Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Resting-State Functional Magnetic Resonance Imaging Reveals Overactivation of the Habitual Control Brain System in Tobacco Dependence
Authors Tan Q , Li S, Niu J, Liu S, Li Y, Lu Y, Wang Z, Xu W, Wei Y , Guo Z
Received 15 August 2021
Accepted for publication 27 November 2021
Published 19 December 2021 Volume 2021:17 Pages 3753—3768
DOI https://doi.org/10.2147/NDT.S334403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jun Chen
Qiaowen Tan,1 Shaoke Li,2 Juan Niu,3 Shien Liu,2 Yaling Li,1 Yujie Lu,1 Zhihong Wang,1 Wanqun Xu,1 Yalin Wei,1 Zongjun Guo1
1Department of Geriatrics, Affiliated Hospital of Qingdao University, Qingdao, 266003, Shandong Province, People’s Republic of China; 2Department of Medical Imaging, Affiliated Hospital of Qingdao University, Qingdao, 266003, Shandong Province, People’s Republic of China; 3Clinical Psychology Department, Affiliated Hospital of Qingdao University, Qingdao, 266003, Shandong Province, People’s Republic of China
Correspondence: Zongjun Guo; Shaoke Li Email [email protected]; [email protected]
Introduction: We studied the regulatory mechanism of the habitual brain network in tobacco dependence to provide a theoretical basis for the regulation and cessation of tobacco dependence.
Methods: We used resting-state functional magnetic resonance imaging (rs-fMRI) to explore the Fractional amplitude of low-frequency fluctuations (fALFF) and functional connectivity (FC) of the habitual brain network in tobacco-dependent subjects and to evaluate the relationship between the FC level and tobacco selection preference behavior. In total, 29 male tobacco-dependent participants and 28 male nonsmoking participants were recruited. rs-fMRI was used to collect blood oxygen level-dependent signals of the participants in the resting and awake states. After rs-fMRI, all subjects completed cigarette/coin selection tasks (task 1 and task 2).
Results: Compared with the control group, the tobacco dependence group showed increased fractional amplitude values of fALFF in the left posterior cingulate cortex and right parahippocampus. FC in the tobacco-dependent group was increased in the right inferior temporal gyrus, left middle frontal gyrus, left cingulated gyrus, and bilateral superior frontal gyrus, compared with that in the control group. Moreover, the preference selection behavior was associated with the enhancement of FC about parts of the brain regions in the habitual brain network of the tobacco-dependent participants. Thus, habitual network activity was significantly enhanced in tobacco-dependent participants in the resting state. Moreover, a positive correlation was found between the cigarette selection preference of the smokers and certain brain regions related to the habitual network.
Discussion: This suggests that increased activity of the habitual brain network may be essential in the development of tobacco-dependent behavior.
Keywords: tobacco dependence, behavioral decision preference, habitual brain network, resting-state functional magnetic resonance imaging, functional connectivity, fractional amplitude of low-frequency fluctuations
Introduction
Among the risk factors for chronic diseases in the elderly, behavioral and habitual factors account for approximately 60% of the total risk.1 The incidence of chronic diseases, such as hypertension, diabetes, and arteriosclerosis, is increased in the long term in people who smoke, drink, and are overweight. Thus, behaviors and habits have an important influence on the occurrence and development of chronic diseases in the elderly, and changing bad behaviors and habits can prevent more than 75% of the chronic diseases.1 Behaviors are controlled by the goal-directed and habitual control systems in the brain.2,3 Normally, behavioral responses are formed through close cooperation and smart switches between the goal-directed and habitual control systems in response to changes in the external environment.3 Although habits are an effective manner of processing information, excessive reliance on habitual control may result in addiction4,5 and alterations in habitual brain network functional activities, which may be an important cause for the formation and maintenance of bad habits or dependent behaviors.6,7
Tobacco dependence is a major public health problem worldwide and the main cause of preventable death in most countries. Although most people who depend on tobacco hope to stop, few succeed in doing so. Berrendero et al8 found that tobacco dependence is a chronic brain disorder characterized by forced tobacco use and loss of control of smoking behavior. Nicotine is the main addictive component of tobacco. Nicotine exerts pharmacological effects by activating nicotinic acetylcholine receptors (nAChRs), which are widely distributed in the central nervous system.9 The activation of nAChRs by nicotine increases the release of various neurotransmitters, including dopamine, glutamate, and gamma aminobutyric acid (GABA),10–13 thereby altering a large number of physiological processes and producing many behavioral responses directly related to addiction, including reward effects and physical dependence.14,15 Everitt and Robbins16 found that, at the neurological level, addiction involves a transition from prefrontal cortical to striatal control and from ventral to dorsal striatal control in a process mediated by the dopaminergic nerves. Dopaminergic innervation forms an ascending spiral between the different striatal regions: the shell in the ventromedial striatum affects the nucleus, the nucleus affects the central striatum, and the central striatum affects the dorsolateral striatum, creating a hierarchy of information flows and providing an anatomical basis for dependence.17 Glutamate and GABA are involved in regulating this process.18 Several studies have investigated brain activation in response to smoking-related stimuli using functional magnetic resonance imaging (fMRI). Baker et al19 found that compared with monetary rewards, cigarette rewards induced a higher reward response in smokers. This suggests that addicts show deficits in the recruitment of brain reward pathways.20 A study on the neurological effects of electronic cigarette smoking using task-state fMRI showed brain activation in the motor cortex, cingulate cortex, putamen, thalamus, globus pallidum, and cerebellum, and relative inactivation of the ventral striatum and orbitofrontal cortex.21 Together, these findings show that overreliance on the habitual system may contribute to the development of tobacco dependence behavior.
