500 color Doppler ultrasound system (Philips, Amsterdam, Netherlands). Heart rate (HR), left ventricular ejection fractions (EF) and left ventricular fractional shortening (FS) were recorded and analyzed to figure the degree of heart failure out.
Hematoxylin and Eosin staining
After the treatment, the mice were sacrificed and the hearts were removed. The myocardial tissue was circumcised at 1/4 of the apex of the left ventricle and fixed with 10% formaldehyde. After dehydration, it was embedded in conventional-dehydrated paraffin, cut into 5-μm slices, and stained with Hematoxylin and Eosin. After that, it was made into a pathological section and microscopically observed under an optical microscope. Arrangement of cardiomyocytes, interstitial cells, cell necrosis and inflammatory cell infiltration were observed under 200-fold microscopy.
Statistical analysis method
Statistical analysis was performed by SPSS22.0 statistical software (SPSS Inc., Chicago, IL, USA). The data were expressed as mean ± standard deviation (x ± s). If the data is in a normal distribution and the variance is homogeneous, the LSD method is used for comparison between the groups. P<0.05 indicates that the difference is statistically significant.
Results
Optimization and characterization of Res-SLN
Central composite design-response surface method
As shown in Table 3, the influence of three factors (A: lecithin dosage; B: Res drug dosage, and C: water volume) and five levels on the response variables (Y1: drug loading and Y2: particle size) were studied. The quadratic polynomial regression equation was fitted by Design Export 7.0 software (Stat-Ease Inc., Minneapolis, MN, USA). Regression analysis was performed for determining the optimal region for response studies (Figure 2A). The model equation is: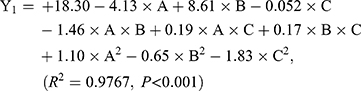
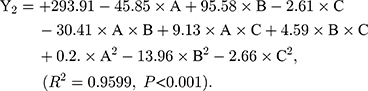
 |
Table 3 The factors, levels and results of central composite design-response surface method |
The R2 values are closer to 1 (>0.95), showing that the model fits well. And, P-values are<0.001, which indicates that the equation model is significant and has better correlation.
Particle size, zeta potential and drug loading efficiency
The optimal prescription of Res-SLN was as follows: the concentration of lecithin was 47.17%, the concentration of Res was 23.98%, and the volume ratio of water phase and oil phase was 6.174. The average diameter of Res-SLN was 271.13 nm with a ζ-potential of −25.8±0.33 mV (Figure 2B and C). The TEM observations showed that the Res-SLN was spherical solid particles with uniform size and essentially no adhesion between nanoparticles (Figure 2B).
Stability
To quantitatively detect Res in non-biological samples, HPLC was introduced. Res was in good linearity over the concentration range of 1.08–54.00 μg/mL and the linear equation was 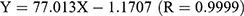 .
.
The long-term storage experiment showed that the particle size of Res-SLN would grow up from 271.1 to 389.8 nm as time increases at 25°C, and the drug loading would be reduced slightly (Figure 2D). While both particle size and drug loading were stable at 4°C (Figure 2D). Obviously, the prepared Res-SLN is more stable at 4°C which indicated that it is more suitable for storing at 4°C rather than room temperature.
In vitro release of Res-SLN
The in vitro release of Res-SLN was investigated by dialysis in simulated gastric juice. The release of optimized Res-SLN in simulated gastric juice was relatively stable. As shown in Figure 2E, over 80% of Res was released into the medium after 30 hrs. The Res-SLN release profile revealed a sustained property.
