Back to Journals » Drug Design, Development and Therapy » Volume 13
Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells
Authors Du L, Chen E, Wu T, Ruan Y, Wu S
Received 11 July 2018
Accepted for publication 25 December 2018
Published 22 February 2019 Volume 2019:13 Pages 747—755
DOI https://doi.org/10.2147/DDDT.S179894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Cristiana Tanase
Ligen Du,1–3 Enping Chen,2 Ting Wu,4 Yunjun Ruan,1 Saizhu Wu1
1Department of Geriatrics, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China; 2Department of Cardiology, The Second People’s Hospital of Longgang District, Shenzhen, Guangdong, China; 3Department of Cardiology, Longgang District People’s Hospital of Shenzhen, Guangdong, China; 4Guangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, Guangdong, China
Purpose: Resveratrol (RESV; trans-3,5,4'-trihydroxystilbene) has emerged as a potential new therapeutic for age-related atherosclerotic diseases. However, the effect of RESV on cellular aging and its underlying mechanisms remain unknown. Therefore, the aim of this study was to examine whether RESV can delay cellular aging through upregulation of autophagy.
Materials and methods: Human umbilical endothelial vein cells (HUVECs) were divided into four groups: the control group, and the hydrogen peroxide (H2O2) alone, H2O2 + RESV pretreatment, and H2O2 + 3-methyladenine (3-MA) + RESV pretreatment intervention groups. The cell viability was evaluated by a cell counting kit-8 assay. Superoxide dismutase (SOD) activity and intracellular reactive oxygen species (ROS) levels were tested using commercial kits. Senescence-related β-galactosidase activities were detected by immunohistochemical staining. The expression levels of aging-related and autophagy-related markers, including phosphorylated Rb (p-Rb), LC3, and p62, with or without RESV were measured by Western blotting.
Results: Pretreatment with 10 µM RESV increased the cell viability and SOD levels. The remarkably higher positive rate of senescence-associated β-galactosidase and increased intracellular ROS levels in the H2O2 treatment group were reversed by treatment with 10 µM RESV. As compared to the H2O2 treatment group, 10 µM RESV could upregulate autophagy through the regulation of p-Rb, LC3, and p62 levels. The anti-aging effect of RESV via an autophagy regulation mechanism was further confirmed by the suppression of these effects with 3-MA treatment.
Conclusion: RESV may reverse and delay the aging process of HUVECs via upregulation of autophagy and could be a candidate therapeutic for age-related atherosclerotic diseases.
Keywords: oxidative stress, senescence, LC3, p62, p-Rb
Introduction
The incidence of cardiovascular diseases, which is likely to be exacerbated by an aging population, is increasing worldwide. Epidemiology data have shown that aging is an independent risk factor of cardiovascular diseases, in addition to other traditional risk factors such as diabetes, hypertension, hyperlipidemia, and hyperuricemia.1–4 Thus, controlling the risk factors of aging would help to reduce the incidence of cardiovascular disease. Accordingly, it is of great importance to understand the pathophysiological process of age-related cardiovascular disease for identifying a suitable therapeutic intervention to delay aging.
Oxidative stress participates in the pathological process of cardiac aging by producing excessive amounts of oxygen free radicals, which destroy mitochondrial DNA, alter mitochondrial function, trigger cell senescence, and activate apoptotic signals.5–8 Our previous study demonstrated that dehydroepiandrosterone reduced the expression level of endothelial nitric oxide synthase and weakened the function of oxidation resistance in rat arteries,9 indicating that drug intervention can reduce oxidative stress injury. More recently, we reported that estrogen can resist oxidative stress, protect endothelial function, and delay cellular aging.10 Several studies have also suggested the cardioprotective effects of estrogen, and its potential for the treatment of coronary heart disease.11,12 However, the long-term use of estrogen might pose serious health risks, including increasing the risk of cancer or thromboembolic disease,13,14 thereby limiting its clinical application.