In recent years, with the development of resting-state functional magnetic resonance imaging (rs-fMRI) technology, a large number of studies have been carried out to clarify the effects of tobacco dependence on brain circuits and activation of brain regions, thus providing objective evidence for human understanding of the changes in brain function caused by tobacco dependence. Stein et al22 used fMRI imaging to examine the acute effects of intravenous nicotine on the central nervous system. Three groups were intravenously injected with different doses of nicotine and normal saline (control group) at different times, and the brain activation pattern was observed within 1min after each dose. They found increased neural activity in several brain regions with increasing doses of nicotine, including nucleus accumbens, amygdala, cingulate gyrus and frontal lobe, and these activated brain areas have the nature of human behavior awakening and behavior strengthening. Due et al23 found that activation of dopamine reward circuitry in limbic midbrain (right posterior amygdala, posterior hippocampus, ventral tegmental area, central thalamus and other brain regions) increased when tobacco addicts viewed smoking pictures compared with non-smoking pictures. For tobacco addicts, exposure to tobacco cues often produces a range of physical and psychological responses, including impulsivity and craving. McClernon et al24 showed that the activation of parietal lobe, prefrontal lobe, occipital lobe, central cortex, putamen, thalamus and other brain regions were enhanced when smokers in withdrawal state were shown smoking pictures and general pictures. Lee et al25 observed changes in brain functional activities of tobacco addicts in simulated real smoking scenes, and found that signals in the prefrontal cortex, anterior cingulate gyrus and left supplementary motor area were enhanced. With the deepening of the understanding of nicotine dependence, fMRI technology is used to reveal the changes of neurotransmitters and neural circuits of nicotine dependence. Tobacco dependence affects the reward circuitry of the brain and the goal-directed network that directs cognitive and behavioral behavior. Does it also affect the habitual brain network?
When a dependent behavior is formed, the functional activity of the goal-directed network in the brain weakens.21,26,27 However, it is not clear how the habitual network changes. Task-state fMRI does not allow to explore whether habitual brain network remodeling occurs after habit formation. rs-fMRI can be used to measure the blood oxygen level-dependent (BOLD) response of patients in the resting and awake states and can reflect the association of the functions of specific brain regions and neural networks with the anatomic structure of the network. This method may also allow observing internal relationships between disease characteristics and behavioral representations.28,29
The striatum is a group of subcortical nuclei that has been associated with motor control since the 19th century.30 In 1877, Sir David Ferrier showed that the striatum is the center of the integration of voluntary and involuntary movements. Since then, substantial research has been conducted on behavior and movement related to the striatum. Through fMRI studies in rodents, a functional model of behavioral regulation strategies has been gradually established. The dorsolateral striatum (DLS: mainly putamen) and dorsomedial striatum (DMS: mainly caudate) receive projections from different cortical regions to regulate behavior. Interaction between the DMS and the prefrontal cortex (PFC) mainly regulates goal-directed behavior, whereas interaction between the DLS and the sensorimotor cortex mainly regulates habitual behavior.31 The two pathways are independent but interact with each other. The goal-directed system mainly selects behaviors through prefrontal–DMS–globus pallidus and reticula nigra–thalamus–prefrontal pathways, whereas the habit learning system acts mainly through sensorimotor–DLS–globus pallidus and reticula nigra–thalamus–sensorimotor area pathways to modulate repetitive behaviors.32,33 Studies have shown that the putamen and caudate are the core regions of the habitual and goal-directed networks.34–36 Dysfunction of both structures is considered the neuroanatomical basis of dependence.37
In this study, rs-fMRI technology was used to analyze the activity of the putamen and whole-brain resting-state connectivity, using 6-mm-diameter spherical seeds centered on the putamen region of interest (ROI).34 Our aim was to observe the activity pattern of the habitual brain network in smoke-dependent subjects in the resting state and to verify the hypothesis that increased activity in this network promotes approach behavior towards tobacco cues in these subjects.
