Back to Journals » Clinical Ophthalmology » Volume 15
Results of Pars Plana Vitrectomy for Different Types of Macular Holes
Authors Ghoraba HH, Leila M , Zaky AG, Wasfy T, Maamoun Abdelfattah H, Elgemai EM, Mohamed El Gouhary S, Mansour HO, Ghoraba HH, Heikal MA
Received 4 November 2020
Accepted for publication 23 December 2020
Published 12 February 2021 Volume 2021:15 Pages 551—557
DOI https://doi.org/10.2147/OPTH.S290404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hammouda Hamdy Ghoraba,1,2 Mahmoud Leila,3 Adel Galal Zaky,4 Tamer Wasfy,1 Haithem Maamoun Abdelfattah,2,5 Emad Mohamed Elgemai,2,6 Sameh Mohamed El Gouhary,4 Hosam Osman Mansour,7 Hashem Hammouda Ghoraba,1,2 Mohamed Amin Heikal8,9
1Ophthalmology Department, Tanta University, Tanta City, Gharbia, Egypt; 2Ophthalmology Department, Magrabi Eye Hospital, Tanta City, Gharbia, Egypt; 3Retina Department, Research Institute of Ophthalmology, Cairo, Egypt; 4Ophthalmology Department, Menoufia University, Shebein Elkoom City, Menoufia, Egypt; 5Ophthalmology Department, Benha Teaching Hospital, Benha City, Qualuopia, Egypt; 6Ophthalmology Department, Damanhour Teaching Hospital, Dmanhour City, Albehaira, Egypt; 7Alazahr University, Damietta City, Damietta, Egypt; 8Ophthalmology Department, Benha University, Benha City, Qualuopia, Egypt; 9Vitreoretinal Department, Magrabi Eye Hospital, Khober City, Eastern Province, Kingdom of Saudi Arabia
Correspondence: Mohamed Amin Heikal
Ophthalmology Department, Benha University, Benha City, Qualuopia, 13511, Egypt
Tel +00201007131431
Email [email protected]
Purpose: To compare different types of macular holes regarding the anatomic and functional success following pars plana vitrectomy (PPV) and internal limiting membrane (ILM) removal.
Methods: A retrospective review of all patients with macular holes treated by PPV, ILM removal with gas tamponade from January 2014 to July 2017 in Magrabi Eye Hospital.
Results: One hundred fifty-seven eyes of 153 patients were analyzed. The eyes were classified according to the etiology of macular hole into four groups: 79 eyes with idiopathic macular hole (IMH), 51 eyes with traumatic macular hole (TMH), 16 eyes with macular hole in diabetic patients (DMH) and 11 eyes with myopic macular hole (MMH). We classified the IMH group based on the International Vitreomacular Traction Study Classification according to size into 3 subgroups; subgroup 1: ≤ 250μ, subgroup 2: > 250 to 400μ and subgroup 3: ≥ 400 μ. All types of macular hole showed statistically significant postoperative improvement in BCVA compared to the baseline except cases with MMH. Anatomic postoperative hole closure was achieved in 86.1%, 60.7%, 43.65%, an 45.46% of eyes with IMH, TMH, DMH and MMH, respectively. In eyes with IMH, closure rate in subgroup 1 was significantly higher than in subgroups 2, and 3.
Conclusion: PPV, ILM peel and C2F6 technique yielded variable anatomic and functional outcomes in different types of macular holes. Anatomic results were most favorable in IMH and least favorable in MMH. The smaller the diameter of the hole the better the results. The underlying pathogenetic mechanisms that lead to different types of macular holes are pivotal in determining the final outcome.
Keywords: ILM peeling, macular holes, pars plana vitrectomy
Introduction
Full thickness macular hole is defined as a full-thickness defect that involves all layers of the neurosensory retina from the internal limiting membrane (ILM) to the photoreceptors.1 Macular holes may be idiopathic, traumatic, myopic with or without retinal detachment or secondary to proliferative diabetic retinopathy (PDR).2–4 Pars plana vitrectomy (PPV), removal of the posterior hyaloid, ILM peel and gas tamponade is the standard surgical treatment for different types of macular hole with closure rate more than 90%.5–7 The aim of the present study is to compare the anatomic and functional success rates of PPV, ILM peel and gas tamponade in different types of macular hole.
Patients and Methods
We conducted a retrospective review of all patients with macular holes treated by PPV, and ILM peel with gas tamponade from January 2014 to July 2017 in Magrabi Eye Hospital, Tanta, Egypt. The Institutional Review Board (IRB) and Research Ethics Committee of Magrabi Eye Hospitals required that all patients participating in the study signed an informed consent prior to enrollment. The consent included a statement that authorized the authors to publish the data of the patients in an anonymous manner that does not reveal their identity. The study adhered strictly to the tenets of the Declaration of Helsinki (the 2013 revision).
