Back to Journals » Drug Design, Development and Therapy » Volume 15
Resveratrol Pretreatment Inhibits Myocardial Apoptosis in Rats Following Coronary Microembolization via Inducing the PI3K/Akt/GSK-3β Signaling Cascade
Authors Li T, Chen Z, Zhou Y , Li H, Xie J, Li L
Received 6 June 2021
Accepted for publication 31 August 2021
Published 7 September 2021 Volume 2021:15 Pages 3821—3834
DOI https://doi.org/10.2147/DDDT.S323555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Tao Li, Zhiqing Chen, You Zhou, Haoliang Li, Jian Xie, Lang Li
Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University & Guangxi Key Laboratory Base of Precision Medicine in Cardio-Cerebrovascular Diseases Control and Prevention & Guangxi Clinical Research Center for Cardio-Cerebrovascular Diseases, Nanning, People’s Republic of China
Correspondence: Lang Li
Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University & Guangxi Key Laboratory Base of Precision Medicine in Cardio-Cerebrovascular Diseases Control and Prevention & Guangxi Clinical Research Center for Cardio-Cerebrovascular Diseases, 6 Shuangyong Road, Nanning, 530021, Guangxi, People’s Republic of China
Tel/Fax +86-771-5331171
Email [email protected]
Purpose: Coronary microembolization (CME) is associated with progressive cardiac dysfunction, myocardial inflammation, and apoptosis. Resveratrol (RES) has a considerable role in cardioprotection. However, the contribution and possible mechanisms of RES in CME have not been clearly understood.
Methods: In the current study, 40 SD rats were randomly selected and categorized into various groups including CME, CME + resveratrol (CME + RES), CME + resveratrol+ LY294002 (CME + RES + LY), and sham groups (10 animals in each group). The inert plastic microspheres (42 μm) were injected into the rats’ left ventricle for developing the CME model. Then resveratrol (25 mg/kg/d) was given to the rats in the CME + RES and CME + RES + LY groups for one week before CME induction. Furthermore, LY294002 (10 mg/kg) was intraperitoneally injected into the rats of the CME + RES + LY group 0.5 hours before CME modeling. The cardiac functions, serum levels of myocardial injury biomarkers, myocardial histopathology, and mRNA and proteins associated with myocardial apoptosis were all assessed 12 hours after surgery.
Results: The results revealed that resveratrol pretreatment alleviated the CME-induced myocardial damage by improving cardiac dysfunction, and lowering the serum level of myocardial injury biomarkers, myocardial microinfarct size, and cardiomyocyte apoptotic index. Pretreatment with resveratrol reduced the level of proteins and mRNAs associated with the pro-apoptosis in myocardial tissues and increased the levels of proteins and mRNAs associated with the anti-apoptosis. Moreover, the combined treatment of resveratrol and LY294002 reversed the observed protective effects.
Conclusion: Resveratrol can inhibit cardiomyocyte apoptosis, thus attenuating the CME-induced myocardial injury by triggering the PI3K/Akt/GSK-3β cascade.
Keywords: resveratrol, myocardial apoptosis, PI3K/Akt/GSK-3β, coronary microembolization, cardiac dysfunction
Introduction
Coronary microembolization (CME) commonly occurs during thrombolytic therapy or percutaneous coronary intervention. CME is caused by atherosclerotic unstable plaques rupturing accidentally or small thrombus or plaque debris blocking the distal end of the coronary artery.1,2 CME may slow or no-reflow in the myocardium, resulting in fatal arrhythmia and local myocardial contractile failure, both of which have severe consequences for the patient’s cardiac function and prognosis.3–5 Microinfarctions occur in the local myocardium during the acute condition of CME, and apoptotic and necrotic cells grow in cardiomyocytes. Many studies have been revealed that the apoptotic process of the cardiomyocytes significantly contributes to CME-induced systolic dysfunction, thus inhibition of cardiomyocyte apoptosis can reduce CME-induced myocardial damage.6,7 According to the Kong et al8 study on rats, myocardial apoptosis and inflammation can potentially result in cardiac failure and myocardial damage after CME induction. By activating the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathways, pretreatment with puerarin considerably decreased the CME-induced myocardial inflammation and apoptotic process, thus alleviating myocardial damage and improving cardiac contractile efficiency.9
Resveratrol (RES), a polyphenolic compound, found in several plants, has many pharmacological properties. Studies suggested that resveratrol has relatively fewer adverse effects and is highly effective.10 According to the reported data on cardiovascular diseases, Resveratrol has many cardiovascular-associated therapeutic activities, including platelet aggregates inhibition,11 blood lipid control,12 anti-oxidative stress,13 anti-myocardial ischemia-reperfusion damage, and anti-apoptosis.14 However, the mechanism of action of resveratrol and its targeting pathway associated with CME-induced myocardial apoptosis has not been clearly understood. Furthermore, Mao et al15 reported that RES can elevate the Sirtuin-1 (SIRT-1) expression in myocardial tissue, resulting in a significant reduction in p53 acetylation and thereby inhibiting p53’s pro-apoptotic activity in myocardial tissues, which leads to the reduction in cardiomyocyte apoptosis induced by CME. Another study reported by Zhang et al16 demonstrated that resveratrol preconditioning could help patients with cardiac failure after resuscitation. The inhibitory potential of resveratrol against nitrative stress was linked to this effect, which may concern the PI3K/Akt signaling cascade in part. To use resveratrol for CME treatment, an extensive study of the association between resveratrol and cardiomyocyte apoptosis is needed. This study was designed to study whether resveratrol therapy could attenuate myocardial apoptosis (CME-induced) by triggering the PI3K/Akt/GSK-3β cascade.