Participants and Methods
General Data Acquisition
Between October 2018 and February 2020, 31 male tobacco-dependent subjects (tobacco dependence group) were recruited from the Health Care Department and Physical Examination Center of the Affiliated Hospital of Qingdao University, and 30 sex-, age-, and educational level-matched non-smokers were recruited as the control group. Three subjects (two in the tobacco dependence group and one in the control group) were excluded due to head movement exceeding 1.5°, and one case in the control group quit halfway during the study. Thus, we collected data from 57 cases, including 29 cases in the tobacco dependence group and 28 cases in the control group. All subjects were of Han ethnicity. This study was approved by the Ethics Committee of Medical College of Qingdao University, and all subjects provided informed consent. All subjects met the following inclusion criteria: (1) no contraindication for undergoing MRI examination; (2) no abnormal brain structure on routine MRI examination (such as space-occupying infarction or ischemic focus); (3) no serious diseases of the heart, lungs, liver, kidneys, and brain; and (4) no cognitive disorder and an intelligence quotient (IQ) score of above 90. In addition, subjects in the tobacco dependence group met the following criteria: (1) smoking -dependence diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria.38 They were all right-handed, and were free of medication and of any other psychiatric diagnosis; (2) smoked ≥10 cigarettes per day, ≥2 years of smoking or <3 months of smoking cessation in the past year; (3) completed the questionnaire survey, including age at initiation of smoking, daily cigarette consumption, and years of smoking; and (4) completed Fagerström test for nicotine dependence (FTND).39
Procedure
All subjects completed the questionnaire surveys, including the FTND and a mental cognitive assessment. Mental cognition, including (1) IQ; (2) cognitive function: Mini-Mental State Examination (MMSE) for the rapid screening of cognitive function; and (3) emotion detection: Hamilton Anxiety Scale and Hamilton Depression Scale (HAMD) for determining anxiety and depression, respectively, was evaluated by trained geriatric physicians. The participants underwent resting-state MRI scans. After the scanning, two cigarette/coin decision tasks were completed in the office.
Cigarette/Coin Decision Task
The E-Prime 2.0 software was used to compile a cigarette/coin decision task. Before the task began, the participants practiced the operation steps based on Iowa gambling and multiple-choice procedure tasks.40–42 The detailed procedure was as follows. The first task was a cigarette/coin preference selection task. A picture of a cigarette or coin was randomly presented on the computer screen for 4 s. The subjects were asked to select the items in the picture:
press key “1” for choice and key “3” to give up. Whether you choose or give up, you should press as many buttons as possible within the specified time to get more cigarette or coin rewards.
After the test, the total number of cigarettes or coins selected was displayed on the computer screen for 2 s, which was used as the basis for giving rewards in kind after the experiment. If the subjected pressed key 3 to give up, there was no reward or loss. Each image was repeated 15 times, for a total of 30 rounds. The computer automatically recorded the subjects’ choice mode (choose or give up), and the total number of choices and types of items were collected (Figure 1 task 1). The second task was a cigarette/coin forced selection task. The pictures of cigarettes and coins seen in the first task were randomly and simultaneously presented on the computer screen. The subjects were asked to choose one of the two pictures: “press key “1” to select the left picture and press key “3” to select the right picture.” The picture was presented for 4 s at a time, for a total of 14 times. There was no result feedback after each choice (Figure 1 task 2). The computer automatically recorded the subjects’ choice mode, which was expressed as the number of cigarettes or coins selected.
 |
Figure 1 n Each button selects the total number of cigarettes or money; 1 On behalf of want; 3 Do not want. |
rs-fMRI
The subjects were awake and lying flat on an examination bed, with the eyes closed, and breathing normally. Brain scans were acquired using the GE Discovery MR750 3.0T (GE Healthcare, Waukesha, MI, USA) superconducting whole-body magnetic resonance imaging system. For three-dimensional magnetization-prepared rapid gradient echo (3D MP-RAGE) sequence scanning, T1-weighted image parameters were as follows: TE = 2.3 ms, Prep Time = 450 ms, FA = 150, band with = 19.23, FOV = 23 × 23, slice thickness 1.0 mm, spacing 0 mm, matrix 230 × 230, NEX 1.0, 178 slices. For gradient echo planar imaging, T1-weighted image parameters were as follows: TE/TR = 30/2000 ms, slice thickness = 4.0 mm, slice spacing = 0 mm, matrix 64 × 64, FA = 90°, FOV = 23×23, NEX = 1, 45 slices in total. The scanning time was 5 min and 10 s.
Data Analysis
Data of the cigarette/coin decision task were statistically analyzed using SPSS 17.0 software. Quantitative data are expressed as the mean ± standard deviation. Means of the two groups were compared using the two-sample t-test. Qualitative data are expressed in cases and percentages and were compared using the chi-square test. Pearson or Spearman correlation was used for correlation analysis. P < 0.05 was considered statistically significant. Data are shown in Table 1.