Inclusion Criteria
Eyes were classified according to the etiology into four groups: idiopathic macular hole (IMH), traumatic macular hole (TMH), macular hole in diabetic patient (DMH), and macular hole in myopic patient (MMH). Patients presenting with TMH were included only after the lapse of one-month observation period to allow for spontaneous closure of the hole to happen. The study required that all participants complete a minimum follow-up period of 3 months after surgical intervention.
Exclusion Criteria
The study excluded all patients presenting with macular hole retinal detachment (MHRD), lamellar macular hole, previous surgery for macular hole, and macular holes other than the 4 specified groups.
Examinations
Preoperative evaluation included detailed history, best-corrected visual acuity (BCVA) assessment using Snellen chart and logMAR equivalent for statistical analysis, intraocular pressure measurement (IOP), slit-lamp anterior segment examination, dilated fundus examination using indirect ophthalmoscope and slit-lamp biomicroscopy using 90D lens. Color fundus photo and optical coherence tomography (OCT) were done using Cirrus OCT (v 4.0.7; Carl Zeiss, Meditec, Inc, Dublin, CA). On OCT, the size of the macular hole was assessed by measuring the minimum linear diameter (MLD), which was defined as the distance in microns between the narrowest points of the macular hole in the mid-retina.
Surgical Procedures
A single surgical technique was used for all patients. A single surgeon (HG) performed all surgeries at the same center. The surgical technique consisted of 3-port PPV using transconjunctival cannulated 20 or 23 gauge. Lensectomy was done if the lens was cataractous or if lens touch occurred intraoperatively. Core vitrectomy was performed and followed by induction of posterior vitreous detachment (PVD). PVD continued anteriorly as safely as possible. ILM was peeled in all cases using Eckardt end-gripping forceps. ILM peel was extended over at least an area of 2 disc diameters centered onto the macular hole. Triamcinolone acetonide (TA) was used to assist visualization during peeling of ILM. The signs of ILM removal were whitening of the retina with or without petechial hemorrhage, and dull appearance of denuded retina. Brilliant Blue stain, 0.2 mL (Brilliant Peel; Geuder, Heidelberg, Germany) was used if the ILM could not be identified by TA. The surgeon did not attempt to perform an inverted ILM flap technique in any case. Laser barrage 360° was done routinely, followed by air-fluid exchange and gas injection (16% C2F6; Hexafluoroethane). We used C2F6 in all macular holes included regardless of the diameter because we wanted to standardize the treatment approach in all patients and explore the efficacy of this standard approach across different categories of holes. Patients were instructed to maintain face-down position for 1 week after surgery. All patients were examined on the 1st postoperative day. Postoperative follow-up was scheduled at 1 week, 3 weeks, 6 weeks and 8-weekly thereafter. Fundus examination, color fundus photography and OCT were done whenever possible.
Statistical Analysis
Results were collected, tabulated and statistically analyzed by an IBM compatible personal computer with SPSS statistical package version 23 (SPSS Inc. Released 2015. IBM SPSS statistics for windows, version 23.0, Armonk, NY: IBM Corp.). Kruskal–Wallis test was used for comparison of quantitative variables between more than two groups of not-normal distributed data with Tamhane’s test as post hoc test. Wilcoxon test was used to compare different readings of not-normally distributed data in the same group. Chi-square test (χ2) was used to study association between qualitative variables. Whenever any of the expected cells were <5, Fischer’s Exact test with Yates correction was used.
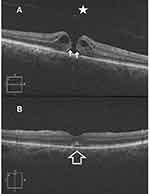
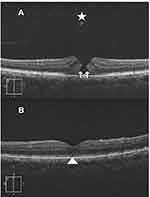
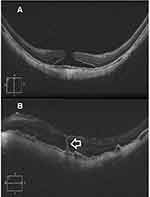
Results (Tables 1–4, Figures 1–3)
The study reviewed the medical records of 201 patients with macular hole. Thirty-one patients with MMH associated with retinal detachment were excluded. Another 17 patients who did not complete the required minimum follow-up period were excluded.