Materials and Methods
Preparation of Animals
The Clinical and Animal Research Ethics Committee of Guangxi Medical University approved the use of animals in this study. All experimental procedures were carried out following the NIH Guidelines for the Use of Laboratory Animals. Forty male Sprague Dawley (SD) rats with 250 to 300g per body weight (8-week-old) were procured from the Experimental Animal Center, Guangxi Medical University. The rats were freely allowed water and food. The temperature and humidity of the animal house were kept at 23 °C, and 50 to 60%, respectively. Furthermore, the light and dark cycle of 12 h dark: 12 h light was maintained.
The CME Model Categorization and Establishment
Rats were randomized into different groups (10 per group): CME, Sham, CME + resveratrol (CME + RES), and CME + resveratrol + LY294002 (CME + RES + LY). Before inducing CME, rats in the CME +RES and CME + RES + LY groups were given a daily dose of 25 mg/kg of resveratrol (Sigma Chemical Co, St Louis, Missouri) for 7 consecutive days. Previous studies and our preliminary experimental results were used to determine the resveratrol dose.17,18 The CME+RES+LY group received a 10 mg/kg intraperitoneal injection of LY294002 (a particular inhibitor of PI3K) (APExBIO, Houston, USA) 30 minutes before CME surgery. The rats were anesthetized with 30–40 mg/kg intraperitoneal pentobarbital sodium as reported by Chen et al.9 To promote aerobic respiration, small animal ventilators were used to keep the rats alive. A thoracotomy was carried out on the left side of the sternum in the 3rd and 4th intercostal spaces. The ascending aorta was then completely isolated and exposed before being clamped for 10 sec via a vascular clamp. The suspension of three thousand polyethylene microspheres (42 μm diameter, BioSphere Medical Inc., USA) was prepared in 0.1 mL normal saline, followed by rapidly injecting into the cardiac apex of the animals in the CME, CME + RES, and CME + RES + LY groups simultaneously. After normal breathing and heart rate, the chest was cautiously sutured followed by the isolation of the endotracheal tube. Following that, penicillin (800,000 IU) was injected intraperitoneally. The sham group animals were treated with 0.1 mL normal saline instead of microspheres while other experimental and surgical procedures were the same. 12 hours after the procedure, all of the animals in the 4 groups were sacrificed.
Evaluation of Rat Cardiac Function
As reported previously, rats’ heart function is at an all-time low 12 hours post-CME modeling.19 In this regard, the current study evaluated cardiac function simultaneously. The left ventricular fractional shortening, left ventricular ejection fraction, left ventricular end-systolic diameter, and left ventricular end-diastolic diameter (indicated as LVFS, LVEF, LVESd, and LVEDd, accordingly) were all calculated via a 12 MHz transducer on a Hewlett Packard Sonos 7500 ultrasound (Philips Technologies, USA). Furthermore, these parameters were averaged over 3 cardiac cycles, followed by echocardiographic recording in the presence of an expert to obtain their echocardiogram individually.
Tissue Collection and Sample Processing
Each rat was treated with an overdose of anesthesia (60 mg/kg) of pentobarbitone sodium intraperitoneally after 12 hours of CME modeling and cardiac function measurements. Blood was collected from each rat via the abdominal aorta to test serum myocardial injury markers. In the end, all rats were sacrificed and easily removed after cardiac arrest, and each heart tissue was segmented into three sections: upper, mid, and bottom section. The upper and mid sections were then placed at −80 °C for RT-qPCR analysis and immunoblotting. Eventually, the bottom sections were fixed in paraformaldehyde (4%), embedded in paraffin, followed by serially slicing into 4 μm small sections for hematoxylin-basic fuchsin-picric acid (HBFP) staining, hematoxylin-eosin (H&E) staining, and TdT-mediated dUTP nick end labeling (TUNEL) staining.