Resveratrol (RESV) is a natural phytoestrogen that exhibits estrogen-like effects but with fewer adverse effects. In particular, RESV can promote cell proliferation through estrogen receptor and extracellular signal-regulated kinase activation.15 Moreover, RESV-mediated neuroprotective effects may be mediated by estrogen receptor.16 Our previous study showed that estrogen can delay the senescence of H2O2-induced endothelial cells, and more recent studies support that RESV has an estrogenic effect. Therefore, we hypothesized that RESV may inhibit oxidative stress, improve endothelial function, and delay aging.
Although the protective effects of RESV on the cardiovascular system have been revealed,17,18 the underlying mechanisms are not clear. Autophagy is an evolutionary conserved self-digesting process, which can degrade retired proteins and organelles to regenerate energy and nutrition.19,20 Moreover, RESV has been found to protect against neurologic diseases and tumors via regulating autophagy.21–24 However, few studies have focused on the role of RESV-induced autophagy in age-related cardiovascular disease. As reported, RESV has been suggested to be an autophagy inducer. RESV was shown to induce basal autophagy and demonstrated a protective effect in atherosclerosis via an autophagic pathway. In 2010, Kao et al reported that RESV protects human endothelium from H2O2-induced oxidative stress and senescence via SirT1 activation.25 Moreover, in 2013, Chen et al revealed the underlying signaling pathway through which RESV induces autophagy in human umbilical endothelial vein cells (HUVECs) under inflammation via the cAMP signaling pathway.26 In our study, we established an H2O2-induced cellular aging model to examine whether RESV can delay the senescence of HUVECs via autophagy in a new degree. Rb is a crucial regulator of cell cycle progression, which can be modulated by posttranslational modifications such as phosphorylation. In senescent cells, the level of p-Rb is reduced due to the inactivation of upstream kinases. Thus, the age-related Rb protein and its phosphorylated levels, along with the expression of key autophagy-related proteins LC3 and p62, were detected to examine the effect of RESV intervention on autophagy-related cellular aging. These results could provide a theoretical foundation for the potential of RESV as targeted therapy for aging-related atherosclerotic diseases.
Materials and methods
Cell culture and drug treatment
The HUVECs (ScienCell, San Diego, CA, USA) were cultured in endothelial cell culture medium (ECM; ScienCell) supplemented with 5% FBS (ScienCell), 1% endothelial cell growth supplement (ScienCell), and 1% penicillin/streptomycin solution (ScienCell), followed by incubation at 37°C in a humidified 5% CO2 incubator. For all experiments, HUVECs were used between the third and fifth passages. The cells were cultured for 24 hours in the presence and absence of the potential autophagy agonist RESV (Sigma-Aldrich Co., St Louis, MO, USA) or 5 mM of the autophagy inhibitor 3-methyladenine (3-MA) (Sigma) prior to H2O2 intervention.
First, the HUVECs were incubated for 24 hours in ECM containing different concentrations of H2O2 (25–200 μM) and then the optimized concentration (100 μM) was selected to build a cellular aging model according to the references and our previous data. Second, the cells were incubated with 5, 10, or 50 μM of RESV in the presence of 100 μM H2O2 for 24 hours to determine the effect of RESV on H2O2-induced cellular senescence. Finally, to examine the effects of RESV on cellular aging, HUVECs were divided into the following four groups: 1) control group: the cells were cultured in ECM only; 2) H2O2 intervention group: the cells were exposed to 100 μM H2O2 in ECM for 24 hours; 3) RESV intervention group (H2O2 + RESV): cells were treated with 10 μM RESV 1 hour before stimulation with H2O2; and 4) 3-MA intervention group (H2O2 + RESV + 3-MA): cells were treated with 5 mM 3-MA 1 hour before the 10 μM RESV intervention and subsequent H2O2 stimulation. Five parallel controls were set up for each group.
Cell viability assays
HUVECs with density of 1×104 cells per well were seeded in 96-well plates and exposed to different concentrations of H2O2 in the absence and presence of RESV. The cells were then incubated with 5% CO2 at 37°C for 24 hours. Subsequently, 10 μL of cell counting kit-8 reagent (Beyotime, Jiangshu, China) and 100 μL of fresh ECM were added to each well after changing the medium, followed by incubation at 37°C, 5% CO2 for 2 hours. The cell viability was measured at 450 nm on a microplate reader.