 |
Table 1 Demographic, Preoperative BCVA, Postoperative BCVA and Functional Outcomes of the Different Groups |
 |
Table 2 Comparison Between Pre- and Postoperative BCVA of Different Groups of Macular Holes |
 |
Table 3 Anatomic and Functional Outcomes of Subgroups of Idiopathic Macular Hole |
 |
Table 4 Postoperative VA Stratified According to Macular Hole Status in Different Groups of Macular Holes |
Baseline Characteristics of the Study Population
One hundred fifty-seven eyes of 153 patients were analyzed. The eyes were classified according to the etiology of the macular hole into four groups: 79 eyes with IMH, 51 eyes with TMH, 16 eyes with DMH and 11 eyes with MMH. We further classified the IMH group based on the International Vitreomacular Traction Study Classification according to size into 3 subgroups. Subgroup 1, included eyes with hole size ≤250 µ; n = 36. Subgroup 2, included eyes with hole size ranging from >250–400µ; n = 33. Subgroup 3, included eyes with hole size ≥400 µ; n = 10. The mean preoperative BCVA was distributed among the 4 groups as follows; In the IMH group; 1.10 logMAR ± 0.31, in the TMH group; 1.2 logMAR ± 0.3, in the DMH; 1.21 logMAR ± 0.4 and in the MMH group; 1.1 ± 0.2. Eyes with IMH size ≤250µ had significantly better preoperative BCVA (0.9 logMAR ± 0.3) than eyes with hole size >250–400µ (1.2 logMAR ± 0.2) and eyes with hole size ≥400µ (1.4 logMAR ± 0.04). Eyes with hole size 250–400µ had better BCVA than eyes with hole size ≥400µ.
Postoperative Visual Outcome
Mean postoperative BCVA was 0.7 logMAR ± 0.35, 0.9 logMAR ± 0.3, 1.1 logMAR ± 0.3, and 0.9 logMAR ± 0.3 in IMH, TMH, DMH, and MMH groups, respectively. All macular holes showed statistically significant postoperative improvement in BCVA compared to baseline except cases with MMH (p< 0.05in IMH, TMH, DMH and 0.08 in MMH).
Postoperative Closure Outcome
Macular hole closure was achieved in 86.1%, 61%, 44%, and 45.4% of eyes with IMH, TMH, DMH, and MMH groups, respectively. The rate of hole closure was significantly higher in IMH than other types of holes. Subgroup analysis of the IMH group revealed macular hole closure rate of 97.2%, 79%, and 70% in subgroups 1, 2, and 3, respectively. Macular hole closure rate was significantly higher in subgroup 1 than in subgroups 2, and 3. The postoperative VA stratified according to macular hole status in different groups of macular holes is shown in Table 4.
Discussion
Different pathologies lead to different types of macular hole. IMH may occur due to the anteroposterior and tangential vitreomacular traction exerted by the posterior vitreous cortex at the fovea.8,9 DMH may occur due to tangential traction of the premacular fibrosis superimposed on long standing cystoid macular edema, rupture of the cyst or traction on the already weakened ischemic fovea.3,10 The pathogenesis of TMH is related to the avulsion force applied to the fovea by the vitreous due to anterior-posterior compression or persistent vitreoretinal adhesion.11,12 In MMH, tangential traction with posterior staphyloma may be a predisposing factor.13 In the current study we compared the anatomic and functional outcomes in patients with IMH, TMH, DMH and MMH that underwent PPV and ILM peeling with gas injection. Patients with IMH had significantly higher age than all other types of macular hole. There was a significant improvement in postoperative BCVA of all types of macular holes as compared to baseline except in MMH group (P <0.05 in IMH, TMH, DMH and 0.08 in MMH). In IMH group, we detected that eyes in subgroups 1, and 2 had significant visual improvement (P <0.001). On the other hand, we did not detect similar significant improvement in visual acuity in eyes in subgroup 3 (P=0.05). In addition, visual acuity improvement was related to the size of the hole in the sense that the smaller the hole diameter the better the visual outcome. These results are in accordance with those of Huang et al11 who reported that larger IMHs (basal and apical area) were associated with worse visual acuity. Similarly, previous studies reported that postoperative visual acuity improvement was more frequently demonstrated in patients with small hole sizes <350 µm.14–19 Classifying patients with IMH by the size of the hole into <400 µm group or >400 µm might be clinically significant for counselling the patients about possible improvement after surgery.20 This was evident in present study as the closure rate was 97.2%, 78%, 70% in IMH subgroups 1, 2, and 3, respectively. In addition, we found that the closure rate of small IMH <250µ was statistically significant than the other 2 groups (P= 0.02). Liu et al20 found that the rate of Type 1 closure for patients with smaller preoperative MHs (≤ 250µ) was 100%; whereas rate of closure of holes with size 250µ and ≤ 400µ, was 97%). These figures were significantly better than the closure rate of IMHs ≥400µ (89%). In DMH group, there was statistically significant improvement of the mean logMAR postoperative BCVA compared to baseline (from 1.21 ± 0.39 to1.09 ± 0.34) (P= 0.02). The closure rate was 44% of eyes. Ghoraba3 reported 4 cases with full thickness macular holes without retinal detachment in diabetic patients with vitreous hemorrhage that was discovered intraoperatively. The author did not peel the ILM in any case. Visual acuity improved, though did not exceed 20/200. Macular hole closure rate was 50% and he suggested that diabetic ischemic changes at the macula, prolonged macular detachment, toxicity of sub-macular blood and severe cystoid macular edema might explain the poor visual prognosis in DMH patients. In the TMH, there was highly significant improvement in the mean visual acuity (p <0.001) compared to the diabetic and myopic types (p=0.02and 0.08 respectively). The closure rate in TMH was 61% versus 44% and 45.4% for diabetic and myopic types, respectively. This relatively good recovery of TMH cases might be due to early diagnosis compared to the other 2 types and to better healing in younger patients.21–23 The postoperative BCVA was significantly better in cases with closed macular holes in idiopathic and traumatic groups (p 0.008 and <0.001 respectively); however, in PDR and myopic macular holes there was no significant difference in the BCVA between closed or opened postoperative holes. (p=0.142 and 0.286 respectively).