Evaluation of Myocardial Injury Biomarkers in Serum
Before euthanizing the rats, blood samples were obtained from the abdominal aorta 12 hours after CME induction or sham procedure. The cardiac troponin I (cTnI) levels in serum were then measured using standardized ELISA kits (Bio-Swamp Biological Technology Co., Ltd, China) as per the manufacturer’s suggested protocol. Furthermore, the creatine kinase myocardial band isoenzyme (CK-MB) and lactate dehydrogenase (LDH) levels were measured using an automated biochemical analyzer (Olympus 5400, Olympus, Japan).
Electron Microscopy of Heart Tissues
After 12 hours of surgery, the animals were euthanized, and then the myocardial tissues were cut into a 1 mm3 pieces. Myocardial tissue pieces were then treated with glutaraldehyde (3%) for 24 hours at 4 °C. The pieces were rinsed thricely with PBS (pH 7.4) before being post-fixed for two hours in osmium tetroxide (1%). Next, the samples were rinsed (multiple times) and dehydrated with PBS and a series of graded ethanol (50~100%), accordingly. Samples were then immersed in resin, further cut into extremely thin sections (50 nm), and stained with lead citrate and uranyl acetate, followed by evaluation and then photographed the observations using a Hitachi H-7650 electron microscope.
Measurement of Myocardial Microinfarct Size
HBFP staining is considered an important staining approach for detecting myocardial ischemia in its earlier stages. Post staining via HBFP, the erythrocytes and ischemic cardiomyocytes were colored red, while the nucleus and cytoplasmic parts of healthy cardiomyocytes were colored blue, and yellow, correspondingly. Using a DMR-Q550 pathological image analyzer (Leica, Germany), five non-overlapping fields were randomly picked from each section to compute the infarction area. Subsequently, using the following calculations, the percent relative ischemic area was measured as: ischemic area/total area× 100%.20
TUNEL Staining for the Evaluation of Myocardial Apoptosis
TUNEL apoptosis detection kit (Roche, USA) was used to quantitatively analyze cardiomyocyte apoptosis to evaluate the apoptotic index, as suggested by the guidelines provided by the manufacturer. In this procedure, the normal cell nuclei were found to be light blue in myocardial tissues while the cells that were apoptotic in nature were found to be yellow-brown. In addition, five randomly chosen fields in each slice were selected for evaluating the number of apoptotic as well as total cardiomyocytes in distal non-infarcted, micro-infarcted, and marginal infarcted regions. The rate of myocardial apoptosis was evaluated using the formula: apoptotic cardiomyocytes/total cardiomyocytes × 100%.21
Immunofluorescence (IF) Staining
Post 12 hours of CME modeling, rats were euthanized. Thick sections (4μm) were prepared, followed by immunofluorescence staining as suggested by the manufacturer. These sections were rinsed thricely in PBS, and then they were blocked in BSA for 0.5 hours at ~25 °C, followed by incubating the sections with following primary antibodies for 24 hours (at 4 °C): cleaved caspase-3 (#9661, Cell Signaling Technologies, Danvers, MA, USA), Bax (ab53154, Abcam, Cambridge, UK), and Bcl-2 (ab194583, Abcam) followed by five times washing with PBS. The sections were then subjected to incubation with fluorescent secondary antibodies at ~ 25°C for 50 minutes. DAPI was used for counterstaining of nuclei for 7 minutes. A fluorescence microscope (Olympus, Japan) was employed to obtain the images.
qRT-PCR Analysis
The TRIzol RNA extraction method was carried out for obtaining the total RNA from the heart tissues, as suggested by the instructions provided by the manufacturer. Next, the NanoDrop (Thermo Fisher Scientific Inc., USA) was employed to measure the concentration of the total RNA. The PrimeScript™ RT reagent Kit with a gDNA Eraser (Perfect Real Time) (TaKaRa, Japan) was employed for the synthesis of cDNA. Moreover, the ABI PRISM-7500 system (CA, USA) was used to perform qRT-PCR using the TB Green® Premix Ex Taq™ II (TaKaRa, Japan). The relative expression of the target genes was evaluated by the 2−ΔΔCt method and GAPDH was used as an endogenous control. Table 1 indicates all the sequences of primer.
