Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Resveratrol Inhibits Lipopolysaccharide-Induced Extracellular Matrix Accumulation and Inflammation in Rat Glomerular Mesangial Cells by SphK1/S1P2/NF-κB Pathway
Authors Gong W, Li J, Chen W, Feng F, Deng Y
Received 22 August 2020
Accepted for publication 28 October 2020
Published 20 November 2020 Volume 2020:13 Pages 4495—4505
DOI https://doi.org/10.2147/DMSO.S278267
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Wenyan Gong,1,* Jie Li,2,* Wenying Chen,3 Fuzhen Feng,3 Yanhui Deng3
1Department of Cardiology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 310000, People’s Republic of China; 2Medical Research Center, Guangdong Second Provincial General Hospital, Guangzhou 510317, People’s Republic of China; 3Department of Pharmacy, The Third Affiliated Hospital of Southern Medical University, Guangzhou 510630, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanhui Deng
Department of Pharmacy, The Third Affiliated Hospital of Southern Medical University, Guangzhou 510630, People’s Republic of China
Tel +86 20 62784810
Email [email protected]
Purpose: Chronic inflammation plays a key role in the pathogenesis of various diseases such as diabetic nephropathy (DN). Resveratrol (RSV), a natural polyphenol, has been proven to have renoprotective effects. In this study, we used a lipopolysaccharide (LPS)-induced rat glomerular mesangial cells (RMCs) model, to elucidate the renoprotective effect of RSV on sphingosine kinase 1 (SphK1)/sphingosine 1-phosphate receptor 2 (S1P2)/NF-κB activation and the expression of downstream inflammatory mediators, such as intercellular adhesion molecule-1 (ICAM-1), inducible nitric oxide synthase (iNOS) and fibronectin (FN) protein expression in RMCs.
Methods: Cell proliferation was tested by 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide (MTT). The protein levels of FN, ICAM-1, iNOS, SphK1, S1P2 and NF-κB p65 in RMCs were detected by Western blot. The DNA-binding activity of NF-κB was detected by electrophoretic mobility shift assay (EMSA). SphK1 activity and S1P content were measured by using sphingosine kinase activity assay kit and ELISA assay, respectively.
Results: We first found that LPS could stimulate SphK1/S1P axis activation, whereas this occurrence was significantly blocked by RSV pretreatment. RSV obviously repressed LPS-induced upregulated expression of fibronectin (FN), intercellular adhesion molecule-1 (ICAM-1) and inducible nitric oxide synthase (iNOS) in RMCs. Moreover, RSV markedly reduced SphK1 activity and its protein expression, and attenuated S1P content in LPS-induced RMCs. Furthermore, RSV could block LPS-induced upregulation of NF-κB p65 and DNA-binding activity of NF-κB. And this phenomenon was notably attenuated by SphK1 inhibitor and S1P2 inhibitor.
Conclusion: RSV inhibited LPS-induced RMCs’ proliferation and inflammation and FN expression by SphK1/S1P2/NF-κB pathway, suggesting that RSV may be independent of its hypoglycemic effect on preventing or delaying the development of mesangial cell fibrosis.
Keywords: diabetic nephropathy, resveratrol, lipopolysaccharide, sphingosine kinase 1, sphingosine 1-phosphate, NF-κB
Introduction
Diabetes mellitus, due to its rapidly increasing prevalence and huge economic burden, is widely recognized as a serious public health problem worldwide. In 2019, the global diabetes prevalence was estimated to be 9.3% (463 million adults), rising to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045.1 Diabetic nephropathy (DN), which occurs in approximately 30–40% of diabetic patients, is one of the most severe microvascular complications of diabetes. At present, it is a leading cause of end-stage renal disease,2 and considered as a chronic inflammatory disease.3–5 The most important pathological changes of DN are glomerular mesangial cells’ proliferation and extracellular matrix (ECM) accumulation, such as fibronectin (FN).6,7 Along with the activation of inflammatory signals and the abnormal expression of inflammatory factors, such as intercellular adhesion molecule-1 (ICAM-1) and inducible nitric oxide synthase (iNOS), it would further worsen. As a result, diabetic kidney damage would be further accelerated, finally leading to renal tubular interstitial fibrosis.5 Although many studies have been performed, the exact mechanism of the pathogenesis of DN has not been fully explained.