Measurement of SOD levels
HUVECs were seeded in 24-well plates at a density of 1×105 cells per well in ECM and incubated for 24 hours. The measurement of superoxide dismutase (SOD) levels was performed using a kit from Nanjing Jianchen (Jiangsu, China) according to the manufacturer’s protocol.
Measurement of intracellular ROS levels
Intracellular reactive oxygen species (ROS) accumulation was measured using an ROS assay kit (Nanjing Jianchen) according to the manufacturer’s protocol. Cultured cells were incubated with 100 μM H2O2 for 24 hours in the absence and presence of 10 μM RESV. Treated cells were then washed three times with appropriate ECM to remove extracellular fluorescent substances. The cells were then incubated with 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA) at 37°C for 20 minutes; the DCF fluorescence of treated cells was detected by a fluorescence microscope directly.
SA-β-galactosidase (SA-β-gal) staining
The cells were fixed by incubating in 4% paraformaldehyde and 0.2% glutaraldehyde at 4°C for 5 minutes and were then stained overnight with freshly prepared senescence-associated SA-β-gal (1 mg/mL X-gal, 40 mM citric acid/sodium phosphate [pH 6.0], 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2) at 37°C. The next day, the stained cells were quantified under an optical microscope.
Western blot analysis
The cells were lysed in RIPA lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% sodium deoxycholate, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, and 1 mM ethylenediaminetetraacetic acid. Total protein content in the supernatant was measured using a bicinchoninic acid protein assay kit (Beyotime). Samples of cell lysate supernatant (50 μg protein) were resolved by SDS-PAGE and electrotransferred onto a polyvinylidene fluoride membrane (Millipore, Burlington, MA, USA) and then blocked with 5% skim milk for 1 hour, followed by overnight incubation with specific antibodies against LC3 (catalog no. 3868), p62 (catalog no. 8025), p-Rb (catalog no. 8516 ), Rb (catalog no. 9313 ) and β-actin (catalog no. 4970) (1:1,000 dilution; all from Cell Signaling Technology, Danvers, MA, USA) at 4°C. The following day, the membranes were washed three times for 30 minutes each and then incubated with the secondary antibody horseradish peroxidase-conjugated anti-IgG for 1 hour. After washing with buffer, the membranes were incubated with enhanced chemiluminescence reagents (Applygen, Beijing, China) to visualize the protein signals. Quantification of band intensity was carried out using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The band intensity of β-actin was used as a control.
Statistical analyses
Data for three independent experiments are expressed as the mean ± SD. Multiple comparisons between groups were performed using one-way ANOVA followed by the least-significant difference test. Differences between the treatment groups were analyzed by a two-tailed Student’s t-test. P-values of <0.05 were considered statistically significant.
Results
RESV improved cell viability
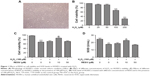
Figure 1A shows the morphology of HUVECs incubated in ECM with 5% CO2 at 37°C. H2O2 markedly inhibited cell viability (Figure 1B) in a dose-dependent manner, with a significant difference in the degree of reduction in viability between the 100 μM H2O2-treated group and 200 μM H2O2-treated group compared to the control group. This suggested that a high concentration (200 μM) of H2O2 may induce cell growth arrest, leading to cell apoptosis or necrosis; therefore, treatment of 100 μM of H2O2 for 24 hours was selected for subsequent experiments.
Moreover, pretreatment with 10 μM of RESV significantly increased cell viability compared to that in the H2O2 group (Figure 1C). However, pretreatment with 50 μM of RESV did not significantly increase cell viability compared to that in the H2O2 group (Figure 1C). Therefore, 10 μM was selected as the concentration of RESV treatment for subsequent experiments.
RESV increased SOD levels
As shown in Figure 1D, H2O2 significantly decreased the SOD concentration compared to that of the control group, and the addition of 10 μM RESV reversed this decrease in H2O2-induced SOD levels. However, there were no statistically significant differences between the 5 and 50 μM RESV groups as compared with the H2O2 group.