 |
Table 1 Sequences of the Used Primers |
Western Blotting Assay
A lysis buffer was utilized to extract the total protein from the heart sample, followed by measuring its concentration by bicinchoninic acid (BCA) assay kit (PC0020, Solarbio, China). The separation of equal quality proteins was carried out by using 10~15% SDS-PAGE. These proteins were electrophoretically moved onto PVDF membranes. Furthermore, 5% BSA or skimmed milk were used for membrane blockage (60 min), followed by incubation with the underlined primary antibodies with a dilution of 1: 1000 at 4 °C for 24 hours: p-PI3K, total PI3K, total GSK-3β, total Akt, p-GSK-3β, p-Akt, cleaved caspase-3, Bax, GAPDH, or Bcl-2. Abcam provided the primary antibodies against total PI3K (ab40776), p-PI3K (Tyr607) (ab182651), Bax (ab53154), and Bcl-2 (ab194583). While the primary antibodies against p-Akt (Ser473) (#9271), total Akt (#9272), p-GSK-3β (Ser9) (#9322), total GSK-3β (#9315), GAPDH (#5174), and cleaved caspase-3 (#9661) were provided by Cell Signaling Technology. Post incubation for 24 hours, a 1×TBST buffer solution was used for membrane washing (five times) at room temperature, followed by treating with secondary antibodies (ab6721, 1:10000, Abcam) conjugated with horseradish peroxidase for 2 hours. An enhanced chemiluminescence detection system (Pierce, Rockford, IL) was employed for detecting the protein band in each group. The ImageJ software (NIH, USA) was used for determining the level of expressions.
Statistical Evaluation
The SPSS version 23.0 (IBM, Chicago) was employed to conduct the statistical analyses, and the obtained data were indicated as mean ± standard deviation (SD). One-way ANOVA was used to compare multiple groups, followed by the Student-Newman-Keuls post hoc analysis. A P-value < 0.05 was regarded as statistically considerable.
Results
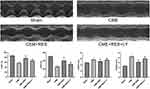
Resveratrol Enhanced Cardiac Functions Following CME
Figure 1 shows the echocardiographic results obtained from each group of rats. Relative to the sham group, the CME group had considerably impaired cardiac contractility with elevated LVEDd and LVESd but decreased LVFS and LVEF (P < 0.05). On the contrary, treatment with resveratrol considerably improved the dysfunction of the heart, as shown by lower values of LVEDd and LVESd but elevated LVFS and LVEF in the CME+RES group, relative to the CME group (P < 0.05). LY294002 treatment reversed the cardioprotective effects of resveratrol.
Resveratrol Lowered the Myocardial Injury Biomarkers in the Serum
The serum levels of cTnI, CK-MB, and LDH were used to determine myocardial damage following CME induction, and the results are shown in Figure 2. The obtained findings revealed that the rats in the CME group had considerably elevated levels of serum cTnI, CK-MB, and LDH relative to the sham group. While pretreatment with resveratrol substantially reduced myocardial damage post CME, as evidenced by lower serum levels of cTnI, CK-MB, and LDH in the CME + RES group relative to the CME group. The combined therapy of resveratrol and LY294002 reversed the effects of resveratrol on serum cTnI, CK-MB, and LDH expression levels.
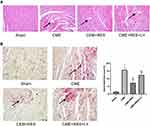
CME Histopathology
H&E and HBFP staining indicated sporadic subendocardial ischemia in the sham group with no visible infarction, however, numerous microinfarctions were found in the CME, CME + RES, and CME + RES + LY groups, as indicated in Figure 3A and B. Furthermore, H&E staining revealed that the nuclei of cardiomyocytes vanished or dissolved in areas of microinfarction (caused by CME), along with cytoplasmic red staining and degeneration. In addition, peripheral inflammatory cell infiltration, erythrocyte exudation, peripheral myocardial edema, and arteriole microembolism were observed. Remarkably, resveratrol administration considerably reduced these conditions. While the combined therapy of resveratrol and LY294002 considerably reversed these beneficial effects. The microinfarct lesions were mostly wedge-shaped, localized, and non-transmural, as indicated by the HBFP staining. They were mostly found in the left ventricle and sub-endocardium. The CME + RES group had a considerably smaller infarct size (P < 0.05) than the CME group, suggesting that resveratrol pretreatment could have attenuated the myocardial microinfarct region post CME induction in rats. The effect of resveratrol on the myocardial microinfarct area was eliminated when a combination of resveratrol and LY294002 was used.
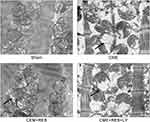
Resveratrol Decreased CME-Induced Mitochondrial Injury
Myocardial mitochondria in the CME group had considerably vacuolated degeneration and swelling, as indicated by the transmission electron microscopy (TEM) analysis of heart tissues. However, post-treatment with resveratrol, the ultrastructural variations observed in the CME group were considerably reduced, and the mitochondrial integrity was maintained, as depicted in Figure 4. A combined treatment of resveratrol and LY294002 reversed the protective effect of resveratrol on mitochondria. These findings suggested that resveratrol pretreatment can help to mitigate mitochondrial damage.