Accumulating reports have shown that inflammation is a major pathogenetic mechanism in DN.4 Therefore, regulating the inflammatory processes in DN progression has aroused great interest for researchers today. Sphingosine kinase 1 (SphK1)/sphingosine 1-phosphate (S1P) pathway, which could be activated by a multitude of growth factors and cytokines,8,9 plays significant roles in the inflammatory responses including early diabetic nephropathy.10 S1P2, the most abundant S1P receptor in kidney tissues of diabetic models, suggests that SphK1/S1P2 pathway is activated in early diabetic nephropathy.11 Furthermore, studies in the past have verified that under diabetic conditions, the increments in SphK1 activity and S1P content could stimulate mesangial cells’ proliferation and then activate some nuclear transcription factors, such as nuclear factor-kappaB (NF-κB).9,12 NF-κB can be activated by many stimulators such as high glucose, lipopolysaccharide (LPS), and further regulate the expression of gene products.13 A clinical study demonstrated that high serum LPS activity is associated with the development of DN.14 LPS as a main component of the outer membrane of Gram-negative bacteria is commonly used to induce inflammatory responses in mesangial cells in vitro.15 However, the role of LPS-induced SphK1/S1P2 pathway in DN has not yet been reported. Additionally, NF-κB acts as a key downstream mediator of SphK1/S1P axis and may exert an important role in the pathogenesis of DN.16 Thus, it is interesting to investigate whether NF-κB activation is dependent of SphK1/S1P2 pathway under LPS stimulation.
Resveratrol (trans-3, 4ʹ, 5-trihydroxystilbene, RSV), a polyphenol that can better penetrate the cell membrane,17 is naturally present in various plants, such as Polygonum cuspidatum, peanuts, grapes and mulberries. Some studies have shown that it could reduce levels of fasting blood glucose and has renoprotective effects in DN with its powerful anti-inflammatory and antioxidant capacities.18–21,29 Recently, we found that RSV prevented high glucose-induced rat glomerular mesangial cells’ (RMCs) proliferation, inflammatory factors’ expression and the activation of AP-1 and NF-κB through SphK1/S1P2 pathway.22 Nevertheless, it has not been clarified whether the inhibitory effect of RSV on SphK1/S1P2 is indirectly associated with its hypoglycemic effect or not. In addition, it has not been addressed whether the protective effect of RSV on DN is due to SphK1/S1P2-dependent depression of NF-κB. Therefore, we used LPS-induced RMCs proliferation model and SphK1/S1P2 inhibitors to examine and provide extensive exploration.
Materials and Methods
Chemicals and Reagents
RSV (trans-, used in cell experiments) was supplied by Kechuang (purity>99%; Beijing, China). SphK1 inhibitor (SK-II) and S1P2 inhibitor (JTE-013) were purchased from Merck-Millipore and Selleck respectively. Bovine serum albumin (BSA, Fraction V) was from Mbchem (Shanghai, China). D-glucose was from Amresco (Solon, OH, USA). LPS, streptomycin, trypsin, DMSO, penicillin, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) and α-tubulin were from Sigma (St. Louis, MO). Nuclear Extract Kit was from Active Motif (Carlsbad, CA, USA). Antibodies against FN, ICAM-1, NF-κB p65 and S1P2 were from Santa Cruz Biotech, Inc. (CA, USA). Monoclonal antibodies against iNOS and SphK1 (rabbit) were from Abcam Inc. (Cambridge, UK) and Cell Signaling Technology Inc. (Boston, MA, USA) respectively. Horseradish peroxidase conjugated secondary antibodies were purchased from Promega (Madison, USA). Biotin labeled EMSA probe for NF-κB was from Beyotime (Shanghai, China). EMSA kit was from Invitrogen Corporation (Carlsbad, CA). Both SphK1 activity kit and S1P ELISA kit were from Echelon (Salt Lake City, CT, USA).
Cell Line and Cell Culture
The rat glomerular mesangial cell line (HBZY-1) was purchased from Center of Type Culture Collection, Wuhan University, Wuhan, China. RMCs were maintained in DMEM (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum at 37°C in a standard humidified incubator with 5% CO2. Cell experiments were performed between 3rd and 8th passages. 80% confluent cells were made quiescent by serum-free starvation for 24 h incubation in DMEM before LPS treatment (100 ng/mL).