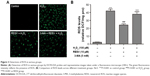
RESV suppressed the accumulation of H2O2-induced SA-β-gal
The SA-β-gal staining assay was used to detect the level senescence in HUVECs induced by H2O2. As illustrated in Figure 2, there was a significant difference in the positive staining rates of cells among the four groups (P<0.001). Compared to the control group, the positive rate of cells in the H2O2 group increased significantly (P<0.001), and this increase was reversed by pretreatment with RESV (P<0.001). However, pretreatment with the autophagy antagonist 3-MA partially blocked this anti-aging effect of RESV (P<0.001).
RESV suppressed H2O2-induced ROS generation
The ROS levels significantly differed among the four groups (P<0.001, Figure 3A and B). The intracellular ROS levels in the cells treated with H2O2 were increased significantly as compared to those of the control group (P<0.001), and decreased levels of ROS were detected in the RESV intervention group compared to those of the H2O2 group (P<0.001). However, pretreatment with 3-MA significantly increased the ROS levels compared to those of the RESV intervention group (P<0.001).
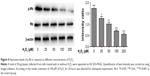
The protective effect of RESV was associated with autophagy regulation
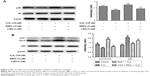
As shown in Figure 4, the levels of p-Rb decreased as the H2O2 concentrations increased from 25 to 200 μM (P<0.05) compared to those of the control group.
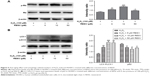
The autophagy indexes of the LC3 II/LC3 I ratio and p62 expression were measured by Western blotting, demonstrating that the protective effect of RESV was largely dependent on the autophagy progress: the LC3 II/LC3 I protein ratio increased significantly, while the expression level of p62 decreased significantly in the 10 μM RESV treatment group compared to the H2O2 group (P<0.05, Figure 5A and B). However, there were no significant differences in p62 expression levels between the 5 and 50 μM RESV groups and the H2O2 group (Figure 5A and B). These results suggested that the progress of autophagic degradation of the substrates was blocked, and the ability of autophagy was limited in the presence of 5 and 50 μM RESV (Figure 5A and B).
Moreover, the concentrations of p-Rb and the LC3 II/LC3 I ratio decreased (P<0.05, Figure 6A and B), while the expression of p62 increased in the H2O2 group compared to those of the control group (P<0.05, Figure 6A and B). These results indicated that HUVECs senescence was associated with a decrease in autophagy to accelerate aging. In contrast, after combined H2O2 and RESV treatment, there was a significant increase in the LC3 II/LC3 I protein ratio and p-Rb levels and a reduction in p62 expression levels (P<0.05) as compared to those in the H2O2 treatment-only group. These results indicated that RESV (10 μM) could upregulate autophagy and delay aging in H2O2-induced HUVECs.
3-MA suppressed the anti-aging effect of RESV by inhibiting autophagy
To further confirm the relationship between the anti-aging effect of RESV and autophagy, the cells were treated with the autophagy inhibitor 3-MA along with RESV. After pretreatment with 3-MA, the levels of p-Rb and LC3 proteins decreased (Figure 6A and B), while the expression level of p62 protein increased as compared to those of the RESV treatment group (Figure 6B). These results further suggested that RESV may delay the senescence of HUVECs via the autophagic pathway.