Previous studies reported significant improvement in visual acuity, and in closure rates that ranged from 42% to 82%.4,24,25 In our series, the visual outcome of MMH patients was poorer than that reported by other studies. Moreover, our closure rate was 45.46%, which was clearly less than that reported by other authors and that exceeded 60%.26,27 Several reasons may explain these results. Firstly, these studies relied only on clinical assessment to determine hole closure and not on OCT finding. Secondly, all myopic eyes included in our study had extensive retinal pigment epithelial atrophy, which limited visual recovery. Thirdly, all myopic eyes were associated with posterior staphyloma, which rendered ILM peeling difficult and required several attempts to perform it and that eventually led to damage of the inner retinal tissues. Finally, all studies that reported the outcome of MMH were limited by small number of cases.
Conclusion
PPV, ILM peel and C2F6 technique yielded variable anatomic and functional outcomes in different types of macular holes. Anatomic results were most favorable in IMH and least favorable in MMH. The smaller the diameter of the hole the better the results. The underlying pathogenetic mechanisms that lead to different types of macular holes are pivotal in determining the final outcome.
Summary
Macular hole is a full-thickness defect that involves all layers of the neurosensory retina. Macular hole leads to central visual loss. The aim of our study is to evaluate the surgical outcome of different types of macular hole; Idiopathic macular hole, traumatic macular hole, myopic macular hole and diabetic macular hole. We did vitrectomy, internal limiting membrane peeling and gas injection to allow the closure of the hole and restore the central vision. We detected different closure rates in the different types of macular holes. The difference in the results was related to the pathology of each type. We found that Idiopathic macular holes had the best anatomic results; whereas myopic macular holes had the least favorable anatomic outcome. The postoperative visual acuity was significantly better in cases with closed macular holes in idiopathic and traumatic groups but not in diabetic and myopic macular holes. The smaller the hole diameter the better the results.
Data Sharing Statement
The data of patients used to support the findings of this study are restricted by the (Magrabi research ethics board). Data are available for researchers who meet the criteria for access to confidential data upon request from Professor Hammouda Ghoraba, Head of vVtreoretinal Department, Magrabi Eye Centers. Email: [email protected].
Disclosure
This work was self-funded by the authors. The authors report no conflicts of interest for this work.