MTT Assay
In brief, 104 cells per well in a 96-well plate were pretreated with or without different concentrations of RSV (5 μM, 10 μM, 20 μM) for 2 h, and then incubated with or without 100 ng/mL LPS for another 24 h. 20 μL of MTT (5 mg/mL) was added to each well and incubated for another 4 h at 37°C. Then each sample well was carefully removed the medium and added 150 μL DMSO to dissolve the formazan crystals. The absorbance of solubilized blue formazan was measured at 570 nm using a microplate reader.
Western Blot Assay
Western blot assay was done as previously described,22 mainly to detect the protein expression of FN, ICAM-1, iNOS, SphK1, S1P2 and NF-κB p65. Briefly, RMCs for total proteins were lysed in RIPA lysis buffer, nuclear and cytosolic proteins were obtained with a Nuclear Extraction Kit according to the manufacturer’s instruction. Then protein concentration was determined by using a BCA Protein Assay Kit (Pierce, USA). Then, 25 μg total protein samples and 10 μg nuclear protein samples were separated on 8% SDS-PAGE and then transferred onto PVDF membranes (Millipore, MA, USA). After blocking with 5% non-fat dry milk in 0.1% Tween-20/TBS (TBST) for 1 h at room temperature and then washing with TBST, the blots were incubated overnight with the primary antibody at 4°C. After washing three times with TBST for 10 min each, the membranes were incubated with the corresponding HRP-conjugated secondary antibodies (anti-rabbit IgG, anti-mouse IgG or anti-goat IgG 1:10,000) for 1 h at room temperature. Labeled protein spots were visualized by ECL kit (Amersham Biosciences) and captured by Gel Doc XR System (Bio-Rad Laboratories, USA), and then quantified by Quantity One Protein Analysis Software (Bio-Rad Laboratories, USA).
Assay of SphK1 Activity
SphK1 activity was performed according to the manufacturer’s instructions of SphK activity assay kit. 30 μg protein extracts from each sample were maintained in reaction buffer supplemented with 100 μm sphingosine and 10 μm ATP for 1 h incubation at 37°C. Then luminescence attached ATP detector was added to stop the kinase reaction. Kinase activity was tested using Lumistar Optima luminometer (BMG LABTECH, Offen-burg, Germany).
Assay of S1P Levels
S1P levels were performed according to the manufacturer’s instructions and tested by using a specific ELISA kit. Data were analyzed by using a standard curve of known concentrations of S1P standards. The levels were presented as pmol/µg of protein.
Nuclear Protein Extraction and Electrophoretic Mobility Shift Assay (EMSA)
Briefly, nuclear proteins for EMSA were prepared using a Nuclear Extraction Kit following the procedure as instructed by the manufacturer. 5 µg proteins from the nuclei were incubated with 1× binding buffer (Light Shift Chemiluminescent EMSA kit; Pierce, Rockford, IL, USA) in the presence of 50 ng/µL poly (dI-dC), 0.05% NP-40, 5 mM MgCl2, and 2.5% glycerol for 10 min. Then the mixture was kept at room temperature for another 20 min incubated with 1 pmol biotin-labeled NF-κB consensus oligonucleotide (5ʹ-AGTTGAGGGGACTTTCCCAGGC-3ʹ). The reaction mixture was subjected to 6% non-denaturing SDS-PAGE, transferred onto nylon hybridization transfer membrane (Amersham, Piscataway, NJ, USA), and then DNA was cross-linked for 10 min. After blocking for 1 h, the blots were incubated with HRP-conjugated streptavidin antibodies (1:300) for 15 min. Peroxidase activity was tested by using an enhanced chemiluminescence substrate system. Images were captured and analyzed using a GE Image Quant LAS 4000mini.
Statistical Analysis
Values were presented as means ± SEM. Multiple comparisons among the groups were analyzed by one-way ANOVA (Graphpad Prism 5.0). Independent experiments were performed in triplicate. A difference was considered statistically significant at P<0.05.
Results
RSV Suppressed LPS-Induced RMCs’ Proliferation
As shown in Figure 1, RMCs’ proliferation was not altered by RSV treatment under normal condition, suggesting that the concentrations of RSV at 5, 10, and 20 μM were not toxic to RMCs. Compared with control, LPS treatment for 24 h could significantly promote RMCs’ proliferation by approximately 1.6 fold (Figure 1, P<0.01), which was obviously suppressed by 10 μM RSV and 20 μM RSV (63.8% decrement in 20 μM RSV group, P<0.01; 56.3% decrement in 10 μM RSV group, P<0.05).