Discussion
Oxidative damage to cells is considered to be one of the major causes of aging.27,28 Oxidative stress can be caused by endogenous ROS overproduction and impairment of mitochondrial homeostasis. Such excessive accumulation of abnormal substances may cause lipid peroxidation and destruction of the cell membrane, endoplasmic reticulum, and mitochondrial membrane,29,30 resulting in cell cycle stagnation if the cell repair mechanism is impaired. Persistent and severe oxidative stress can also initiate apoptotic signaling by releasing pro-apoptotic proteins, thereby activating the downstream caspase family proteins through a series of cascade amplification reactions, and eventually leading to cell death.31 Our previous study showed that endothelial cells can induce cellular senescence after stimulation with H2O2.10 The present study further demonstrated that the intensity of oxidative stress is associated with the positive rate of aging, and H2O2-induced oxidative stress affected HUVECs senescence in a concentration-dependent manner. In addition, we found that induction of autophagy was closely related to the concentrations of RESV. When cells were in the aging state, ROS levels increased (Figure 3A and B), the positive rate of senescent cells increased (Figure 2), and the level of the aging-related protein p-Rb decreased gradually (Figure 4), which suggested that excessive oxidative stress can trigger cellular senescence and accelerate cell death. Moreover, the level of the antioxidant protein SOD decreased during oxidative stress (Figure 1D), suggesting an imbalance between oxidation and anti-oxidation during the aging process. Our study also revealed that 50 μM of RESV did not significantly improve cell viability and SOD levels after H2O2 treatment. We speculate that the antioxidant capacity of RESV may be related to cell concentration and that excessive RESV may inhibit cell growth. In a healthy physiological state, the production and elimination of oxygen free radicals in the human body are dynamically regulated to achieve a balance. Antioxidant enzymes can catalyze the decomposition of free radicals and thus maintain a constant internal environment in the body.32 However, when the production of ROS exceeds the capacity of the antioxidant defense systems, the aging process can be accelerated. We here demonstrated that cell senescence decreased the autophagy ability, while RESV treatment could delay senescence by regulating the autophagy pathway. Thus, RESV shows potential as an effective therapeutic option to prevent oxidative injury to aging cells and maintain endothelial cell survival.
Our previous study confirmed that estrogen protects endothelial cells and delays cellular senescence, and subsequent studies confirmed the role of estrogen in protecting cardiac function.33,34 However, the long-term usage of estrogen can lead to serious side effects such as increasing the risk of stroke, breast cancer, ovarian cancer, and others.13,14,35 Therefore, the clinical application of estrogen is limited, pointing to the potential for RESV as a new phytoestrogen with estrogenic effects but without the side effects and risks.36,37
Although the underlying mechanisms of RESV in delaying the senescence of HUVECs are currently not clear, our results suggest that RESV can upregulate autophagy, reduce oxidative stress, and delay the aging of cells. Autophagy can remove misfolded or aggregated proteins, eliminate intracellular pathogens, and degrade damaged organelles, including the mitochondria, endoplasmic reticulum, and peroxisomes. Some studies have also suggested that RESV can protect neurological functions and inhibit tumor development through induction of autophagy.38,39 To further confirm the anti-aging effect of RESV, we observed the changes in autophagy-related markers and cellular senescence between the RESV and 3-MA intervention groups. The autophagy effect of RESV was blocked by 3-MA treatment, which further established that RESV may play an important role in anti-aging treatment through the autophagy pathway. The mechanism may be that oxidative stress causes damage to cells by inducing endothelial cell barrier dysfunction, thereby blocking the autophagic pathway so that abnormal generation of ROS exceeds the body’s ability to metabolize them to ultimately accelerate the aging process. By reducing the ROS levels, RESV could decrease DNA damage accumulation by removal of damaged mitochondria and toxic free radicals.40–42 Therefore, RESV can inhibit oxidative stress, promote autophagy, improve vascular endothelial function, maintain cellular homeostasis of the internal and external environment, and eventually delay the aging process.
The aging of endothelial cells induces vascular senescence and changes the molecular biology of cells. Vascular aging is one of the risk factors for atherosclerosis, which can increase arterial stiffness and weaken elasticity, leading to endothelial dysfunction and the occurrence of atherosclerotic disease.43,44 RESV, through promoting the autophagy of aging cells, can degrade harmful substances such as damaged proteins and organelles, delay the pathological progress of aging, and prevent the occurrence of aging cardiovascular diseases. These findings provide new insights into the prevention and treatment of aging-related diseases, indicating that RESV has good application prospect and suggesting that it will be a novel lead compound for the treatment of aging-related atherosclerotic diseases.
The current understanding of RESV-mediated autophagy is still in its preliminary stage, and there are some limitations of our study. First, we only examined the effect of RESV on aged HUVECs at the protein level. Second, further studies on the specific autophagic pathways triggered by RESV are needed. More experimental data are required to support the use of RESV in the treatment of aging-related atherosclerotic diseases.
Conclusion
Oxidative stress plays an important role in the cellular aging progress, whereas RESV could delay aging through upregulating autophagic pathways in HUVECs. These findings support the potential of RESV for the prevention and treatment of age-related arteriosclerotic diseases.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81771507), Natural Science Foundation of Guangdong Province (2016A030313755), and Special Fund for Economic and Technological Development of Longgang District in Shenzhen (201606031610304).