Resveratrol Decreased the Apoptotic Process of Cardiomyocyte Post CME
TUNEL staining was carried out to evaluate the apoptotic process in cardiomyocytes, and the results are shown in Figure 5. Normal and apoptotic nuclei were found to be light blue and yellow-brown (TUNEL positive), accordingly. In the sham group, myocardial apoptosis was sometimes noticed in the papillary and subendocardium muscles. In comparison to the sham group, the number of TUNEL-positive cells elevated considerably in the CME group (P < 0.05). Following CME induction, the underlined apoptotic cardiomyocytes were mostly found in the myocardial peri-infarct and micro-infarct areas. Furthermore, the TUNEL positive cells in the peri-infarct and micro-infarct zones of rats were considerably decreased in the CME +RES group than those in the CME group (P < 0.05). The anti-apoptotic effects of resveratrol were reversed by the combined therapy of resveratrol and LY294002.
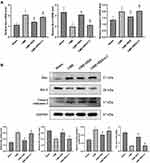
Pretreatment with Resveratrol Decreased Apoptotic Markers
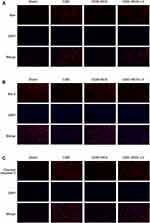
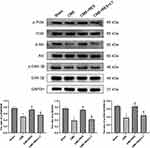
qRT-PCR and immunoblotting were employed to determine the expression of Bax, Bcl-2, and cleaved caspase-3 in the rats’ myocardium of each group to confirm the existence of apoptotic process in cardiomyocytes following CME. The results are shown in Figure 6A and B. The transcriptional and translational levels of cleaved caspase-3 and Bax were considerably elevated in the CME group relative to the sham group, while the transcriptional and translational levels of Bcl-2 were considerably reduced (P < 0.05). Furthermore, in comparison with the CME group, the transcriptional and translational levels of cleaved caspase-3 and Bax were considerably decreased in the CME+RES group, whereas the transcriptional and translational levels of Bcl-2 were considerably elevated (P < 0.05). However, when resveratrol was combined with LY294002, the impact of resveratrol on the expression of Bax, Bcl-2, and cleaved caspase-3 was reversed. Furthermore, the IF findings from myocardial tissues showed consistency with the results obtained from qRT-PCR and immunoblotting, as depicted in Figure 7. These findings indicated that resveratrol pretreatment could effectually minimize the CME-induced apoptotic process in cardiomyocytes.
Attenuation of Myocardial Apoptosis by Resveratrol Linked with the PI3K/Akt/GSK-3β Signaling Cascade
Immunoblotting showed no considerable variations in total PI3K, total Akt, or total GSK-3β between the four groups, as depicted in Figure 8. As compared with the sham group, myocardial expression of p-Akt, p-PI3K, and p-GSK-3β was considerably decreased in the CME group (P < 0.05), while in the CME+RES group, myocardial expression of p-Akt, p-PI3K, and p-GSK-3 was considerably elevated (P < 0.05) relative to the CME group. Moreover, the influence of resveratrol on the expression of p-Akt, p-PI3K, and p-GSK-3β was reversed by the combined treatment of resveratrol and LY294002. These results indicate that resveratrol pretreatment could efficiently decrease myocardial apoptosis following CME, which was linked with the stimulation of the PI3K/Akt/GSK-3β cascade.
Discussion
The data provided in this work demonstrate the following 3 points. Firstly, myocardial apoptosis was found to be a critical factor in myocardial damage and cardiac dysfunction in rat following CME modeling. Secondly, resveratrol pretreatment effectively suppressed CME-induced myocardial apoptosis and myocardial injury. Thirdly, triggering of the PI3K/Akt/GSK-3β signaling cascade was found to be an important factor in resveratrol’s protective mechanism against CME-related myocardial injury. Generally, the study for the first time revealed that resveratrol can attenuate CME-induced myocardial apoptosis by controlling the PI3K/Akt/GSK-3β cascade, thus alleviating CME-associated cardiac dysfunction and decreasing myocardial damage. As a result, resveratrol pretreatment can be useful in the treatment of myocardial injury induced by CME.
CME became a focus of interest approximately a decade ago,22 when it was revealed that CME and its sequelae are common iatrogenic consequences of percutaneous coronary procedures.23 Moreover, myocardial apoptosis has an important role in myocardial damage and advanced cardiac dysfunction following CME. Hence, inhibiting myocardial apoptosis has the potential to considerably decrease cardiac dysfunction.24,25 In the current study, the obtained results suggested that our CME rat model indicated a high level of serum markers, increased microinfarct areas, cardiac dysfunction, and apoptotic cardiomyocytes in peri-infarct and microinfarct areas, which showed consistency with the pathophysiological variations of CME.