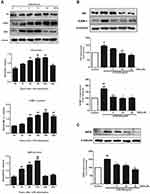
RSV Decreased FN, ICAM-1 and iNOS Expression in LPS-Induced RMCs’ Proliferation
It is well known that the main characteristic of renal fibrosis is mesangial cell proliferation and ECM accumulation leading to the accumulation of FN in mesangium.23 Simultaneously, abnormal expression of inflammatory mediators, such as ICAM-1 and iNOS, could further promote the development of renal fibrosis. In this study, LPS treatment could significantly enhance the protein expression of FN, ICAM-1 and iNOS in a time-dependent manner (Figure 2A). The increment in FN and ICAM-1 expression both reached the maximum at 24 h under LPS stimulation (Figure 2A, P<0.01), while the increment in iNOS expression reached the maximum at 12 h (P<0.01). Such enhanced expression of FN was significantly inhibited by RSV at 10 μM and 20 μM, whereas all the indicated concentrations of RSV statistically reduced ICAM-1 and iNOS expression (Figure 2B and C).
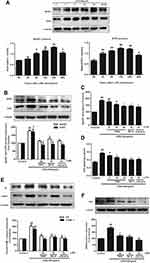
Effects of RSV on SphK1/S1P2 Pathway in LPS-Induced RMCs’ Proliferation
Previous studies confirmed that SphK1/S1P pathway activation is implicated in the development of DN.12,24 Our research group illuminated that RSV could effectively ameliorate the renal function of STZ-induced diabetic rats,31 and attenuate cell proliferation and inflammation via inhibiting high glucose-induced SphK1/S1P2 pathway.22 To investigate whether the potential mechanism of RSV was independent of its hypoglycemic effect, we observed the effect of RSV on the activation of SphK1/S1P2 pathway in RMCs under LPS stimulation.
Firstly, SphK1 and S1P2 expression were examined at different times under LPS stimulation. As indicated in Figure 3A, LPS stimulation time-dependently, statistically increased the expression of SphK1 and S1P2, and both of them reached the maximum at 24 h (P<0.01). Secondly, we chose RSV at 20 μM which manifested the best inhibitory effect on cell proliferation and inflammation. And SK-II (10 μM) as an SphK1-specific inhibitor, JTE-013 (10 μM) as a selective S1P2 inhibitor, were both used as positive controls. RSV at 20 μM inhibited the increments of SphK1 and S1P2 levels, which is similar to this effect of JTE-013 but stronger than that of SK-II. And there was no statistically significant difference in SphK1 and S1P2 expression among 20 μM RSV group, SK-II and JTE-013 (Figure 3B, P<0.01). Thirdly, we detected SphK1 activity and S1P content. As indicated in Figure 3C and D, LPS significantly enhanced SphK1 activity and S1P level (P< 0.01), while RSV dose-dependently reversed the increments in SphK1 activity and S1P level (P<0.05). This occurrence was also blocked by SK-II and JTE-013. RSV at 20 μM significantly decreased the increments of SphK1 activity and S1P level, which was also similar to that of SK-II and JTE-013. All the results are similar to the variation of SphK1 and S1P2 proteins’ expression.
In addition, to further investigate whether the effect of RSV on FN, ICAM-1 and iNOS expression is associated with SphK1/S1P2 pathway, we also used SK-II and JTE-013 as positive controls to measure these proteins’ expression. As shown in Figure 3E and F, RSV at 20 μM also markedly decreased FN and ICAM-1 expression, stronger than that of SK-II and similar to that achieved by JTE-013, but there was no statistically significant difference in iNOS expression among 20 μM RSV group, SK-II and JTE-013.
Overall, these results suggested that inhibiting LPS-induced SphK1/S1P2 pathway in RMCs might be a new underlying mechanism for RSV and there may be a direct close relationship between RSV and S1P2 that should be further investigated.