 |
Table 1 Demographic Data, Psychological Data, and Cigarette/Coin Decision Task Data |
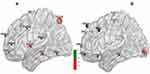
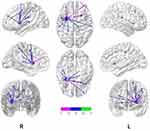
For resting-state image processing and functional connectivity analysis, all rs-fMRI data were analyzed on the MATLAB 2010b platform, using DPARSF 2.1, SPM 8, REST 1.6, and BrainNet Viewer software packages for data processing, low-frequency amplitude analysis, functional connectivity value calculation, correlation analysis, and simulated brain mapping. Image preprocessing included removal of the first 10 time points to reduce magnetization disequilibrium, followed by time correction, spatial normalization, smoothing, head movement correction, and filtering. After processing, a data file for each examinee was automatically generated. If the subject’s head moved more than 1.5 mm on any of the x, y, and z axes, or the rotation range exceeded 1.5°, the subject was excluded. Nuisance covariates, including six head motion parameters and signals of white matter and cerebrospinal fluid, were regressed out. The sampling rate was (3 × 3×3) mm3, and spatial Gaussian smoothing (Full Width at Half Maximum, FWHM) of the functional image used (6 × 6×6) mm3 as the smoothing kernel. Detrending and temporal band-pass filtering (0.01–0.08 Hz) were performed to remove respiratory and cardiovascular noise. Using REST 1.6 software, a single-sample t-test was used to compare the tobacco dependence and control groups, and P < 0.001 was considered significant. A whole-brain Fractional amplitude of low-frequency fluctuations (fALFF) activation map (using the whole-brain default mask, corrected using Alphasim, cluster ≥ 13 voxels) with higher-than-average values was generated for the two groups at resting state. Using the BrainNet Viewer software, the single-sample t-test brain maps for both groups were accurately and clearly presented in stereo brain maps. The green nodes in the map indicate the brain regions that contribute to the goal-directed system, and the red nodes are the regions that participate in the habitual control system. The node size was set according to the t value for each brain region. The REST software was used to combine the two brain regions based on the formula (i1 > 0+i2 > 0) > 0 to make a mask. A two-sample t-test was utilized to identify brain regions with a difference in low-frequency amplitude between the two groups (using the union mask, corrected using Alphasim, cluster ≥ 41 voxels). The size of the ROI (putamen) was selected on the basis of a previous study.34 Montreal Neurological Institute (MNI) coordinates (28, 5, and 2) were applied to draw a sphere with a diameter of 6 mm. Time courses of the ROI were extracted by averaging the time courses of all voxels within the ROI. Subsequently, correlation coefficients between the ROI and the whole brain were calculated. Fisher’s r-to-z transform was applied for the appropriate degrees of freedom. After the removal of covariates, the functional connectivity between the putamen and the whole brain was calculated. By the same method, a single-sample t-test was used to compare the tobacco dependence and control groups with REST 1.6, and a functional connectivity network (the habitual system brain network) and the activity intensity were obtained, using the putamen as the seed point. BrainNet Viewer was employed to render a 3D brain image. In the image, green represents the putamen. The pink nodes and lines indicate the functional connectivity network in the tobacco dependence group, with the putamen as the ROI. The blue nodes and lines indicate the functional connectivity network of the control group. Node size was set according to the r value for each brain region: the larger the node, the stronger the activity. Union masks for the two groups were generated, and a two-sample t-test was conducted to obtain the brain regions with a significant difference in functional connectivity between the two groups (P < 0.01, threshold = 2.7116, df = 55, corrected using Alphasim, cluster ≥ 41 voxels).
The REST software was utilized for simple linear correlation analysis of the FTND score and the strength of the functional connectivity with the putamen in the tobacco dependence group. In addition, the correlation between the number of cigarette choices in the cigarette/coin selection preference task and the intensity of the habitual network activity in the tobacco dependence group were analyzed (P < 0.01, cluster ≥ 41 voxels, threshold = 0.56144, corrected using Alphasim). To this end, we identified the brain regions that were significantly correlated with the FTND score, with the total number of cigarette choices in the tobacco dependence group in the first cigarette/coin selection preference task, and with the number of cigarette choices in the tobacco dependence group in the second cigarette/coin selection preference task.
Results
Comparison of Demographic and Neuropsychological Data
There were no significant differences in age (P = 0.09), educational level (P = 0.46), IQ (P = 0.43), and MMSE (P = 0.83), HAMA (P = 1.00), and HAMD (P = 0.80) scores between the two groups (Table 1).
Comparison of Cigarette/Coin Selection Preference Data
In the first task, there was a significant difference in the total number of cigarette choices between the tobacco dependence group (115.66 ± 33.03 times) and the control group (31.61 ± 22.15 times) (P = 0.00). Likewise, in the second task, there was a significant difference in the number of cigarette choices between the tobacco dependence group (12.52 ± 2.13 times) and the control group (2.68 ± 2.94) (P = 0.00). These data indicated that the tobacco dependence group was more inclined to choose cigarettes than the control group.
Comparison of Habitual Brain Networks
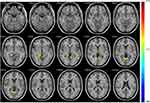
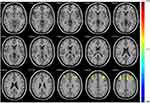
In the tobacco dependence group, the fALFF values in the left posterior cingulate gyrus and right parahippocampal gyrus were increased (P < 0.01) (Table 2, Figure 2). Compared with the control group, the functional connectivity between the whole brain and the putamen was increased in the right inferior temporal gyrus, left middle frontal gyrus, left cingulate gyrus, and bilateral superior frontal gyrus in the tobacco dependence group (P < 0.01) (Table 3, Figure 3). The results of fALFF and functional connectivity (FC) analysis by a single-sample t-test for the two groups are shown in Figures 4 and 5.
 |
Table 2 Comparison of fALFF Between the Tobacco-Dependent and Control Groups |
 |
Table 3 Comparison of FC Between the Tobacco Dependence and Control Groups |
Correlation Analysis of FC, Cigarette/Coin Decision Making, and Cigarette Dependence
We took smoking duration as a covariable. In the tobacco dependence group, the brain regions where FC values of the whole brain and putamen were positively correlated with the total number of cigarette choices in the first task were the anterior central gyrus of the left frontal lobe and the right precuneus lobe. The brain regions that were positively correlated with the number of cigarette choices in the second task were the left middle temporal gyrus and left posterior central gyrus. (all P < 0.01; Tables 4, 5, Figures 6 and 7).
 |
Table 4 Correlation Between FC in the Tobacco Dependence Group and the Number of Cigarette Choices in the First Cigarette/Coin Decision Task |
 |
Table 5 Correlation Between FC in the Tobacco Dependence Group and the Number of Cigarette Choice in the Second Cigarette/Coin Decision Task |
 |
Table 6 Correlation Between the FTND Score and FC Value in the Tobacco Dependence Group |
We use FTND score as covariable. In the tobacco dependence group, FC values in the whole brain and putamen were not positively correlated with the total number of cigarette choices in the first task. The Inferior Parietal Lobule was negatively correlated (r= −0.84). The brain regions that were positively correlated with the number of cigarette choices in the second task was the Precuneus (Table 6, Figure 8, r=0.77).