In vivo pharmacokinetics evaluation
UPLC-MS/MS
UPLC in tandem with MS was introduced to detect Res in rat plasma. Res in rat plasma was in good linearity over the concentration range of 1.368–684 ng/mL with a linear equation of y=0.02083x-0.1167 (r=0.9996). The UPLC-MS/MS method developed for Res was specific (Figure 3A) and in good precision, accuracy, recovery and matrix effect which listed in Table 4. Meanwhile, Res was stable in rat plasma at room temperature, −20°C and under freezing and thawing conditions. (Table 5)
 |
Table 4 The data of methodological verification of UPLC-MS/MS for Res |
 |
Table 5 The stability of Res in rat plasma under different conditions (n=5) |
Pharmacokinetics of Res-SLN in rats
The pharmacokinetics study was performed in rats following intragastric administration of Res-SLN and Res-lipid physical mixture, respectively. Concentration–time profiles of Res are shown in Figure 3B, and the pharmacokinetics parameters are presented in Table 6. Compared with the Res-lipid physical mixture, Cmax and AUC of Res-SLN were significantly increased and Tmax was significantly shortened (P<0.05), suggesting that entrapping of Res in the Res-SLN may promote the oral absorption of Res and SLN as a carrier may promote oral bioavailability of insoluble drugs.
 |
Table 6 Pharmacokinetic parameters of Res-SLN and Res-lipid physical mixture in rats (n=5) |
Pharmacodynamic study of Res-SLN
Behavior, weight and survival rate
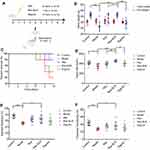
Fifty mice were divided equally into five groups. (Figure 4A) All of them were in good spirits and behaved normally in the first 3 days of the experiments. After injected with Dox (or saline) to induce heart failure, the mice in the model group started losing weight and the action of them was slower (Figure 4B). At the 7th day, the survival rate was lower. As for the groups injected with different drugs, the mice were better than the model group to some extent. The mice in Res-SLN group lost least weight (P<0.01) and the survival rate was significantly higher than other drug groups. According to the data showed in Figure 4C, Res owns the benefit of inhibiting Dox-induced heart failure and entrapping Res in the SLN was helpful for improving its efficiency.
Cardiac function detection
The cardiac function was examined by Doppler ultrasonic diagnostic apparatus and HR, EF and FS were recorded in Figure 4D–F.
Heart function and HR decreased (P<0.05) in mice in the model group which was injected with Dox to induce heart failure but not with therapeutic drugs. The HRs of Res-SLN and Digoxin group were increased compared to the model group (P<0.01) in which the HR of Res-SLN was better. On the contrary, free Res cannot increase the HR of Dox-induced heart failure mice (P>0.05).
The EF and FS in echocardiography are introduced as indicators in the clinical evaluation of cardiac function.20 EF and FS were decreased in the model group (P<0.01), suggested that the Dox had induced the heart failure in mice. Both EF and FS in Res-SLN were increased compared to the model group (P<0.01). Res-SLN can improve the EF and FS of left ventricle, while Digoxin only improves the EF.
It can be concluded that Res-SLN can improve the decrease of cardiac indexes (HR, EF, FS) induced by DOX in mice.
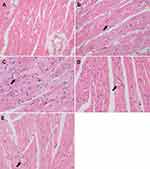
Pathological observation
Microstructure of myocardial histopathology showed that the myocardial fibers of the mice in the control group were well arranged and no abnormality appeared in the myocardial cells (Figure 5A). In the model group, the myocardial fibers were distorted and the cells showed obvious vacuoles degeneration of different sizes (Figure 5B). After the mice were treated with drugs (free Res, Res-SLN and Digoxin), the myocardial fibers were arranged regularly and a few vacuoles degenerated in the myocardial cells (Figure 5C–E).
Discussion
In recent years, studies on the antagonism of DOX cardiotoxicity by botanicals have become a hot topic,21 such as some important active ingredients, Astragalus polysaccharide,22 oxymatrine,23 baicalin,24 rhamnosin,25 Res,26 etc. In this study, we developed a Res-SLN for better absorption and efficacy after orally taken of Res, directing against its poor solubility. The presented results verify an important role as Res-SLN to be a protector against Dox-induced cardiotoxicity.
