Back to Journals » Clinical Interventions in Aging » Volume 12
Response to teriparatide in Chinese patients with established osteoporosis: osteocalcin and lumbar spine bone-mineral density changes from teriparatide Phase III study
Authors Lu C , Chen Y, Zhang B, Chen Y, Bai F, Chen D
Received 3 May 2017
Accepted for publication 9 August 2017
Published 12 October 2017 Volume 2017:12 Pages 1717—1723
DOI https://doi.org/10.2147/CIA.S140900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Chunyan Lu,1 Yun Chen,2 Bin Zhang,2 Yu Chen,2 Fan Bai,2 Decai Chen1
1Department of Endocrinology and Metabolism, West China Hospital, Sichuan University, Chengdu, China; 2Lilly Suzhou Pharmaceutical Co. Ltd, Shanghai, China
Abstract: Teriparatide is the first anabolic agent for osteoporosis, and this analysis aimed to understand responses to teriparatide in Chinese patients with established osteoporosis in subgroups. In this Phase III study of teriparatide in China, 362 patients were randomized at a 2:1 ratio to receive subcutaneous teriparatide (20 µg/day) or intranasal salmon calcitonin (200 IU/day) for 24 weeks. Teriparatide treatment produced a significantly greater increase in lumbar spine bone-mineral density (LS-BMD) in postmenopausal women than calcitonin at the 24-week end point. The relationship between osteocalcin (OCN) and LS-BMD was evaluated, and the greatest correlation was found between absolute OCN change at week 12 and percentage change in LS-BMD for patients in the teriparatide group (r=0.24, P<0.001). The correlation weakened at week 24 (r=0.16, P=0.02) and was negligibly negative for calcitonin-treated patients. Proportions of patients achieving >10 µg/L absolute OCN change from baseline in the teriparatide- and calcitonin-treated groups were 81% and 6% at week 12, respectively (P<0.001). Proportions of patients with increased LS-BMD ≥3% at week 24 from baseline were 71% and 35% in the teriparatide- and calcitonin-treated groups, respectively (P<0.001). Proportions of patients meeting both criteria were 63% for the teriparatide group and 1% for the calcitonin-treated group (P<0.001). Subgroup analysis suggested that significant increases in LS-BMD and OCN can be achieved in patients receiving teriparatide, regardless of baseline age, LS-BMD, and fracture times. The rate of treatment-emergent adverse events in each subgroup was similar to the overall analysis.
Keywords: osteoporosis, teriparatide, lumbar spine bone-mineral density, osteocalcin
Introduction
Osteoporosis is a chronic metabolic bone disease that has a great impact on China, which has the largest elderly population in the world. Li et al showed that the total prevalence of osteoporosis in Chinese over 40 years old was 16.1% and the prevalence rate 11.5% and 19.9% among males and females, respectively.1 The incidence of fragility fractures increases markedly with age, and more than half of women and a third of men will experience osteoporotic fractures during their lives.2 In a study of the osteoporosis-related fractures in China for 2010–2050, it was estimated that fracture numbers will reach 6 million in 2050, with total treatment costs of US$9.45 billion in 2010 and estimated to reach $25.43 billion by 2050.3
Teriparatide is an anabolic agent4 that stimulates osteoblast maturation and function, which in turn activate osteoclasts, thus rebalancing bone formation and resorption.5 Although there are a lot of published clinical studies on the efficacy of teriparatide, very few have studied response to the drug in Chinese patients.4,6,7
It takes about 12 months or more to reflect bone-mineral density (BMD) changes to show efficacy after therapy in men and women.4,6,7 Bone-turnover marker changes may predict anabolic treatment efficacy earlier than BMD. There are data suggesting a relationship between procollagen I N-terminal peptide (PINP) and BMD in Western countries.8–10 As another marker of bone formation, osteocalcin (OCN) may also indicate the efficacy of anabolic medicines in early stages of treatment.11 However, the clinical relevance of changes in OCN and BMD remains unclear. There are limited data on the relationship between OCN and BMD during anabolic therapy. It is also important to understand response to teriparatide in different Chinese patients with respect to baseline characteristics. To gain a better understanding of this, post hoc analysis was performed to explore the clinical significance of a change in OCN with BMD and whether teriparatide is efficacious within different subgroups.
Materials and methods
Study design and participants
Ethical review boards at each center approved the study, and patients gave written informed consent to participate. The study design for the Chinese teriparatide efficacy and safety trial has previously been published.12 Briefly, the trial was an open-label, multicenter, active-comparator, randomized Phase III study (ClinicalTrials.gov identifier NCT00414973) aimed to examine the efficacy, safety, and tolerability of teriparatide in the Chinese population. The subject sample comprised Chinese men and postmenopausal women with established osteoporosis (at least one fracture in the lumbar spine [LS] and BMD T-score ≤2.5). Those who met protocol criteria were randomized 2:1 to teriparatide (20 μg/day) and salmon calcitonin (200 IU/day), self-administered by subcutaneous injection for up to 24 weeks. All patients received approximately 500–650 mg/day elemental calcium and 200 to 400 IU/day vitamin D supplementation. The treatment-compliance rate for teriparatide was greater than 97%, and there was no difference between the two treatment groups. This post hoc analysis was performed using the trial database.