References
1. Frezzotti R, Guerra R. Oftalmologia essenziale.
2. Ho AC, Guyer DR, Fine SL. Macular hole. Surv Ophthalmol. 1998;42(5):393–416. doi:10.1016/S0039-6257(97)00132-X
3. Ghoraba H. Types of macular holes encountered during diabetic vitrectomy. Retina. 2002;22(2):176–182. doi:10.1097/00006982-200204000-00007
4. Ghoraba HH, Ellakwa AF, Ghali AA. Long term result of silicone oil versus gas tamponade in the treatment of traumatic macular holes. Clin Ophthalmol. 2012;6:49–53. doi:10.2147/OPTH.S22061
5. Eckardt C, Eckardt U, Gross S, et al. Removal of internal limiting membrane in macular holes. Clinical and morphological findings. Ophthalmologe. 1997;94:545–551. doi:10.1007/s003470050156
6. Da Mata AP, Burk SE, Riemann CD, et al. Indocyanine green assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology. 2001;108:1187–1192. doi:10.1016/S0161-6420(01)00593-0
7. Ando F, Sasano K, Ohba N, et al. Anatomic and visual outcomes after indocyanine green-assisted peeling of the retinal internal limiting membrane in idiopathic macular hole surgery. Am J Ophthalmol. 2004;137(4):609–614. doi:10.1016/j.ajo.2003.08.038
8. Bainbridge J, Herbert E, Gregor Z. Macular holes: vitreoretinal relationships and surgical approaches. Eye. 2008;22(10):1301–1309. doi:10.1038/eye.2008.23
9. Abdelkader E, Lois N. Internal limiting membrane peeling in vitreo-retinal surgery. Surv Ophthalmol. 2008;53:368–396. doi:10.1016/j.survophthal.2008.04.006
10. Flynn HW. Macular hole surgery in patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1994;112(7):877–878. doi:10.1001/archopht.1994.01090190021011
11. Huang J, Liu X, Wu Z, Sadda S. Comparison of full-thickness traumatic macular holes and idiopathic macular holes by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248(8):1071–1075. doi:10.1007/s00417-009-1226-z
12. Delori F, Pomerantzeff O, Cox MS. Deformation of the globe under high-speed impact: it relation to contusion injuries. Invest Ophthalmol. 1969;8(3):290–301.
13. Oleñik A, Rios J, Mateo C. Inverted internal limiting membrane flap technique for macular holes in high myopia with axial length >30 mm. Retina. 2016:1–6.
14. Gupta B, Laidlaw DA, Williamson TH, et al. Predicting visual success in macular hole surgery. Br J Ophthalmol. 2009;93(11):1488–1491. doi:10.1136/bjo.2008.153189
15. Ezra E, Gregor ZJ; Morfields Macular Hole Study Group. Surgery for idiopathic full-thickness macular hole: two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: moorfields Macular Hole Study Group Report no. 1. Arch Ophthalmol. 2004;122:224–236. doi:10.1001/archopht.122.2.224
16. Ip MS, Baker BJ, Duker JS, et al. Anatomic outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120:29–35. doi:10.1001/archopht.120.1.29
17. Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86:390–393. doi:10.1136/bjo.86.4.390
18. Kusuhara S, Negi A. Predicting visual outcome following surgery for idiopathic macular holes. Ophthalmologica. 2014;231(3):125–132. doi:10.1159/000355492
19. Wakely L, Rahman R, Stephenson J. A comparison of several methods of macular hole measurement using optical coherence tomography, and their value in predicting anatomical and visual outcomes. Br J Ophthalmol. 2012;96(7):1003–1007. doi:10.1136/bjophthalmol-2011-301287
20. Liu L
21. Glaser BM, Michels RG, Kuppermann BD, Sjaarda RN, Pena RA. Transforming growth factor-beta 2 for the treatment of full thickness macular holes. A prospective randomized study. Ophthalmology. 1992;99(7):1162–1173. doi:10.1016/S0161-6420(92)31837-8
22. Smiddy WE, Glaser BM, Thompson JT, et al. Transforming growth factor-beta 2 significantly enhances the ability to flatten the rim of subretinal fluid surrounding macular holes. Preliminary anatomic results of multi-center prospective randomized study. Retina. 1993;13(4):296–301. doi:10.1097/00006982-199313040-00005
23. Thompson JT, Smiddy WE, Williams GA, et al. Comparison of recombinant transforming growth factor beta-2 and placebo as an adjunctive agent for macular hole surgery. Ophthalmology. 1998;105(4):700–706. doi:10.1016/S0161-6420(98)94027-1
24. Bor’i A, Al-Aswad MA, Saad AA, Hamada D, Mahrous A. Pars plana vitrectomy with internal limiting membrane peeling in traumatic macular hole: 14% perfluoropropane (C3F8) versus silicone oil tamponade. J Ophthalmol. 2017;2017:3917696.
25. Miller JB, Yonekawa Y, Eliott D, et al. Long-term follow-up and outcomes in traumatic macular holes. Am J Ophthalmol. 2015;160(6):1255–1258.e1. doi:10.1016/j.ajo.2015.09.004
26. Garcia-Arumi J, Martinez V, Puig J, Corcostegui B. The role of vitreoretinal surgery in the management of myopic macular hole without retinal detachment. Retina. 2001;21(4):332–338. doi:10.1097/00006982-200108000-00006
27. Patel SC, Loo RH, Thompson JT, Sjaarda RN. Macular hole surgery in high myopia. Ophthalmology. 2001;108:377–380. doi:10.1016/S0161-6420(00)00532-7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.