Back to Journals » Cancer Management and Research » Volume 12
Restoration of UPK1A-AS1 Expression Suppresses Cell Proliferation, Migration, and Invasion in Esophageal Squamous Cell Carcinoma Cells Partially by Sponging microRNA-1248
Received 20 November 2019
Accepted for publication 9 March 2020
Published 21 April 2020 Volume 2020:12 Pages 2653—2662
DOI https://doi.org/10.2147/CMAR.S239418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Fang Du,1 Tao Guo,2 Chenghua Cao3
1Department of Hematology and Oncology, No. 988 Hospital of Joint Logistic Support Force of the Chinese People’s Liberation Army, Zhengzhou, Henan Province, People’s Republic of China; 2Pediatric Intensive Care, The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China; 3Translational Research Institute, Henan University, Kaifeng, Henan Province, People’s Republic of China
Correspondence: Fang Du
Department of Hematology and Oncology, No. 988 Hospital of Joint Logistic Support Force of the Chinese People’s Liberation Army, No. 602 Jianshe Road, Zhengzhou, Henan Province, People’s Republic of China
Email [email protected]
Background: Recent evidence suggests that long non-coding RNAs (lncRNAs) are emerging as key determinants of esophageal squamous cell carcinoma (ESCC) progression. This study aimed to investigate the role of lncRNA UPK1A antisense RNA 1 (UPK1A-AS1) in ESCC cell proliferation, invasion, and migration.
Methods: The expression levels of UPK1A-AS1 and miR-1248 were determined using quantitative reverse transcriptase-polymerase chain reaction. The functional role of UPK1A-AS1 in ESCC was investigated using subcellular localization assay, Cell Counting Kit-8 assay, colony formation assay, scratch-healing assay, and transwell invasion assay. The functional interaction between UPK1A-AS1 and miR-1248 was assessed using luciferase reporter and RNA pull-down assays.
Results: Twenty dysregulated lncRNAs were detected in ESCC. Downregulation of UPK1A-AS1 was observed in ESCC tissues and cell lines. Functionally, upregulation of UPK1A-AS1 suppressed the proliferation, migration, and invasion of ESCC cells. Moreover, an inverse correlation between UPK1A-AS1 and miR-1248 expression was observed in ESCC specimens, and miR-1248 was identified as a direct target of UPK1A-AS1. Furthermore, we found that UPK1A-AS1 exerts its anti-cancer effects partially through sponging miR-1248 in ESCC cells.
Conclusion: UPK1A-AS1 suppressed the proliferation, migration, and invasion of ESCC cells partially by sponging miR-1248. Hence, our findings provide novel insights into the regulatory pathway involved in ESCC development.
Keywords: esophageal squamous cell carcinoma, long non-coding RNA, UPK1A antisense RNA 1, miR-1248
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignancies worldwide, accounting for 90% of all esophageal carcinoma cases.1 ESCC is the sixth leading cause of cancer-related deaths, and has become a major global health threat.2 ESCC, unlike other cancers that have been widely studied, has a poor prognosis, with a 5-year survival rate of <25%.3 To date, there are no effective therapies for ESCC.4 Accumulating evidence suggests that genomic and molecular alterations are emerging as key risk factors for developing ESCC, in addition to tobacco smoke, heavy alcohol consumption, and infection with human papillomavirus.5–8 Therefore, a detailed understanding of the genomic and molecular alterations underlying ESCC is of great significance for elucidating its molecular mechanism and contributing to the development of therapeutics against ESCC.