Resveratrol is a powerful antioxidant widely found in many plants including peanuts, and grapes. It has potent biological activity against aging, inflammation, and apoptotic process.26 Furthermore, because of its extensive pharmacological influence, diverse mechanisms, and higher efficacy, it has the potential to be used in cardiovascular diseases. A previous study showed that resveratrol can inhibit the apoptotic process (stimulated by hypoxia) in H9c2 cells and has a direct anti-apoptosis effect on cardiomyocytes by elevating the expression of SIRT-1 in vitro.27 Furthermore, in mice with myocardial ischemia/reperfusion injury, resveratrol enhanced cardiac function and reduced apoptotic process and infarct size, as revealed by the previous study.28 Another study by Mao et al15 found that resveratrol is essential for myocardial defense in CME-induced myocardial injury, as evidenced by increased SIRT-1 expression in myocardial tissue, which lowers p53 acetylation in myocardial tissue, reducing p53’s pro-apoptotic activity and, as a result, decreasing the apoptotic process in CME-induced cardiomyocytes. Resveratrol also reduced cellular apoptosis, elevated the Bcl-2 expression, and lowered the Bax, and cleaved caspase-3 expression. In this study, rats developed left ventricular systolic dysfunction and increased cardiomyocyte apoptosis after CME modeling. These results, like earlier studies, indicate that apoptotic process in cardiomyocyte have a key role in the irreversible injury of cardiomyocytes caused by CME. However, after resveratrol pretreatment, cardiac function was improved and cardiomyocyte apoptosis was reduced in CME rats. These results validate the conclusion that resveratrol improves cardiac function by inhibiting CME-induced cardiomyocyte apoptosis.
Furthermore, PI3K/Akt is not only an important signaling pathway that mediates apoptosis, growth, and survival, but also a common pathway for a variety of cardiovascular drugs to achieve myocardial protection. Activated PI3K inhibits programmed cell death in cells, and Akt, a direct downstream target of PI3K that mediates the PI3K-dependent cell-survival response, is also related to anti-apoptosis.29 Bcl-2 expression is increased when the PI3K/Akt cascade is stimulated, and Bcl-2 can regulate the apoptotic process by this mechanism. The elevated Bcl-2 expression can attenuate cytochrome C release which results in the formation of a stable Bcl-2/Bax heterodimer with Bax, hence attenuating the apoptotic process induced by Bax. On the contrary, the apoptotic process has been enhanced by a higher expression of Bax.30 Recently reported data have revealed that many factors phosphorylate GSK-3β through considerably triggering the PI3K/Akt cascade, attenuating the GSK-3β protein’s biological activity and, as a result, enhancing the survival of cells.31,32 The PI3K/Akt/GSK-3β cascade has been reported as an effective endogenous negative feedback mechanism in the cell which results in a compensatory reaction against unfavorable stimuli to attenuate the apoptotic and inflammatory processes.33,34 The reported studies suggested that the stimulation of the PI3K/Akt/GSK-3β cascade markedly decreased myocardial inflammation, apoptotic process, and myocardial damage, however, it might have opposing effects when it is not activated.35,36
In this study, the data suggested that the pretreatment with resveratrol elevated the expression of Bcl-2, p-Akt, p-PI3K, and p-GSK-3β post CME induction in rats. However, resveratrol considerably attenuated the cleaved caspase-3 and Bax levels. Furthermore, the protective effects of resveratrol were substantially reversed when it was combined with LY294002. Moreover, the data of the current study demonstrated that the attenuation of the PI3K/Akt/GSK-3β cascade resulted in a decrease in cardiac function and elevation in serum cTnI levels, myocardial apoptosis, and microinfarct size, suggesting that the cardioprotective effects of resveratrol could be reversed by LY294002. In general, the results showed that the PI3K/Akt/GSK-3β cascade was correlated with the resveratrol’s protective effects against CME-induced myocardial injury. As a result, resveratrol will likely protect the heart by triggering the PI3K/Akt/GSK-3β cascade and attenuating the myocardial apoptosis.
There are some limitations to this study. In this study, the physical microembolic spheres were injected into coronary microvessels to establish a CME rat model. However, because these plastic microspheres lack biological features like vascular or thrombotic activity, the pathological alterations produced by this plastic material in the CME model differ from those generated in atherosclerosis during clinical practice. Further, this study evaluated the impact of resveratrol on myocardial apoptosis triggered by CME and the PI3K/Akt/GSK-3β cascade by using pretreatment with resveratrol and the LY294002. However, to better understand the effects of resveratrol on the PI3K/Akt/GSK-3β cascade in CME, inhibitors or agonists of PI3K downstream molecules are required. It cannot be ruled out whether other signaling cascades are involved in myocardial damage caused by CME, which requires further investigation.