In the tobacco dependence group, the left superior temporal gyrus was positively correlated with the FC value of the putamen and the FTND score, and the right posterior lobe of the cerebellum was negatively correlated with these indices (Figure 9).
Discussion
rs-fMRI was used to observe neural network activity in the habitual brain system of cigarette addicts in the resting state and to assess the relationship between habitual network activity and cigarette preference. The fALFF value is an index reflecting the intensity of spontaneous brain activity in the resting state.43 Our results showed that activities in the left posterior cingulate gyrus and the right parahippocampal gyrus were higher in the tobacco dependence group than in the control group. Studies have shown that the posterior cingulate gyrus may play an important role in assessing familiarity and personal relevance.44,45 In the functional brain network, the posterior cingulate gyrus is an important functional structure in the self-directed network. In addicted individuals, increased self-directed network activation reportedly is associated with an increase in self-reported desire and use impulse as well as with the formation of dependent behavior.27,46,47 In accordance herewith, the results of the current study indicate that the formation of tobacco dependence is associated with enhanced activation of the habitual system. The parahippocampal gyrus is involved in learning memory storage and memory of reward cues. The prefrontal cortex-hippocampus pathway is an important pathway for the formation of dependent behavior.48 In this study, the level of spontaneous brain activity in the parahippocampal gyrus was increased in the tobacco-dependent group, suggesting that the formation of tobacco-dependent behavior may be related to enhanced hippocampus memory for reward cues. In rodent studies, the marginal cortex has been proven to also promote the implementation and control of habitual behavior,49–52 which was consistent with the increased spontaneous activity level of the hippocampus in tobacco-dependent individuals in this study. The increased spontaneous activity level in the posterior cingulate and parahippocampal gyrus in the tobacco dependence group indicates that the desire for cigarettes and the memory of cigarette stimulation reward promote the occurrence of tobacco dependence.
The purpose of seed point-based FC analysis is to calculate the correlation coefficient of the BOLD time series between the seed point and all other voxels or ROIs in the brain.53 In this study, the putamen of the dorsolateral striatum was used as the ROI34 to study the strength of its FC with the whole brain. The results showed that the FC between the whole brain and the putamen in the tobacco dependence group was increased in the right inferior temporal gyrus, left middle frontal gyrus, bilateral superior frontal gyrus, and left cingulate gyrus. The putamen is the core area of habit formation.34 The habitual system mainly involves the core brain region of the frontal motor cortex-dorsolateral striatum (putamen) circuit loop. Studies have demonstrated that the frontal-striatum-hypothalamus-frontal direct pathway is strongly associated with habit formation,54 and the prefrontal lobe is also associated with addiction.55 The interaction between the prefrontal cortex (PFC) and thalamus plays a key role in sensory processing, multimodal integration, and higher cognition. Cortical sensory processing is mainly achieved through cross-modal modulation of inhibition and de-inhibition circuits mediated by orientation selectivity of layer (L)1 neurons and L2/3 VIP- cells.56 The lateral posterior thalamic nucleus activity can modulate auditory processing in superficial layers of primary auditory cortex. This is achieved by subtractive suppression of auditory evoked responses together with suppression of spontaneous firing activity.57 The thalamus ventrolateral thalamus is densely connected with cognitive control substrates in the prefrontal cortex.58 The hippocampus, posterior cingulate cortex and parietal cortex play a role in attentional guidance of working memory and long-term memory.59,60 All these functions are related to the establishment of habitual control.
According to Brodman areas (BA), the FC in the putamen of the tobacco dependence group was enhanced in the right inferior temporal gyrus, left middle frontal gyrus, left cingulate gyrus, and bilateral superior frontal gyrus. The bilateral superior frontal gyrus is located in the premotor cortex of BA8. The temporal lobe is related to sensory learning. The supplementary motor complex-dorsolateral striatum pathway61 regulates habitual behavior, which is consistent with our results. The FC between the cingulate gyrus (BA32) and putamen was also enhanced in tobacco dependence group, which was consistent with the fALFF values. BA32 lies in the anterior cingulate cortex, which is part of the self-directed network and closely related to the formation of habitual behavior. The left middle frontal gyrus is located in BA10 and belongs to the medial prefrontal cortex (mPFC). The mPFC is responsible for contingency and behavioral result detection to guide behavior.62 Damage of the mPFC renders rats insensitive to a change in reward probability.4 This suggests that impairment of the mPFC may weaken goal-directed behavior and promote the acquisition of habitual behavior. The infralimbic (IL) and prelimbic (PL) cortex reportedly exert opposite effects on the mPFC. The IL cortex participates in the expression of habitual behavior and suppresses the goal reward effect of the basolateral amygdala, highlighting the role of the central amygdala in habitual reinforcement learning.63,64 The PL is involved in goal-directed behavior. A study of PL lesions in rats suggested that the neural input of the PL-dorsal medial striatum is very important for goal-directed learning.65 These results corroborate that chronic smoking may enhance and remodel the habitual brain network.