With hundreds of non-coding RNAs (ncRNAs) being studied and identified, researchers have come to realize the importance of ncRNAs, which account for 90% of the human genome.9 Previous evidence suggests the role of ncRNAs in various human diseases, including cancers, thus offering the prospect of identifying novel therapeutic and diagnostic targets.10 Based on their size, ncRNAs can be classified into microRNAs (miRNAs) and long ncRNAs (lncRNAs). miRNAs are a class of single-strand ncRNAs of about 18–22 nucleotides in length, and play a key role in numerous cellular biological processes.11 miRNAs have been reported to exert their tumor-promoting or -inhibiting roles via inhibition of the target gene by base-pairing with its 3ʹ-untranslated region.12 Although the link between miRNAs and carcinogenesis is well established, only a few miRNAs have been mechanistically characterized. miR-1248 has been shown to play an important role in tumorigenesis.13 However, limited studies have focused on the functional role of miR-1248 in ESCC.
lncRNAs are a group of transcripts more than 200 nucleotides in length, without protein-coding potential. In recent decades, the significance of lncRNAs in carcinogenesis has gradually come to light. lncRNAs are no longer regarded as by-products of RNA polymerase II transcription or genomic noises, but rather as key regulators involved in controlling cellular functions.14,15 Previous evidence suggests that lncRNAs participate in the regulation of tumorigenesis via multiple pathways, including epigenetic modification, transcription modulation, RNA decay, and miRNA decoys.16–18 lncRNAs have also been recognized as potential biomarkers for human cancers.19 Recent evidence suggests that dysregulation of lncRNAs is closely associated with the development of human cancers, including ESCC.20 UPK1A antisense RNA 1 (UPK1A-AS1), a newly discovered lncRNA, is located on chromosome 19 with biased expression in the esophageal tissues.21 However, no study has reported the role of UPK1A-AS1 in ESCC.
In this study, we reported the downregulation of UPK1A-AS1 in ESCC tissues and cell lines. Functionally, upregulation of UPK1A-AS1 suppressed the proliferation, migration, and invasion of ESCC cells. Further, we investigated the molecular mechanism by which UPK1A-AS1 influences ESCC carcinogenesis and found that the UPK1A-AS1/miR-1248 axis serves as a novel regulatory axis in ESCC progression.
Materials and Methods
Patients’ Specimens
Following protocols approved by the Institutional Review Board, ESCC tissues and adjacent normal tissues were collected from 30 patients with ESCC who underwent surgery at No. 988 Hospital of Joint Logistic Support Force of the Chinese People's Liberation Army. All patients met the following criteria: ESCC diagnosis was confirmed by pathological examination, no adjuvant therapy was given prior to surgery. Written informed consent was obtained from each participant.
Cell Culture and Transfection
Human ESCC cell lines (EC109, EC9706, KYSE30 and KYSE150) and human immortalized esophageal epithelial cell line (SHEE) were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China). All cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS; Solarbio, Beijing, China) at 37°C and 5% CO2.
The UPK1A-AS1 sequence was introduced into pcDNA-3.1 vector to construct the overexpressing plasmid of UPK1A-AS1 (UPK1A-AS1), and empty pcDNA-3.1 vector (vector) served as the control. miR-1248 mimic and its negative control (miR-NC) were synthesized by Sangon Biotech (Shanghai, China). These plasmids and miRNA mimics were transfected into EC109 and KYSE30 cells using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s recommendations.
Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
RNA isolation was performed using the RNeasy Plus Mini Kit as per the manufacturer’s instructions. cDNA was synthesized using TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), followed by qRT-PCR analysis based on TaqMan® MicroRNA Assay (Applied Biosystems). The expression of UPK1A-AS1 was determined on an ABI 7600 FAST thermal cycler using One Step TB Green™ PrimeScript™ RT-PCR Kit (Takara, Dalian, China). All primer sequences were as follows: UPK1A-AS1 forward: 5ʹ-GAG CGG TGG GTT AGG AAG GT-3ʹ, reverse: 5ʹ-CGT GGG GGT CGG TTG TCT-3ʹ; GAPDH forward: 5ʹ-GGG AGC CAA AAG GGT CAT-3ʹ, reverse: 5ʹ-GAG TCC TTC CAC GAT ACC AA-3ʹ; miR-1248 forward: 5ʹ-GTC CAC CTT CTT GTA TAA GCA CT-3ʹ, reverse: 5ʹ-GCA GGG TCC GAG GTA TTC-3ʹ; U6 forward: 5ʹ-GCT TCG GCA GCA CAT ATA CTA AAA T-3ʹ, reverse: 5ʹ-CGC TTC ACG AAT TTG CGT GTCA T-3ʹ. Target gene expression was calculated using the 2–ΔΔCt method and normalized to glyceraldehyde-3-phosphate dehydrogenase or U6 expression. UPK1A-AS1 expression level was evaluated by the ratio of UPK1A-AS1 level/GAPDH mRNA level. miR-1248 expression level was evaluated by the ratio of miR-1248 level/U6 level.