A role for SLN is to be a submicron drug delivery system for delivering Res, leading to release gradually and distribute better in the body. Our Res-SLN made up with egg yolk lecithin and GMS released approximately 80% of Res within 24 hrs, which is similar to those with glyceryl behenate.15,16 The findings of pharmacokinetics demonstrated that Res-SLN resulted in better distribution in rat than Res-lipid physical mixture. Other strategy was reported that Res-loaded liposome was also able to improve the efficacy of Res on stimulating the proliferation but preventing its cytotoxicity.27
This role for Res-SLN is not limited to it, Res-SLN triggers more credible improvement on cardiac function of cardio-damaged mice by DOX than free Res. An established DOX-induced cardiotoxicity model was employed to evaluate the Res-SLN; 20 mg/kg DOX was intraperitoneally injected into mice, which caused severe damage to cardiac myocytes. As reported, after treated by 20 mg/kg Dox, the left ventricular myocardium of a rat appeared massive fragmentation and lysis of the myofibrils, which is similar to the model mice in our study.28 The most promising therapeutics meaning of our findings is that Res-SLN can mitigate the collapse of the heart caused by DOX, evidenced by restoring the HR, ES, FE of heart, which is also proved by the myocardial tissue biopsy of mice.
Conclusion
In our study, SLN encapsulating Res was successfully prepared and optimized by emulsification-diffusion method followed by sonication. The prepared Res-SLN has a credible therapeutic effect on DOX-induced cardiotoxicity in mice by protecting the cardiomyocytes and improving the HR, EF, and FS values in mice. However, Res-SLN’s research on anti-myocardial toxicity still requires deeper and multi-faceted mutual verification. Based on the promising evidence that we have already found, we will be able to keep working on elucidating the correlation between multiple mechanisms that Res-SLN is involved.
Abbreviation list
Dox, doxorubicin; Res, resveratrol; SLN, solid lipid nanoparticles; GMS, glycerol monostearate; TEM, transmission electron microscopy; IS, internal standard; UPLC, ultra-high performance liquid chromatography; HR, heart rate; EF, ejection fractions; FS, fractional shortening.
Acknowledgments
The work was supported by grants from Shanghai Committee of Science and Technology (17401902300 and 18401931400), the Program of Shanghai Academic/Technology Research Leader (18XD1403700), Shanghai Three-year Action Plan for the Development of Traditional Chinese Medicine [ZY(2018-2020)-CCCX-2001-04] and the National Scientific and Technological Major Special Project of China (2018ZX09201008-002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barry E, Alvarez JA, Scully RE, et al. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–1058. doi:10.1517/14656566.8.8.1039
2. Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi:10.1016/j.pcad.2006.10.002
3. Lin ST, Chou HC, Chen YW, Chan HL. Redox-proteomic analysis of doxorubicin-induced altered thiol activity in cardiomyocytes. Mol Biosyst. 2013;9:447–456. doi:10.1039/c2mb25367d
4. Tallaj JA, Franco V, Rayburn BK, et al. Response of doxorubicin-induced cardiomyopathy to the current management strategy of heart failure. J Heart Lung Transplant. 2005;24:2196–2201. doi:10.1016/j.healun.2004.12.108
5. Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi:10.1038/nm.2919
6. Oktem G, Uysal A, Oral O, et al. Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol. 2012;64:471–479. doi:10.1016/j.etp.2010.11.001
7. Salehi B, Mishra AP, Nigam M, et al. Resveratrol: a double-edged sword in health benefits. Biomedicines. 2018;6:E91. doi:10.3390/biomedicines6030091
8. Zhang C, Feng Y, Qu S, et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res. 2011;80:538–545. doi:10.1093/cvr/cvr022
9. Gu J, Hu W, Song ZP, et al. Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. IntImmunopharmacol. 2016;32:1–7. doi:10.1016/j.intimp.2016.01.002
10. Khan AR, Yang X, Fu M, Zhai G. Recent progress of drug nanoformulations targeting to brain. J Control Release. 2018;291:37–64. doi:10.1016/j.jconrel.2018.10.004
11. Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196.