Effect of RSV on NF-κB Activation in LPS-Induced RMCs’ Proliferation
NF-κB is a key downstream mediator of SphK1/S1P axis, which could play a critical role during cell proliferation and inflammatory response. The expression of fibrotic components such as FN, and inflammatory molecules such as ICAM-1 and iNOS, should be regulated by NF-κB activation. Here, we further examined whether the protective effect of RSV on DN contributed to SphK1/S1P2-dependent depression of NF-κB. At first, we detected DNA-binding activity of NF-κB by using EMSA assay. As shown in Figure 4A, the DNA-binding activity of NF-κB was enhanced in LPS-induced RMCs and evidently blocked by RSV at 20 μM. SK-II and JTE-013 inhibited the DNA-binding activity of NF-κB more obviously than 20 μM RSV group. Then, we detected NF-κB p65 expression in the nucleus. LPS could markedly promote NF-κB p65 transferring into the nucleus (Figure 4B, P<0.01) and this occurrence was intervened by RSV pretreatment. Furthermore, we found that the inhibitory effect of JTE-013 was stronger than that of 20 μM RSV group and SK-II (Figure 4B, P<0.05). This occurrence is consistent with EMSA results. However, there was no statistically significant difference in NF-κB p65 expression in nucleus among 20 μM RSV group, SK-II and JTE-013. These results revealed that RSV could also inhibit LPS-induced DNA-binding activity and protein expression of NF-κB in RMCs, which may contribute to SphK1/S1P2-dependent.
Discussion
The present study has clearly shown that RSV could decrease LPS-induced mesangial cell proliferation and attenuate the upregulated expression of FN, ICAM-1 and iNOS. Additionally, RSV, SK-II and JTE-013 pretreatment all obviously decreased LPS-induced SphK1 activity and the expression of SphK1 and S1P2, and then reduced the transcriptional activity of NF-κB and NF-κB p65 expression that could regulate FN, ICAM-1 and iNOS expression, suggesting that SphK1/S1P2/NF-κB pathway may have a pivotal role in regulation of RMC proliferation and inflammation, which is inhibited by RSV. In addition, our previous study demonstrated that such elevated activity and expression of SphK1 and S1P were reversed by RSV in RMCs transfected with wild type SphK1 under normal condition.20 Taken together, these results have further suggested that the renal protective effect of RSV was independent upon its hypoglycemic effect. So far, our studies have implied that RSV has renoprotective effects on RMCs’ proliferation and inflammation via SphK1/S1P pathway in different mesangial cell models from different perspectives, which may provide novel strategies for DN therapy.
The typical characteristics of DN include mesangial cell proliferation, early glomerular hypertrophy, ECM accumulation such as FN, finally leading to renal fibrosis.6,7 Some other molecules and cytokines, such as ICAM-1 and iNOS, could also be secreted by glomerular mesangial cells.25 ICAM-1, an important cellular adhesion molecule associated with inflammation, promotes cellular inflammatory response and subsequently accelerates diabetic glomerulosclerosis.26,27 iNOS, another inflammatory mediator, is normally expressed at low level in glomerular mesangial cells, but highly expressed when stimulated by cytokines and endotoxins. It may lead to NO overproduction, which is associated with glomerular dilatation and capillary dilatation during the early stage of diabetes.28,29 Thus, these events can worsen abnormal kidney hemodynamic processes, and then result in diabetic glomerular hyperfiltration, ECM accumulation, and renal impairment.30 Furthermore, there is no specific medicine for diabetic nephropathy in clinical treatment as yet.
RSV, a natural polyphenolic substance with its anti-oxidant, anti-inflammatory and anti-fibrotic capabilities, has attracted wide attention as a novel therapeutic approach for many inflammatory diseases, including DN. RSV could reduce renal hypertrophy and ECM accumulation in diabetic model animals,13,31–33 and suppresses mesangial cells’ proliferation under high glucose conditions.13,22,34 Here, we showed that RSV obviously reversed LPS-stimulated mesangial cell proliferation. RSV at the concentration over 40 µM exhibited cytotoxicity in RMCs (data not shown). Simultaneously, referring to other reference,19 lower concentrations of RSV (5–20 µM) were used in our experiments. In addition, LPS could time-dependently upregulate FN, ICAM-1 and iNOS expression, which could be dose-dependently downregulated by RSV.