Cell Counting Kit-8 (CCK-8) Assay
After transfection, EC109 and KYSE30 cells were seeded in 96-well plates and cultured for 24 h at 37°C in 5% CO2. All cells were incubated with CCK-8 solution for 2 h. Cell viability was evaluated by measuring the absorbance at 450 nm.
Colony Formation Assay
After transfection, EC109 and KYSE30 cells (1 × 103 cells per well) were seeded in a 6-well plate. Colonies were fixed and stained with crystal violet solution (Solarbio) following 2 weeks of incubation. Colonies (>50 cells) were counted under a microscope. For soft-agar colony formation assay, the 6-well plate was coated by the basement gel containing 0.6% soft agar in culture medium. Mixture of agar (0.35%) and cells was plated on top of the bottom layer for two-layer gel formation. After 2 weeks of culture, colonies were stained with crystal violet reagent (Solarbio). The number of colonies >100 μm in diameter was scored using Image J software (National Institutes of Health, NY, USA).
Scratch-Healing Assay
After transfection, EC109 and KYSE30 cells (3 × 105 cells per well) were seeded in a 6-well plate and cultured in serum-free medium for 24 h. The cell monolayer was then scraped using a micropipette tip to create a straight line scratch, followed by rinsing with phosphate-buffered saline. After 24 h of culture, the distance between the sides of the scratch was determined using ImageJ software (National Institutes of Health, NY, USA).
Transwell Invasion Assay
After transfection, EC109 and KYSE30 cells (2.5 × 104 cells per well) were suspended in serum-free medium and seeded in the upper chamber pre-coated with Matrigel (Corning, Steuben County, NY, USA). Medium containing 10% FBS was added to the lower chamber and served as a chemoattractant. Following 24 h of incubation, the cells were fixed with formaldehyde, permeated with methanol, and stained with 0.1% crystal violet for 30 min. The non-invasive cells were removed with a cotton swab and the invasive cells were quantified under a light microscope.
Subcellular Localization Fractionation Assay
Subcellular localization assay was performed using the PARIS Kit (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. qRT-PCR analysis was then performed as described above.
Luciferase Reporter Assay
Wild-type (WT)-UPK1A-AS1 constructs were generated by introducing the UPK1A-AS1 sequence into psiCHECK2 vector (Promega, Madison, WI, USA). Mutations were introduced into the miR-1248 binding sites in the UPK1A-AS1 sequence, followed by fusion with psiCHECK2 vector to generate MUT-UPK1A-AS1 constructs. EC109 and KYSE30 cells were co-transfected with WT-UPK1A-AS1 or MUT-UPK1A-AS1 and miR-1248 or miR-NC, and luciferase activity was measured at 48 h post-transfection using the Dual-Luciferase Reporter Assay System (Promega).
RNA Pull-Down Assay
The RNA pull-down assay was conducted as described.22,23 EC109 and KYSE30 cells were seeded and cultured for 24 h before transfection. Biotinylated miR-1248 (Bio-miR-1248) and miR-NC (Bio-miR-NC) were synthesized from Sangon Biotech, and transfected into EC109 and KYSE30 cells at a final concentration of 50 nM using LipofectamineTM 2000 (Invitrogen). At 48 h post-transfection, EC109 and KYSE30 cells were lysed in RIPA lysis buffer, and then the cell lysates were incubated with streptavidin beads at 4°C on the rotator overnight to generate probe-coated beads. After binding process, the beads were incubated with the elution buffer at 37°C with agitation for 1 h. The eluted pulled-down RNA complex in the supernatant was purified using TRIzol reagent (Invitrogen) and analyzed for UPK1A-AS1 enrichment by qRT-PCR assay.