12. Shrotriya SN, Ranpise NS, Vidhate BV. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contace dermatitis. Drug Deliv Transl Res. 2017;7:37–52. doi:10.1007/s13346-016-0350-7
13. Wang W, Zhang L, Chen T, et al. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules. 2017;22:E1814. doi:10.3390/molecules22111814
14. Barbosa JP, Neves AR, Silva AM, et al. Nanostructured lipid carriers loaded with resveratrol modulate human dendritic cells. Int J Nanomed. 2016;11:3501–3516. doi:10.2147/IJN.S108694
15. Singh A, Ahmad I, Ahmad S, et al. A novel monolithic controlled delivery system of resveratrol for enhanced hepatoprotection: nanoformulation development, pharmacokinetics and pharmacodynamics. Drug Dev Ind Pharm. 2016;42:1524–1536. doi:10.3109/03639045.2016.1151032
16. Jose S, Anju SS, Cinu TA, et al. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int J Pharm. 2014;474:6–13. doi:10.1016/j.ijpharm.2014.08.003
17. Oliveira RR, Carriao MS, Pacheco MT, et al. Triggered release of paclitaxel from magnetic solid lipid nanoparticles by magnetic hyperthermia. Mater Sci Eng C Mater Biol Appl. 2018;92:547–553. doi:10.1016/j.msec.2018.07.011
18. Wu X, Hu Z, Nizzero S, et al. Bone-targeting nanoparticle to co-deliver decitabine and arsenic trioxide for effective therapy of myelodysplastic syndrome with low systemic toxicity. J Control Release. 2017;268:92–101. doi:10.1016/j.jconrel.2017.10.012
19. Gu J, Fan YQ, Zhang HL, et al. Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochem Pharmacol. 2018;150:202–213. doi:10.1016/j.bcp.2018.02.025
20. Wu Z, Chen Q, Ke D, et al. Emodin protects against diabetic cardiomyopathy by regulating the AKT/GSK-3beta signaling pathway in the rat model. Molecules. 2014;19:14782–14793. doi:10.3390/molecules190914782
21. Shabalala S, Muller CJF, Louw J, Johnson R. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017;180:160–170. doi:10.1016/j.lfs.2017.05.003
22. Cao Y, Shen T, Huang X, et al. Astragalus polysaccharide restores autophagic flux and improves cardiomyocyte function in doxorubicin-induced cardiotoxicity. Oncotarget. 2017;8:4837–4848. doi:10.18632/oncotarget.13596
23. Zhang YY, Yi M, Huang YP. Oxymatrine ameliorates doxorubicin-induced cardiotoxicity in rats. Cell Physiol Biochem. 2017;43:626–635. doi:10.1159/000480471
24. Sahu BD, Kumar JM, Kuncha M, et al. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci. 2016;144:8–18. doi:10.1016/j.lfs.2015.11.018
25. Sun J, Sun G, Meng X, et al. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One. 2013;8:e64526. doi:10.1371/journal.pone.0064526
26. Shoukry HS, Ammar HI, Rashed LA, et al. Prophylactic supplementation of resveratrol is more effective than its therapeutic use against doxorubicin induced cardiotoxicity. PLoS One. 2017;12:e0181535. doi:10.1371/journal.pone.0181535
27. Caddeo C, Teskac K, Sinico C, Kristl J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int J Pharm. 2008;363:183–191. doi:10.1016/j.ijpharm.2008.07.024
28. Al-Harthi SE, Alarabi OM, Ramadan WS, et al. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol Med Rep. 2014;10:1455–1460. doi:10.3892/mmr.2014.2384
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.