This study showed that tobacco addicts exhibited preferential cigarette selection behavior. We took smoking duration as a covariable. In the first cigarette/coin decision-making task, the total number of cigarette choices was positively correlated with the FC values of the putamen left anterior central gyrus and putamen left anterior cuneiform lobe (BA6,7). These brain regions are in sensory motor areas, which are also necessary parts of the habit learning system.54 According to Vergara et al 2017,66 the connectivity of precuneus and angular gyrus is increased while putamen and thalamus are decreased connectivity specifically in tobacco dependent subjects. Precuneus is associated with episodic memory and can be used to search for smoking memory under tobacco cues.67 This may promote tobacco choice。The thalamus is a main hub of information in the brain important in the study of nicotine addiction. Lack of connectivity between putamen and thalamus can be an indication of reward dysregulation that enhances nicotine-seeking behavior in smokers.66 This further supports our experiment showing that tobacco dependence is biased towards cigarette choice. We use FTND score as covariable. The brain regions where FC values of the whole brain and putamen were positively correlated with the total number of cigarette choices in the first task were the Inferior Parietal Lobule. A previous study demonstrated that the left inferior parietal lobule, one of the crucial brain regions of the attentional control network, was involved in executive function.68 The current results suggested that such abnormal intrinsic connectivity may indicate cognitive and executive deficits in Tobacco dependent individuals.69 That means the goal-directed function is weakened. As the first task mainly assessed cigarette choice motivation, this experiment showed that the enhanced habitual network activity might be associated with an increased motivation of cigarette choice, which is consistent with findings reported by.42 In the second task, the number of cigarettes selected was positively correlated with the left middle temporal gyrus and left posterior central gyrus, which also indicated that the reinforced effect of cigarette cues on tobacco-dependent behaviors was related to an increase in habitual brain network activity. In the tobacco dependence group, the brain region where the FC value was positively correlated with the FTND score contained the left superior temporal gyrus, which is consistent with the above results for the habitual brain network. A previous study indicated that enhanced putamen-temporal cortex connection may enhance the formation of habitual behavior.70 This suggests that habitual brain network remodeling in tobacco addicts may be associated with the degree of tobacco dependence. In the tobacco dependence group, the brain region that was negatively correlated with all indexes in the three correlation analyses was the right posterior lobe of the cerebellum. The structural volume of the cerebellum and the cognitive level are decreased in tobacco addicts,71 which was consistent with the results of this study.
BrainNet Viewer72 is a brain network visualization tool that can help researchers quickly, easily, and flexibly visualize different levels of structural connectivity and FC patterns. Figure 4 and Table 2 show that the spontaneously activated brain areas in the tobacco dependence group in the resting state mainly were brain areas that process habitual behaviors. The activation intensity and number of brain regions involved in goal-directed behaviors were significantly reduced in tobacco addicts when compared with those in the control group, indicating that the functional activity of the goal-directed system was weakened in this group. Comparison of the two groups using the striatum putamen as the ROI to extract the habitual system brain networks (Figure 3, Table 3) revealed that the connection to the putamen in the tobacco dependence group was biased towards the frontal lobe. According to the results of the two-sample t test (Table 3), the brain area with enhanced FC in the tobacco dependence group was located in the frontal motor cortex, and the frontal motor cortex-dorsolateral striatum pathway is the main pathway for habitual formation.31 These results indicate that the FC of the habitual brain network in smokers is enhanced after the formation of dependence.
Conclusion
In summary, the spontaneous activity and connectivity of the habitual control brain network were enhanced in tobacco-dependent subjects, who were more inclined to choose cigarettes. This preference behavior was remarkably correlated with the FC of the habitual network. The tobacco dependence score was associated with alterations in the FC mode of the habitual network. Our findings indicate that enhanced spontaneous activity and connectivity in the habitual brain network may be the neural functional bases for the occurrence and persistence of tobacco-dependent behavior. This is consistent with a previous finding that an imbalance between the goal-directed and habitual learning systems leads to addiction. In future studies, neuroimaging methods can be used for observational research on the treatment and withdrawal of behavioral disorders.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethics Committee of Medical College of Qingdao University, and all subjects signed informed consent.
Acknowledgments
This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2012HM049) and the Foundation Program of Technology Bureau of Qingdao (Grant No. 15-9-2-74-nsh; Grant No. KZJ-28) and the Foundation Program of Technology Bureau of Huangdao District of Qingdao (Grant No. 2014-1-73).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gao Q. Mechanism of formation and transformation of health habit and prevention of chronic diseases. Nanjing Yike Daxue Xuebao. 2012;12:241–245.