Statistical Analysis
The results were expressed as the mean ± standard error of the mean. Statistical analysis was performed using Student’s t-test or one-way analysis of variance. P values <0.05 were considered statistically significant.
Results
UPK1A-AS1 Expression Is Significantly Downregulated in ESCC Tissues and Cell Lines
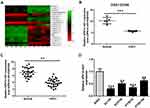
To assess the involvement of lncRNAs in ESCC development, we analyzed the data from GEO120356 database and identified 20 differentially expressed lncRNAs (10 upregulated and 10 downregulated) in ESCC tissues relative to their adjacent normal tissues (Figure 1A). Among them, UPK1A-AS1 was markedly downregulated in ESCC tissues (Figure 1B). To identify the differential expression of UPK1A-AS1 in ESCC, 30 pairs of ESCC tissues and corresponding normal tissues were collected and the expression of UPK1A-AS1 was evaluated by qRT-PCR. As expected, downregulation of UPK1A-AS1 was confirmed in ESCC tissues (Figure 1C). Likewise, UPK1A-AS1 was underexpressed in ESCC cell lines, especially in EC109 and KYSE30 cells, relative to the human immortalized esophageal epithelial cell line SHEE (Figure 1D).
Upregulation of UPK1A-AS1 Inhibits ESCC Cell Proliferation, Migration, and Invasion
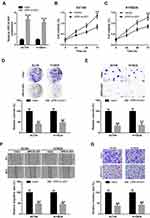
Since UPK1A-AS1 expression was found to be downregulated in ESCC, we overexpressed UPK1A-AS1 in EC109 and KYSE30 cells to further study its functional role. Results showed that, after transfection of EC109 and KYSE30 cells with UPK1A-AS1 overexpressing plasmids, the expression of UPK1A-AS1 was obviously increased (Figure 2A). Under these conditions, an obvious reduction of cell viability was observed in EC109 and KYSE30 cells (Figure 2B and C). Accordingly, overexpression of UPK1A-AS1 repressed the colony-forming ability of EC109 and KYSE30 cells (Figure 2D and E). Meanwhile, scratch-healing assay showed that upregulation of UPK1A-AS1 caused a significant decrease in the migration of EC109 and KYSE30 cells (Figure 2F). In parallel, transwell invasion assay showed that upregulation of UPK1A-AS1 led to a marked decrease in EC109 and KYSE30 cell invasion (Figure 2G).
UPK1A-AS1 Directly Interacts with miR-1248
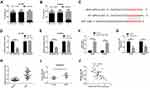
To determine the cellular location of UPK1A-AS1, we isolated the nuclear fraction from the cytoplasm of EC109 and KYSE30 cells, and found that UPK1A-AS1 was predominantly located in the cytoplasm (Figure 3A and B). Moreover, a putative miR-1248 binding site was identified in the UPK1A-AS1 sequence, as predicted by the DIANA tools LncBase (Figure 3C). To investigate the interaction between UPK1A-AS1 and miR-1248, EC109 and KYSE30 cells were co-transfected with WT-UPK1A-AS1 or MUT-UPK1A-AS1 and miR-1248 or miR-NC. Results showed that upregulation of miR-1248 reduced the relative luciferase activity of reporter plasmids containing WT-UPK1A-AS1, but the relative luciferase activity of reporter plasmids containing MUT-UPK1A-AS1 was unaffected (Figure 3D and E). Moreover, the expression of UPK1A-AS1 was higher in the Bio-miR-1248 group compared with that in the Bio-NC group, as shown by RNA pull-down assay (Figure 3F). Additionally, the expression of miR-1248 in EC109 and KYSE30 cells was markedly decreased upon UPK1A-AS1 upregulation (Figure 3G). Meanwhile, miR-1248 was highly expressed in ESCC tissues compared with that in corresponding normal tissues (Figure 3H). In line with this, data from GSE97051 database revealed that miR-1248 was upregulated in ESCC tissues relative to normal tissues (Figure 3I). Furthermore, an inverse correlation between UPK1A-AS1 and miR-1248 expression was observed in ESCC specimens by Spearman correlation coefficient analysis (Figure 3J).