Disclosure
The authors report no conflict of interest in this work.
References
Wu J, Xia S, Kalionis B, Wan W, Sun T. The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res Int. 2014;2014:615312. | ||
de Almeida AJPO, Ribeiro TP, de Medeiros IA. Aging: molecular pathways and implications on the cardiovascular system. Oxid Med Cell Longev. 2017;2017(6):1–19. | ||
Dharmarajan K, McNamara RL, Wang Y, et al. Age differences in hospital mortality for acute myocardial infarction: implications for hospital profiling. Ann Intern Med. 2017;167(8):555–564. | ||
Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89(Pt B):122–135. | ||
Niemann J, Johne C, Schröder S, et al. An mtDNA mutation accelerates liver aging by interfering with the ROS response and mitochondrial life cycle. Free Radic Biol Med. 2017;102:174–187. | ||
Ivanova DG, Yankova TM. The free radical theory of aging in search of a strategy for increasing life span. Folia Med (Plovdiv). 2013;55(1):33–41. | ||
Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial metabolism in aging heart. Circ Res. 2016;118(10):1593–1611. | ||
van de Ven RAH, Santos D, Haigis MC. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med. 2017;23(4):320–331. | ||
Wu S, Ruan Y, Yin M, Lai W. Research on the age-related changes in the nitric oxide pathway in the arteries of rats and the intervention effect of dehydroepiandrosterone. Gerontology. 2007;53(4):234–237. | ||
Ruan Y, Wu S, Zhang L, Chen G, Lai W. Retarding the senescence of human vascular endothelial cells induced by hydrogen peroxide: effects of 17 beta-estradiol (E2) mediated mitochondria protection. Biogerontology. 2014;15(4):367–375. | ||
Poornima IG, Mackey RH, Allison MA, et al; WHI and WHI-CAC Study Investigators. Coronary artery calcification (CAC) and post-trial cardiovascular events and mortality within the women’s health Initiative (WHI) estrogen-alone trial. J Am Heart Assoc. 2017;6(11):e006887. | ||
Cong B, Zhu X, Cao B, Xiao J, Wang Z, Ni X. Estrogens protect myocardium against ischemia/reperfusion insult by up-regulation of CRH receptor type 2 in female rats. Int J Cardiol. 2013;168(5):4755–4760. | ||
Marjoribanks J, Farquhar C, Roberts H. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Db Syst Rev. 2012:CD004143. | ||
Rosenberg L, Bethea TN, Viscidi E, et al. Postmenopausal female hormone use and estrogen receptor-positive and -negative breast cancer in African American women. J Natl Cancer Inst. 2016;108(4):djv361. | ||
Dai Z, Li Y, Quarles LD, et al. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14(12):806–814. | ||
Saleh MC, Connell BJ, Saleh TM. Resveratrol induced neuroprotection is mediated via both estrogen receptor subtypes, ER(α) and ER(β). Neurosci Lett. 2013;548:217–221. | ||
Gliemann L, Schmidt JF, Olesen J, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591(20):5047–5059. | ||
Bird JK, Raederstorff D, Weber P, Steinert RE. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Adv Nutr. 2017;8(6):839–849. | ||
Gao Y, Chu S, Zhang Z, et al. Early stage functions of mitochondrial autophagy and oxidative stress in acetaminophen-induced liver injury. J Cell Biochem. 2017;118(10):3130–3141. | ||
Saha S, Sadhukhan P, Mahalanobish S, Dutta S, Sil PC. Ameliorative role of genistein against age-dependent chronic arsenic toxicity in murine brains via the regulation of oxidative stress and inflammatory signaling cascades. J Nutr Biochem. 2018;55:26–40. | ||
Hu J, Han H, Cao P, et al. Resveratrol improves neuron protection and functional recovery through enhancement of autophagy after spinal cord injury in mice. Am J Transl Res. 2017;9(10):4607–4616. | ||
Kou X, Chen N. Resveratrol as a natural autophagy regulator for prevention and treatment of Alzheimer’s disease. Nutrients. 2017;9:927. | ||