2. Cushman F, Morris A. Habitual control of goal selection in humans. Proc Natl Acad Sci U S A. 2015;112:13817–13822. doi:10.1073/pnas.1506367112
3. Dolan RJ, Dayan P. Goals and habits in the brain. Neuron. 2013;80:312–325. doi:10.1016/j.neuron.2013.09.007
4. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50. doi:10.1146/annurev-psych-122414-033457
5. Sjoerds Z, de Wit S, van den Brink W, et al. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry. 2013;3:e337. doi:10.1038/tp.2013.107
6. de Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci. 2009;29:11330–11338. doi:10.1523/JNEUROSCI.1639-09.2009
7. Eryilmaz H, Rodriguez-Thompson A, Tanner AS, Giegold M, Huntington FC, Roffman JL. Neural determinants of human goal-directed vs. habitual action control and their relation to trait motivation. Sci Rep. 2017;7:6002. doi:10.1038/s41598-017-06284-y
8. Berrendero F, Robledo P, Trigo JM, Martín-García E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010;35:220–231. doi:10.1016/j.neubiorev.2010.02.006
9. Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi:10.1016/S0091-3057(01)00652-9
10. McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi:10.1126/science.7569895
11. Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi:10.1038/382255a0
12. Yang X, Criswell HE, Breese GR. Nicotine-induced inhibition in medial septum involves activation of presynaptic nicotinic cholinergic receptors on gamma-aminobutyric acid-containing neurons. J Pharmacol Exp Ther. 1996;276:482–489.
13. Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi:10.1021/jm050069n
14. Decker MW, Brioni JD, Bannon AW, Arneric SP. Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci. 1995;56:545–570. doi:10.1016/0024-3205(94)00488-E
15. Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009;192:335–367.
16. Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi:10.1038/nn1579
17. Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi:10.1523/JNEUROSCI.20-06-02369.2000
18. Perreault ML, Fan T, Alijaniaram M, O’Dowd BF, George SR. Dopamine D1-D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: regulation of BDNF, GAD67 and VGLUT1/2. PLoS One. 2012;7:e33348. doi:10.1371/journal.pone.0033348
19. Baker TE, Lesperance P, Tucholka A, et al. Reversing the atypical valuation of drug and nondrug rewards in smokers using multimodal neuroimaging. Biol Psychiatry. 2017;82:819–827. doi:10.1016/j.biopsych.2017.01.015
20. Lin X, Deng J, Shi L, et al. Neural substrates of smoking and reward cue reactivity in smokers: a meta-analysis of fMRI studies. Transl Psychiatry. 2020;10:97. doi:10.1038/s41398-020-0775-0
21. Wall MB, Mentink A, Lyons G, Kowalczyk OS, Demetriou L, Newbould RD. Investigating the neural correlates of smoking: feasibility and results of combining electronic cigarettes with fMRI. Sci Rep. 2017;7:11352. doi:10.1038/s41598-017-11872-z
22. Stein EA, Pankiewicz J, Harsch HH, et al. Nicotine—induced limbic cortical activation in the human brain: a functional MRl study. Am J Psychiatry. 1998;155(8):1009–1015. doi:10.1176/ajp.155.8.1009
23. Due DL, Huettel SA, Hall WG, et al. Activation in mesolimbie and visuospatial neural circuits elicited by smoking cues; evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159(6):954–960. doi:10.1176/appi.ajp.159.6.954
24. McClemon FJ, Kozink RV, Lutz AM, et al. 24-h smoking abstinence potentiates fMRI—BOLD activation to smoking CUeS in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204(1):25–35. doi:10.1007/s00213-008-1436-9
25. Lee JH, Lim E, Wiederhold BK, et al. A functional magnetic resonance imaging (FMRI) study of cue—induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005;30(3):195–204. doi:10.1007/s10484-005-6377-z
26. Owens MM, MacKillop J, Gray JC, et al. Neural correlates of tobacco cue reactivity predict duration to lapse and continuous abstinence in smoking cessation treatment. Addict Biol. 2018;23:1189–1199. doi:10.1111/adb.12549
27. Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98:886–903. doi:10.1016/j.neuron.2018.03.048
28. Wang K, Yang J, Zhang S, et al. The neural mechanisms underlying the acute effect of cigarette smoking on chronic smokers. PLoS One. 2014;9:e102828. doi:10.1371/journal.pone.0102828
29. Hu B, Li C, Jing B, Chu S, Peng P, Jiang T. Research on rs-fMRI brain functional connectivity in smokers. Zhongguo Yixue Yingxiang Jishu. 2016;34:486–490.
30. Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–772. doi:10.1038/nrn2915
31. Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi:10.1016/j.bbr.2008.10.034
32. Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi:10.1111/j.1460-9568.2004.03095.x
33. Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi:10.1038/nature04053
34. Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi:10.1111/j.1460-9568.2009.06796.x
35. Watson P, van Wingen G, de Wit S. Conflicted between goal-directed and habitual control, an fMRI investigation. eNeuro. 2018;5:
36. van der Straten A, van Leeuwen W, Denys D, van Marle H, van Wingen G. The effect of distress on the balance between goal-directed and habit networks in obsessive-compulsive disorder. Transl Psychiatry. 2020;10:73. doi:10.1038/s41398-020-0744-7
37. Tau GZ, Marsh R, Wang Z, et al. Neural correlates of reward-based spatial learning in persons with cocaine dependence. Neuropsychopharmacology. 2014;39:545–555. doi:10.1038/npp.2013.189
38. Vieta E. DSM-5.1. Acta Psychiatr Scand. 2016;134:187–188. doi:10.1111/acps.12624
39. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x
40. Griffiths RR, Troisi JR, Silverman K. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. doi:10.1097/00008877-199302000-00001
41. Ert E, Yechiam E, Arshavsky O. Smokers’ decision making: more than mere risk taking. PLoS One. 2013;8:e68064. doi:10.1371/journal.pone.0068064
42. Li L, Lu Y, Li Y, Zhang M, Wang Z, Guo Z. Study on the decision-making behavior preference of middle-aged and senior men’s smoking dependence. Qingdao Daxue Xuebao. 2019;55:295–298.