UPK1A-AS1 Regulates ESCC Cell Proliferation, Migration, and Invasion Partially by Sponging miR-1248
To determine whether the anti-cancer effect of UPK1A-AS1 was mediated by miR-1248, EC109 and KYSE30 cells were transfected with UPK1A-AS1 overexpressing plasmids alone or co-transfected with UPK1A-AS1 overexpressing plasmids and miR-1248 mimic. We found that UPK1A-AS1-induced downregulation of miR-1248 was obviously blocked by miR-1248 upregulation (Figure 4A and B). Moreover, CCK-8 assay showed that the reduction of cell viability induced by UPK1A-AS1 in EC109 and KYSE30 cells was abrogated by transfection with miR-1248 mimic (Figure 4C and D). Colony formation assay revealed that the colony-forming ability of EC109 and KYSE30 cells was inhibited by UPK1A-AS1 upregulation; however, this effect was markedly reversed by miR-1248 upregulation (Figure 4E and E). In parallel, in both EC109 and KYSE30 cells, upregulation of UPK1A-AS1 inhibited cell migration and invasion, as indicated by the scratch-healing and transwell invasion assays, respectively. However, these changes induced by UPK1A-AS1 were partially attenuated by miR-1248 upregulation (Figure 4G and H).
Discussion
ESCC is one of the most common fatal malignancies worldwide, but its pathogenesis is complex and remains unclear. Accumulating evidence suggests that disruption of lncRNAs is tightly associated with the development of human cancers, including ESCC.24,25 Here, we analyzed the data from GEO120356 database in order to identify the lncRNAs involved in tumorigenesis. We identified 20 differentially expressed lncRNAs in ESCC and selected UPK1A-AS1, which has never previously been characterized in ESCC, for functional experiments.
lncRNAs have attracted considerable research interest due to their involvement in regulating biological processes via special modulatory mechanisms. In the last decades, hundreds of lncRNAs have been studied and identified in various human cancers, and abnormal expression of lncRNAs is a key mechanism underlying ESCC carcinogenesis.26 In addition, lncRNAs have been recognized as promising therapeutic targets for cancer.27 For example, high expression of lncRNA MNX1 antisense RNA1 (MNX1-AS1) was closely correlated with ESCC lymph node metastasis. Functional studies revealed that lncRNA MNX1-AS1 facilitated cell proliferation and metastasis and inhibited cell apoptosis by acting as a competing endogenous RNA to target sirtuin 1 via sponging miR-34a in ESCC.28 In addition, a higher expression of small nucleolar RNA host gene 6 was reportedly correlated with prognosis, lymph node metastasis, distant metastasis, and tumor node metastasis stage of ESCC patients. Furthermore, small nucleolar RNA host gene 6 upregulation promoted ESCC progression, whereas its knockdown resulted in inhibition of ESCC progression.29 Although an increasing number of studies have reported lncRNAs as key determinants of carcinogenesis, the functional role of UPK1A-AS1 in ESCC has not yet been characterized.
Commonly associated with their genomic origin and cellular location, lncRNAs exhibit various roles in different types of human cancers.30 Therefore, knowledge regarding their cellular location is crucial for unraveling their biological roles. Nuclear lncRNAs may be implicated in chromatin interactions, transcriptional regulation, and RNA processing, while cytoplasmic lncRNAs may participate in post-transcriptional processes, such as mRNA translation and degradation.31 Here, we found that UPK1A-AS1 was predominantly located in the cytoplasm of EC109 and KYSE30 cells, and upregulation of UPK1A-AS1 was observed in ESCC tissues and cell lines. Using in vitro analyses, we found that UPK1A-AS1 upregulation suppressed the proliferation, migration, and invasion of ESCC cells, thus suggesting a potential role of UPK1A-AS1 in ESCC carcinogenesis.