It has been shown that abnormal activation of SphK1/S1P signaling is closely involved in many diseases such as cancer, diabetes, and fibrosis.9,12,35–37 Several studies have also reported that SphK1/S1P pathway is closely related to the pathogenesis of DN.9,11,12 SphK1 is a lipid kinase which catalyzes the phosphorylation of sphingosine to generate S1P.8 S1P can induce cell proliferation and migration and also upregulate the secretion and expression of pro-fibrotic growth factors such as CTGF and FN.9,12 Elevated levels of SphK1 and S1P have been observed in the kidney tissues of STZ-induced diabetic rats or db/db mice, and high glucose-cultured or advanced glycation end products (AGEs)-cultured glomerular mesangial cells. As far as we know, such observation has not yet been reported in LPS-induced mesangial cells’ proliferation. Our current results showed that LPS significantly enhanced the levels of SphK1 and S1P. Since SK-II at 10 µM seemed to be a suitable concentration, without cytotoxicity, to suppress SphK1 activity in this study and other literature,37 10 µM SK-II treatment was also used in LPS-induced RMCs’ proliferation. As we know, SK-II is a competitive inhibitor of SphK1 and inhibits SphK1 activity mainly by competing with sphingosine (SphK1 substrate) to combine with SphK1. And SK-II markedly suppressed SphK1 activity and expression with the similar effect, and it also significantly reduced S1P production. Furthermore, RSV suppressed SphK1 activity, SphK1 expression and S1P content under LPS stimulation, especially for RSV at 20 µM. In addition, high concentrations of RSV inhibited SphK1 expression more strongly than SK-II, indicating that RSV inhibited those proteins’ expression at least partly through SphK1 pathway. These results were similar with our previous study which was performed in high glucose conditions.22
S1P2, as one of the S1P receptors, is primarily expressed in mesangial cells and play an important role in DN.38 S1P2 receptor mediated S1P-induced mesangial cell proliferation and FN upregulation through activating high glucose-induced MAPK pathway.11 Simultaneously, the increment in cell proliferation and exogenous S1P-induced FN were obviously decreased by S1P2 receptor antagonist JTE-013 in RMCs under normal glycemic and hyperglycemic conditions.12 Additionally, S1P2-siRNA that knocked down S1P2 receptor also significantly inhibited S1P-induced FN augmentation.9,11 Consistent with these observations, we found that both RSV and JTE-013 remarkably attenuated LPS-induced S1P2 protein expression in RMCs, implying that the effect of RSV was closely related to S1P2.
Furthermore, some studies suggested that S1P could activate the NF-κB pathway mainly due to its specific binding to TRAF2 to activate its E3 enzyme,39 which in turn regulates S1P2 expression.40 NF-κB is an inflammatory signaling molecule and its activation was observed in kidneys, retina and heart of diabetic rats and human kidney in DN.41–43 These observations indicated that NF-κB activation may be implicated in DN. However, its specific mechanisms are unclear. Therefore, to determine the exact mechanism whereby RSV deactivates SphK1 activity, further studies are needed. According to previous studies,9,22 NF-κB could be activated by oxidative stress, LPS, hyperglycemia and AGEs, then it binds to κB binding sites on the promoter regions of ICAM-1 and iNOS to initiate target genes’ transcription and proteins’ expression, resulting in obvious inflammatory responses, further accelerating the development of renal fibrosis. Our findings revealed that LPS promoted the translocation of NF-κB p65 to the nucleus and enhanced the DNA-binding activity of NF-κB, whereas these increments were blocked by 20 μM RSV group, SK-II and JTE-013. Especially, JTE-013 was the best one for inhibiting NF-κB p65 expression in nucleus and DNA-binding activity, presuming that there may be a close relationship between S1P2 and NF-κB.
Conclusion
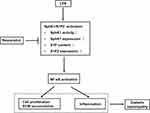
In conclusion, as indicated in Figure 5, this study elucidated that LPS promoted RMCs’ proliferation and ECM accumulation and cell inflammation through SphK1/S1P2/NF-κB pathway, whereas RSV treatment inhibited these increments via blocking this pathway. These results suggested that the protective effect of RSV on renal fibrosis that was independent of its hypoglycemic effect might partially be ascribed to inhibiting SphK1/S1P2/NF-κB pathway. Overall, the findings show new insights into the mechanisms of DN and indicate that targeting this pathway for pharmacological interventions in DN may have broad application prospects. Therefore, further investigation should be done on animal models which may better illustrate the mechanisms of RSV in treatment of DN. And we also believe that RSV should become a potential drug for treating DN in clinical use.