Conclusions
In brief, the current study demonstrated that resveratrol can significantly reduce CME-induced myocardial damage and cardiomyocyte apoptosis, most likely by triggering the PI3K/Akt/GSK-3β signaling cascade. As a result, our findings revealed that resveratrol may be a candidate therapeutic agent for cardioprotection against CME in clinical applications. These results may also serve as a theoretical basis for further evaluating the impact of resveratrol on CME patients.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No.81770346) and the Project for Innovative Research Team in Guangxi Natural Science Foundation (Grant No.2018GXNSFGA281006).
Disclosure
The authors declare no conflicts of interest.
References
1. Heusch G, Kleinbongard P, Bose D, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120(18):1822–1836. doi:10.1161/CIRCULATIONAHA.109.888784
2. Heusch G, Skyschally A, Kleinbongard P. Coronary microembolization and microvascular dysfunction. Int J Cardiol. 2018;258:17–23. doi:10.1016/j.ijcard.2018.02.010
3. Scarpone M, Cenko E, Manfrini O. Coronary no-reflow phenomenon in clinical practice. Curr Pharm Des. 2018;24(25):2927–2933. doi:10.2174/1381612824666180702112536
4. Skyschally A, Gres P, Hoffmann S, et al. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100(1):140–146. doi:10.1161/01.RES.0000255031.15793.86
5. Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117(24):3152–3156. doi:10.1161/CIRCULATIONAHA.107.742312
6. Liu T, Zhou Y, Wang JY, et al. Coronary microembolization induces cardiomyocyte apoptosis in swine by activating the LOX-I-dependent mitochondrial pathway and caspase-8-dependent pathway. J Cardiovasc Pharmacol Ther. 2016;21(2):209–218. doi:10.1177/1074248415599265
7. Chen ZW, Qian JY, Ma JY, et al. TNF-alpha-induced cardiomyocyte apoptosis contributes to cardiac dysfunction after coronary microembolization in mini-pigs. J Cell Mol Med. 2014;18(10):1953–1963. doi:10.1111/jcmm.12342
8. Kong B, Qin Z, Ye Z, Yang X, Li L, Su Q. microRNA-26a-5p affects myocardial injury induced by coronary microembolization by modulating HMGA1. J Cell Biochem. 2019;120(6):10756–10766. doi:10.1002/jcb.28367
9. Chen ZQ, Zhou Y, Huang JW, et al. Puerarin pretreatment attenuates cardiomyocyte apoptosis induced by coronary microembolization in rats by activating the PI3K/Akt/GSK-3beta signaling pathway. Korean J Physiol Pharmacol. 2021;25(2):147–157. doi:10.4196/kjpp.2021.25.2.147
10. Wicinski M, Socha M, Walczak M, et al. Beneficial effects of resveratrol administration-focus on potential biochemical mechanisms in cardiovascular conditions. Nutrients. 2018;10(11):1813. doi:10.3390/nu10111813
11. Yang Y, Wang X, Zhang L, An H, Zao Z. Inhibitory effects of resveratrol on platelet activation induced by thromboxane a(2) receptor agonist in human platelets. Am J Chin Med. 2011;39(1):145–159. doi:10.1142/S0192415X11008713
12. Abbas AM. Cardioprotective effect of resveratrol analogue isorhapontigenin versus omega-3 fatty acids in isoproterenol-induced myocardial infarction in rats. J Physiol Biochem. 2016;72(3):469–484. doi:10.1007/s13105-016-0494-4
13. Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299(1):H18–24. doi:10.1152/ajpheart.00260.2010
14. Xu K, Liu XF, Ke ZQ, Yao Q, Guo S, Liu C. Resveratrol modulates apoptosis and autophagy induced by high glucose and palmitate in cardiac cells. Cell Physiol Biochem. 2018;46(5):2031–2040. doi:10.1159/000489442
15. Mao Q, Liang X, Wu Y, Lu Y. Resveratrol attenuates cardiomyocyte apoptosis in rats induced by coronary microembolization through SIRT1-mediated deacetylation of p53. J Cardiovasc Pharmacol Ther. 2019;24(6):551–558. doi:10.1177/1074248419845916
16. Zhang HH, Wu QQ, Wan Z, Cao Y, Zeng Z. Preconditioning but not postconditioning treatment with resveratrol substantially ameliorates post-resuscitation myocardial dysfunction through the PI3K/Akt signaling pathway. Mol Med Rep. 2019;20(2):1250–1258.