lncRNAs have been shown to participate in the regulation of tumorigenesis through multiple pathways. One of the typical mechanisms of lncRNA function is that it serves as a decoy of miRNAs, antagonizes its function, and results in the upregulation of miRNA target genes that are involved in tumorigenesis.32 Using DIANA tools LncBase, we identified putative miR-1248 binding sites within the UPK1A-AS1 sequence. Previous evidence suggests that miR-1248 plays a crucial role in carcinogenesis. In non-small cell lung cancer, miR-1248 was shown to be involved in the regulation of thymidylate synthase, thereby affecting the treatment outcome of patients with non-small cell lung cancer.33 In our study, we found that miR-1248 was highly expressed in ESCC tissues and cell lines. Consistent with our finding, miR-1248 upregulation was also reported in osteosarcoma and correlated with poor survival. miR-1248 enhanced cell viability and inhibited apoptosis in U2OS cells treated with fluorouracil through inhibiting the expression of apoptotic protein angiotensin II type 1 receptor.34 Based on these findings, we speculated that UPK1A-AS1-induced inhibition of ESCC growth might be mediated by miR-1248. Here, we identified miR-1248 as a direct target of UPK1A-AS1 and found that high expression of UPK1A-AS1 was associated with reduced miR-1248 levels. Furthermore, we provided evidence supporting this proposed mechanism. We found that upregulation of miR-1248 could reverse the inhibitory effect of UPK1A-AS1 on ESCC proliferation and metastasis, suggesting that UPK1A-AS1, as a decoy for miR-1248, acts as a tumor-suppressive factor in ESCC. Hence, our findings suggest the involvement of the UPK1A-AS1/miR-1248 axis in ESCC development.
In conclusion, we demonstrated that UPK1A-AS1 acted as a decoy for miR-1248 and inhibited the proliferation, migration, and invasion of ESCC cells. Our findings suggest UPK1A-AS1/miR-1248 axis as a novel regulatory network in ESCC, providing a better understanding of the pathogenesis of ESCC. Targeting this newly identified UPK1A-AS1/miR-1248 axis may serve as a potential therapeutic strategy for treating ESCC.
Acknowledgment
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
Authors declare that there is no conflict of interest relevant to this work.
References
1. Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3(1):17048. doi:10.1038/nrdp.2017.4810.1038/nrdp.2017.48
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi:10.1002/ijc.29210
3. Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi:10.1016/s0140-6736(12)60643-6
4. Burki TK. Definitions of oesophageal cancer. Lancet Oncol. 2017;18(2):e71. doi:10.1016/s1470-2045(17)30018-9
5. Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509(7498):91–95. doi:10.1038/nature13176
6. Lin DC, Hao JJ, Nagata Y, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46(5):467–473. doi:10.1038/ng.2935
7. Najafi F. Tobacco smoking and alcohol drinking: two clinically significant risk factors for esophageal squamous cell carcinoma. Gastroenterology. 2019;157(3):897. doi:10.1053/j.gastro.2019.04.054
8. Saravanan R, Youshya S, Campbell F, et al. Unique expression of human papilloma virus type 5 and type 16 in esophageal squamous cell carcinoma-a case report. Am J Gastroenterol. 2006;101(10):2423–2426. doi:10.1111/j.1572-0241.2006.00705.x
9. Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi:10.1038/nature11233
10. Xiong XD, Ren X, Cai MY, et al. Long non-coding RNAs: an emerging powerhouse in the battle between life and death of tumor cells. Drug Resist Updat. 2016;26:28–42. doi:10.1016/j.drup.2016.04.001
11. Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37. doi:10.1038/s41580-018-0045-7