Abbreviations
SphK1, sphingosine kinase 1; S1P, sphingosine 1-phosphate; DN, diabetic nephropathy; RSV, resveratrol; RMC, rat glomerular mesangial cell; FN, fibronectin; ICAM-1, intercellular adhesion molecule-1; iNOS, induced nitric oxide synthase; NF-κB, nuclear factor κB.
Acknowledgments
This work was supported by research grants from the National Natural Science Foundation of China (No. 81603355, No. 81900745) and the Youth Foundation of Guangdong Second Provincial General Hospital (No. YQ2018-008).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Saeedi P, Petersohn I, Salpea P, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
2. Park CW. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J. 2014;38(4):252–260. doi:10.4093/dmj.2014.38.4.252
3. Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant. 2005;20(12):2601–2604. doi:10.1093/ndt/gfi155
4. Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–340. doi:10.1038/nrneph.2011.51
5. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124(3):139–152. doi:10.1042/CS20120198
6. Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi:10.1146/annurev.pathol.4.110807.092150
7. Mariappan MM. Signaling mechanisms in the regulation of renal matrix metabolism in diabetes. Exp Diabetes Res. 2012;2012:749812. doi:10.1155/2012/749812.
8. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nat Rev Mol Cell Biol. 2003;4(5):397–407. doi:10.1038/nrm1103
9. Deng Y, Lan T, Huang J, Huang H. Sphingosine Kinase-1/sphingosine 1-phosphate pathway in diabetic nephropathy. Chin Med J (Engl). 2014;127(16):3004–3010.
10. Zhang X, Ritter JK, Li N. Sphingosine-1-Phosphate pathway in renal fibrosis. Am J Physiol Renal Physiol. 2018;315(4):F752–F756. doi:10.1152/ajprenal.00596.2017
11. Liu W, Lan T, Xie X, et al. S1P2 receptor mediates sphingosine-1-phosphate-induced fibronectin expression via MAPK signaling pathway in mesangial cells under high glucose condition. Exp Cell Res. 2012;318(8):936–943. doi:10.1016/j.yexcr.2012.02.020
12. Lan T, Liu W, Xie X, et al. Sphingosine kinase-1 pathway mediates high glucose-induced fibronectin expression in glomerular mesangial cells. Mol Endocrinol. 2011;25(12):2094–2105. doi:10.1210/me.2011-0095
13. Xu F, Wang Y, Cui W, et al. Resveratrol prevention of diabetic nephropathy is associated with the suppression of renal inflammation and mesangial cell proliferation: possible roles of Akt/NF-κB pathway. Int J Endocrinol. 2014;2014:289327. doi:10.1155/2014/289327.
14. Nymark M, Pussinen PJ, Tuomainen AM, Forsblom C, Groop P-H, Lehto M. Lipopolysaccharide activity is associated with the progression of kidney disease in finnish patients with type 1 diabetes. Diabetes Care. 2009;32(9):1689–1693. doi:10.2337/dc09-0467
15. Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3(4):279–288. doi:10.4161/gmic.19625
16. King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–1534. doi:10.1902/jop.2008.080246
17. Wang H, Gao J, Han Y, et al. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine. 2015;22(5):553–559. doi:10.1016/j.phymed.2015.03.014
18. Chang CC, Chang CY, Wu YT, Huang JP, Yen TH, Hung LM. Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci. 2011;18(1):47. doi:10.1186/1423-0127-18-47.
19. Zhang L, Pang S, Deng B, et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int J Biochem Cell Biol. 2012;44(4):629–638.