17. Wilson DN, Schacht SE, Al-Nakkash L, Babu JR, Broderick TL. Resveratrol prevents pulmonary trunk remodeling but not right ventricular hypertrophy in monocrotaline-induced pulmonary hypertension. Pathophysiology. 2016;23(4):243–250. doi:10.1016/j.pathophys.2016.05.004
18. Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68(3):593–601. doi:10.1007/s00280-010-1525-4
19. Su Q, Lv X, Sun Y, Ye Z, Kong B, Qin Z. Role of TLR4/MyD88/NF-kappaB signaling pathway in coronary microembolization-induced myocardial injury prevented and treated with nicorandil. Biomed Pharmacother. 2018;106:776–784. doi:10.1016/j.biopha.2018.07.014
20. Zhu HH, Wang XT, Sun YH, et al. MicroRNA-486-5p targeting PTEN protects against coronary microembolization-induced cardiomyocyte apoptosis in rats by activating the PI3K/AKT pathway. Eur J Pharmacol. 2019;855:244–251. doi:10.1016/j.ejphar.2019.03.045
21. Chen QF, Wang W, Huang Z, et al. Role of high-mobility group B1 in myocardial injury induced by coronary microembolization in rats. J Cell Biochem. 2019;120(3):4238–4247. doi:10.1002/jcb.27709
22. Bose D, von Birgelen C, Zhou XY, et al. Impact of atherosclerotic plaque composition on coronary microembolization during percutaneous coronary interventions. Basic Res Cardiol. 2008;103(6):587–597. doi:10.1007/s00395-008-0745-9
23. Dorge H, Neumann T, Behrends M, et al. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 2000;279(6):H2587–H2592. doi:10.1152/ajpheart.2000.279.6.H2587
24. Sun YH, Su Q, Li L, Wang XT, Lu YX, Liang JB. Expression of p53 in myocardium following coronary microembolization in rats and its significance. J Geriatr Cardiol. 2017;14(5):292–300.
25. Su Q, Lv X, Sun Y, Yang H, Ye Z, Li L. Role of high mobility group A1/nuclear factor-kappa B signaling in coronary microembolization-induced myocardial injury. Biomed Pharmacother. 2018;105:1164–1171. doi:10.1016/j.biopha.2018.06.098
26. Nakata R, Takahashi S, Inoue H. Recent advances in the study on resveratrol. Biol Pharm Bull. 2012;35(3):273–279. doi:10.1248/bpb.35.273
27. Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun. 2009;378(3):389–393. doi:10.1016/j.bbrc.2008.11.110
28. Xu H, Cheng J, Wang X, et al. Resveratrol pretreatment alleviates myocardial ischemia/reperfusion injury by inhibiting STIM1-mediated intracellular calcium accumulation. J Physiol Biochem. 2019;75(4):607–618. doi:10.1007/s13105-019-00704-5
29. Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22(56):8983–8998. doi:10.1038/sj.onc.1207115
30. Tsukahara S, Yamamoto S, Ahmed S, et al. Inhalation of low-level formaldehyde increases the Bcl-2/Bax expression ratio in the hippocampus of immunologically sensitized mice. Neuroimmunomodulation. 2006;13(2):63–68. doi:10.1159/000094829
31. Zhang X, Shi M, Ye R, et al. Ginsenoside Rd attenuates tau protein phosphorylation via the PI3K/AKT/GSK-3beta pathway after transient forebrain ischemia. Neurochem Res. 2014;39(7):1363–1373. doi:10.1007/s11064-014-1321-3
32. Park SJ, Jin ML, An HK, et al. Emodin induces neurite outgrowth through PI3K/Akt/GSK-3β-mediated signaling pathways in Neuro2a cells. Neurosci Lett. 2015;588:101–107. doi:10.1016/j.neulet.2015.01.001
33. Zheng T, Yang XY, Wu D, et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3 pathway. Brit J Pharmacol. 2015;172(13):3284–3301. doi:10.1111/bph.13120
34. Zhang YN, Zhang Z, Wang HT, et al. Neuroprotective effect of ginsenoside Rg1 prevents cognitive impairment induced by isoflurane anesthesia in aged rats via antioxidant, anti-inflammatory and anti-apoptotic effects mediated by the PI3K/AKT/GSK-3 pathway. Mol Med Rep. 2016;14(3):2778–2784. doi:10.3892/mmr.2016.5556
35. Wei DJ, Xu HJ, Gai XD, Jiang Y. Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3 beta signaling pathways. Acta Cir Bras. 2019;34(7). doi:10.1590/s0102-865020190070000008
36. Zhang L, Guo Z, Wang Y, Geng J, Han S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev Res. 2019;80(3):294–309. doi:10.1002/ddr.21495
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.