12. Dong H, Lei J, Ding L, et al. MicroRNA: function, detection, and bioanalysis. Chem Rev. 2013;113(8):6207–6233. doi:10.1021/cr300362f
13. Tanic M, Yanowski K, Gomez-Lopez G, et al. MicroRNA expression signatures for the prediction of BRCA1/2 mutation-associated hereditary breast cancer in paraffin-embedded formalin-fixed breast tumors. Int J Cancer. 2015;136(3):593–602. doi:10.1002/ijc.29021
14. Wu H, Yang L, Chen LL. The diversity of long noncoding RNAs and their generation. Trends Genet. 2017;33(8):540–552. doi:10.1016/j.tig.2017.05.004
15. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi:10.1016/j.cell.2013.02.012
16. Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi:10.1016/j.cell.2007.05.022
17. Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. doi:10.1038/nature10887
18. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi:10.1016/j.ccell.2016.03.010
19. Kunej T, Obsteter J, Pogacar Z, et al. The decalog of long non-coding RNA involvement in cancer diagnosis and monitoring. Crit Rev Clin Lab Sci. 2014;51(6):344–357. doi:10.3109/10408363.2014.944299
20. Li CH, Chen Y. Insight into the role of long noncoding RNA in cancer development and progression. Int Rev Cell Mol Biol. 2016;326:33–65. doi:10.1016/bs.ircmb.2016.04.001
21. Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13(2):397–406. doi:10.1074/mcp.M113.035600
22. Bai Y, Zhang Y, Han B, et al. Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci. 2018;38(1):32–50. doi:10.1523/JNEUROSCI.1348-17.2017
23. Yang L, Han B, Zhang Y, et al. Engagement of circular RNA HECW2 in the nonautophagic role of ATG5 implicated in the endothelial-mesenchymal transition. Autophagy. 2018;14(3):404–418. doi:10.1080/15548627.2017.1414755
24. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981. doi:10.1158/0008-5472.can-16-2634
25. Shen WJ, Zhang F, Zhao X, et al. LncRNAs and esophageal squamous cell carcinoma - implications for pathogenesis and drug development. J Cancer. 2016;7(10):1258–1264. doi:10.7150/jca.14869
26. Chen JL, Lin ZX, Qin YS, et al. Overexpression of long noncoding RNA LINC01419 in esophageal squamous cell carcinoma and its relation to the sensitivity to 5-fluorouracil by mediating GSTP1 methylation. Ther Adv Med Oncol. 2019;11:1758835919838958. doi:10.1177/1758835919838958
27. Lorenzi L, Avila Cobos F, Decock A, et al. Long noncoding RNA expression profiling in cancer: challenges and opportunities. Genes Chromosomes Cancer. 2019;58(4):191–199. doi:10.1002/gcc.22709
28. Chu J, Li H, Xing Y, et al. LncRNA MNX1-AS1 promotes progression of esophageal squamous cell carcinoma by regulating miR-34a/SIRT1 axis. Biomed Pharmacother. 2019;116:109029. doi:10.1016/j.biopha.2019.109029
29. Zhang Y, Li R, Ding X, et al. Upregulation of long non-coding RNA SNHG6 promote esophageal squamous cell carcinoma cell malignancy and its diagnostic value. Am J Transl Res. 2019;11(2):1084–1091.
30. Chen LL. Long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761–772. doi:10.1016/j.tibs.2016.07.003
31. Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46. doi:10.1016/j.cell.2013.06.020
32. Tam C, Wong JH, Tsui SKW, et al. LncRNAs with miRNAs in regulation of gastric, liver, and colorectal cancers: updates in recent years. Appl Microbiol Biotechnol. 2019;103(12):4649–4677. doi:10.1007/s00253-019-09837-5
33. Xu J, Tian S, Yin Z, et al. MicroRNA-binding site SNPs in deregulated genes are associated with clinical outcome of non-small cell lung cancer. Lung Cancer. 2014;85(3):442–448. doi:10.1016/j.lungcan.2014.06.010
34. Zhao Y, Xu K, Liu P. Post-transcriptional control of Angiotensin II Type 1 receptor regulates osteosarcoma cell death. Cell Physiol Biochem. 2018;45(4):1581–1589. doi:10.1159/000487719
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.