Zhu H, Ding J, Wu J, et al. Resveratrol attenuates bone cancer pain through regulating the expression levels of ASIC3 and activating cell autophagy. Acta Biochim Biophys Sin. 2017;49(11):1008–1014. | ||
Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta. 1852;2015:1178–1185. | ||
Kao CL, Chen LK, Chang YL, et al. Resveratrol protects human endothelium from H2O2-induced oxidative stress and senescence via SIRT1 activation. J Atheroslcer Thromb. 2010;17(9):970–979. | ||
Chen ML, Yi L, Jin X, et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy. 2013;9(12):2033–2045. | ||
Schosserer M, Grillari J, Wolfrum C, Scheideler M. Age-induced changes in white, brite, and brown adipose depots: a mini-review. Gerontology. 2018;64(3):229–236. | ||
Diamanti-Kandarakis E, Dattilo M, Macut D, et al; COMBO ENDO TEAM: 2016. Mechanisms in endocrinology: aging and anti-aging: a combo-endocrinology overview. Eur J Endocrinol. 2017;176(6):R283–R308. | ||
Sanz A, Fernández-Ayala DJ, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging. 2010;2(4):200–223. | ||
Yuzefovych LV, LeDoux SP, Wilson GL, Rachek LI. Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: crosstalk, links and signaling. PLoS One. 2013;8(12):e83349. | ||
Yüksel E, Naziroğlu M, Şahin M, Çiğ B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: protective role of selenium. Sci Rep. 2017;7(1):17543. | ||
Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD. Association of oxidative DNA damage, protein oxidation and antioxidant function with oxidative stress induced cellular injury in pre-eclamptic/eclamptic mothers during fetal circulation. Chem Biol Interact. 2014;208:77–83. | ||
Dakin RS, Walker BR, Seckl JR, Hadoke PWF, Drake AJ. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int J Obes (Lond). 2015;39(10):1539–1547. | ||
Zafirovic S, Obradovic M, Sudar-Milovanovic E, et al. 17β-estradiol protects against the effects of a high fat diet on cardiac glucose, lipid and nitric oxide metabolism in rats. Mol Cell Endocrinol. 2017;446:12–20. | ||
Carrasquilla GD, Frumento P, Berglund A, et al. Postmenopausal hormone therapy and risk of stroke: a pooled analysis of data from population-based cohort studies. PLoS Med. 2017;14(11):e1002445. | ||
Nguyen BT, Kararigas G, Wuttke W, Jarry H. Long-term treatment of ovariectomized mice with estradiol or phytoestrogens as a new model to study the role of estrogenic substances in the heart. Planta Med. 2012;78(1):6–11. | ||
Lee GA, Hwang KA, Choi KC. Roles of dietary phytoestrogens on the regulation of epithelial-mesenchymal transition in diverse cancer metastasis. Toxins (Basel). 2016;8(6):162. | ||
Vidoni C, Secomandi E, Castiglioni A, Melone MAB, Isidoro C. Resveratrol protects neuronal-like cells expressing mutant huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem Int. 2018;117:174–187. | ||
Fukuda T, Oda K, Wada-Hiraike O, et al. Autophagy inhibition augments resveratrol-induced apoptosis in Ishikawa endometrial cancer cells. Oncol Lett. 2016;12(4):2560–2566. | ||
Colin DJ, Limagne E, Ragot K, et al. The role of reactive oxygen species and subsequent DNA-damage response in the emergence of resistance towards resveratrol in colon cancer models. Cell Death Dis. 2014;5(11):e1533. | ||
Mikuła-Pietrasik J, Kuczmarska A, Rubiś B, et al. Resveratrol delays replicative senescence of human mesothelial cells via mobilization of antioxidative and DNA repair mechanisms. Free Radic Biol Med. 2012;52(11–12):2234–2245. | ||
Vitale N, Kisslinger A, Paladino S, et al. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PLoS One. 2013;8(11):e80728. | ||
Pant R, Marok R, Klein LW. Pathophysiology of coronary vascular remodeling: relationship with traditional risk factors for coronary artery disease. Cardiol Rev. 2014;22(1):13–16. | ||
North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.