20. Elbe H, Vardi N, Esrefoglu M, Ates B, Yologlu S, Taskapan C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum Exp Toxicol. 2015;34(1):100–113. doi:10.1177/0960327114531995
21. Kim MY, Lim JH, Youn HH, et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. 2013;56(1):204–217. doi:10.1007/s00125-012-2747-2
22. Deng Y, Gong W, Li Q, et al. Resveratrol inhibits high glucose-induced activation of AP-1 and NF-κB via SphK1/S1P2 pathway to attenuate mesangial cells proliferation and inflammation. J Funct Foods. 2019;55:86–94. doi:10.1016/j.jff.2019.02.014
23. Gasparitsch M, Arndt A, Pawlitschek F, et al. RAGE-mediated interstitial fibrosis in neonatal obstructive nephropathy is independent of NF-κB activation. Kidney Int. 2013;84(5):911–919. doi:10.1038/ki.2013.171
24. Geoffroy K, Troncy L, Wiernsperger N, Lagarde M, Bawab SE. Glomerular proliferation during early stages of diabetic nephropathy is associated with local increase of sphingosine-1-phosphate levels. FEBS Lett. 2005;579(5):1249–1254. doi:10.1016/j.febslet.2004.12.094
25. Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14(5):1358–1373. doi:10.1097/01.ASN.0000065640.77499.D7
26. Okada S, Shikata K, Matsuda M, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52(10):2586–2593. doi:10.2337/diabetes.52.10.2586
27. Gu HF, Ma J, Gu KT, Brismar K. Association of intercellular adhesion molecule 1 (ICAM1) with diabetes and diabetic nephropathy. Front Endocrinol (Lausanne). 2013;3:179. doi:10.3389/fendo.2012.00179.
28. Hueper K, Hartung D, Gutberlet M, et al. Assessment of impaired vascular reactivity in a rat model of diabetic nephropathy: effect of nitric oxide synthesis inhibition on intrarenal diffusion and oxygenation measured by magnetic resonance imaging. Am J Physiol Renal Physiol. 2013;305(10):F1428–F1435. doi:10.1152/ajprenal.00123.2013
29. Pflueger AC, Larson TS, Hagl S, Knox FG. Role of nitric oxide in intrarenal hemodynamics in experimental diabetes mellitus in rats. Am J Physiol. 1999;277(3):R725–R733.
30. Cosenzi A, Bernobich E, Bonavita M, Trevisan R, Bellini G, Campanacci L. Early effects of diabetes on inducible nitric oxide synthase in the kidney. Acta Diabetol. 2002;39(2):91–96. doi:10.1007/s005920200019
31. Huang K, Huang J, Xie X, et al. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528–540. doi:10.1016/j.freeradbiomed.2013.07.029
32. Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60(2):634–643. doi:10.2337/db10-0386
33. Chen KH, Hung CC, Hsu HH, Jing YH, Yang CW, Chen JK. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-β/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem Biol Interact. 2011;190(1):45–53. doi:10.1016/j.cbi.2011.01.033
34. Ding DF, You N, Wu XM, et al. Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Am J Nephrol. 2010;31(4):363–374. doi:10.1159/000300388
35. Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. doi:10.1016/j.tcb.2011.09.003
36. Li W, Yu CP, Xia JT, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15(4):1393–1399. doi:10.1158/1078-0432.CCR-08-1158
37. Koch A, Völzke A, Wünsche C, Meyer zu Heringdorf D, Huwiler A, Pfeilschifter J. Thiazolidinedione-dependent activation of sphingosine kinase 1 causes an anti- fibrotic effect in renal mesangial cells. Br J Pharmacol. 2012;166(3):1018–1032. doi:10.1111/j.1476-5381.2012.01824.x
38. Imasawa T, Kitamura H, Ohkawa R, Satoh Y, Miyashita A, Yatomi Y. Unbalanced expression of sphingosine 1-phosphate receptors in diabetic nephropathy. Exp Toxicol Pathol. 2010;62(1):53–60. doi:10.1016/j.etp.2009.02.068
39. Alvarez SE, Harikumar KB, Hait NC, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–1088. doi:10.1038/nature09128
40. Huang K, Liu W, Lan T, et al. Berberine reduces fibronectin expression by suppressing the S1P-S1P2 receptor pathway in experimental diabetic nephropathy models. PLoS One. 2012;7(8):e43874. doi:10.1371/journal.pone.0043874.
41. Chen S, Khan ZA, Cukiernik M, Chakrabarti S. Differential activation of NF-kappa B and AP-1 in increased fibronectin synthesis in target organs of diabetic complications. Am J Physiol Endocrinol Metab. 2003;284(6):E1089–E1097. doi:10.1152/ajpendo.00540.2002
42. Mezzano S, Aros C, Droguett A, et al. NF-κB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19(10):2505–2512. doi:10.1093/ndt/gfh207
43. Schmid H, Boucherot A, Yasuda Y, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55(11):2993–3003. doi:10.2337/db06-0477
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.