43. Chen R, Guan M, Lou J, et al. Aberrant hippocampus functional connectivity in college students with mobile phone dependence with resting state fMRI. Zhongguo Yixue Yingxiang Jishu. 2017;33:193–197.
44. Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233. doi:10.1016/j.neuroimage.2011.05.028
45. Lockwood PL, Wittmann MK. Ventral anterior cingulate cortex and social decision-making. Neurosci Biobehav Rev. 2018;92:187–191. doi:10.1016/j.neubiorev.2018.05.030
46. Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi:10.1111/j.1460-9568.2010.07590.x
47. Wilson SJ, Sayette MA. Neuroimaging craving: urge intensity matters. Addiction. 2015;110:195–203. doi:10.1111/add.12676
48. Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107:14811–14816. doi:10.1073/pnas.1007779107
49. Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146:167–174. doi:10.1016/j.bbr.2003.09.025
50. Haddon JE, Killcross S. Inactivation of the infralimbic prefrontal cortex in rats reduces the influence of inappropriate habitual responding in a response-conflict task. Neuroscience. 2011;199:205–212. doi:10.1016/j.neuroscience.2011.09.065
51. Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79(2):361–374. doi:10.1016/j.neuron.2013.05.038
52. Smith KS, Virkud A, Deisseroth K, et al. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109(46):18932–18937. doi:10.1073/pnas.1216264109
53. Yousaf T, Dervenoulas G, Politis M. Advances in MRI methodology. Int Rev Neurobiol. 2018;141:31–76.
54. Dong C, Liang J, Dong Y, Zheng Z, Peng Z. Neural mechanism of objective-directed and habit learning system. Xinli Kexue Jinzhan. 2018;26:667–677.
55. Zhou Y, Ye J, Meng Z. Neural-molecular mechanism of learning of opium addiction. Zhonguo Yaowu Lanyong Fangzhi Zazhi. 2004;6:344–346.
56. Ibrahim LA, Mesik L, Ji XY, et al. Cross-modality sharpening of visual cortical processing through Layer-1-mediated inhibition and disinhibition. Neuron. 2016;89(5):1031–1045. doi:10.1016/j.neuron.2016.01.027
57. Chou XL, Fang Q, Yan L, et al. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. Elife. 2020;6(9):e54157. doi:10.7554/eLife.54157
58. Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi:10.1038/nn1075
59. Stokes MG, Atherton K, Patai EZ, Nobre AC. Long-term memory prepares neural activity for perception. Proc Natl Acad Sci USA. 2012;109:E360–E367. doi:10.1073/pnas.1108555108
60. Soto D, Greene CM, Kiyonaga A, Rosenthal CR, Egner T. A parieto-medial temporal pathway for the strategic control over working memory biases in human visual attention. J Neurosci. 2012;32:17563–17571. doi:10.1523/JNEUROSCI.2647-12.2012
61. Gläscher J, Hampton AN, O’Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb Cortex. 2009;19:483–495. doi:10.1093/cercor/bhn098
62. Busti. D, Geracitano R, Whittle N, et al. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 2011;31:5131–5144. doi:10.1523/JNEUROSCI.6100-10.2011
63. McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi:10.1111/j.1749-6632.1999.tb09275.x
64. Hart G, Bradfield LA, Balleine BW. Prefrontal corticostriatal disconnection blocks the acquisition of goal-directed action. J Neurosci. 2018;38:1311–1322. doi:10.1523/JNEUROSCI.2850-17.2017
65. Haynos AF, Hall LMJ, Lavender JM, et al. Resting state functional connectivity of networks associated with reward and habit in anorexia nervosa. Hum Brain Mapp. 2019;40:652–662.
66. Vergara VM, Liu J, Claus ED, Hutchison K, Calhoun V. Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage. 2017;1(151):45–54. doi:10.1016/j.neuroimage.2016.11.012
67. Ye Q, Zou F, Lau H, Hu Y, Kwok SC. Causal evidence for mnemonic metacognition in human precuneus. J Neurosci. 2018;38(28):6379–6387. doi:10.1523/JNEUROSCI.0660-18.2018
68. Monge ZA, Geib BR, Siciliano RE, Packard LE, Tallman CW, Madden DJ. Functional modular architecture underlying attentional control in aging. NeuroImage. 2017;155:257–270. doi:10.1016/j.neuroimage.2017.05.002
69. Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi:10.1176/appi.ajp.157.11.1789
70. Cardenas VA, Hough CM, Durazzo TC, Meyerhoff DJ. Cerebellar morphometry and cognition in the context of chronic alcohol consumption and cigarette smoking. Alcohol Clin Exp Res. 2020;44:102–113. doi:10.1111/acer.14222
71. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. doi:10.1371/journal.pone.0068910
72. de Wit S, van de Vijver I, Ridderinkhof KR. Impaired acquisition of goal-directed action in healthy aging. Cogn Affect Behav Neurosci. 2014;14:647–658. doi:10.3758/s13415-014-0288-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.