Outcome measures
LS-BMD was measured at baseline and week 24 by dual-energy X-ray absorptiometry using Hologic (Marlborough, MA, USA), Lunar (GE Healthcare, Little Chalfont, UK) or Norland (Swissray, Piscataway, NJ, USA) scanners. Manufacturer-specific BMD data, not scanner-generated standardized data, were collected. Standardized BMD (sBMD) data were the basis for any statistical analysis. The following standardization methodology was used:13
- for Hologic instruments: sBMD (mg/cm2) = 1,000 [1.091 × BMDHologic − 0.016]
- for Lunar instruments: sBMD (mg/cm2) = 1,000[BMDLunar − 0.0552]
- for Norland instruments: sBMD (mg/cm2) = 1,000 [1.005 × BMDNorland + 0.07]
As a biochemical marker of bone formation, OCN was measured at baseline, week 12, and week 24. Due to the inherent liability of OCN, all samples were tested at the National Center for Clinical Laboratories using an Elecsys 2010 immunoassay analyzer (Hoffman-La Roche, Basel, Switzerland) to ensure consistency of methods. To ensure accurate, complete, and reliable data, interlaboratory-standardization methods and quality-assurance procedures were applied. Investigators had to document their review of each laboratory report. During the study, adverse events (AEs) were collected at every visit. Treatment-emergent AEs (TEAEs) were defined as any event reported after the beginning of the treatment that was worse in severity than at baseline.
Statistical analyses
Statistical analyses of this post hoc analysis were based on the intent-to-treat population. All tests were two-tailed with a significance level of 0.05. As for changes in OCN, Wilcoxon rank-sum tests were applied to compare differences between the teriparatide- and calcitonin-treatment groups. Wilcoxon signed-rank tests were performed to compare OCN change within treatment groups. Analysis of covariance (ANCOVA) adjusting for investigator and baseline LS-BMD was used to test treatment difference on percentage change in LS-BMD from baseline to week 24. Spearman ranked correlations were used to test the correlation between OCN and LS-BMD, and correlation coefficients and P-values are provided.
To determine the best cutoff point of absolute OCN change at week 12 to predict week 24 LS-BMD, sensitivity, specificity, and accuracy were calculated. As an exploratory analysis, cutoff points of 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 μg/L were tested and responses defined as percentage change in LS-BMD ≥3% at week 24. Fisher’s exact test was used to assess the two treatment groups on percentages of patients achieving OCN increases >10 μg/L and LS-BMD increases ≥3%.
Subgroup categories were age (<75 or ≥75 years old), baseline LS-BMD (≤ median or > median), and baseline fractures (one or more). For subgroup analyses, ANCOVAs adjusting for investigator and baseline LS-BMD were used to test treatment differences in percentage change in LS-BMD between the teriparatide- and calcitonin-treated groups. Adjusted mean differences and 95% CIs are presented. For analysis of the bone marker OCN, where results can deviate significantly from the normality assumption, ranked ANCOVA adjustment for investigator and baseline OCN was used to test the treatment-difference percentage change in OCN. The proportion of patients with any TEAE was compared within subgroups using logistic regression. Adjustments for multiplicity were not made, due to the exploratory purpose. All subgroup analyses were based on 24-week data and performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Demographic and baseline clinical characteristics
A total of 362 patients were included in this post hoc analysis. Table 1 summarizes the baseline demographic characteristics of all intent-to-treat patients, shown separately for the teriparatide- and calcitonin-treated groups. Baseline characteristics between the two treatment arms were similar.
Relationship between OCN and LS-BMD
Serum OCN increased significantly after teriparatide treatment (Table 2). Compared to baseline, OCN was significantly increased at week 12 (median of absolute change and percentage change at week 12 were 22.3 μg/L and 113%, P<0.001) after teriparatide treatment and continued to increase at week 24 (median of absolute change and percentage change at week 24 were 29.2 μg/L and 135%, P<0.001). In the calcitonin-treated group, OCN was decreased at week 12 and 24 from baseline. The differences between the two treatment arms were statistically significant (P<0.001).
  | Table 2 Absolute and percentage changes in OCN at weeks 12 and 24 |
At week 24, LS-BMD had increased significantly from baseline for those patients taking teriparatide (mean percentage change ± SEM 6.28%±0.45%, P<0.0001). There was a slight but statistically significant LS-BMD increase in the calcitonin-treatment group (mean percentage change ± SEM 1.92%±0.61%, P=0.0018). The elevation in the teriparatide treatment group was greater (P<0.0001).
Based on these analyses, the correlation between change in OCN and percentage change in LS-BMD at week 24 was assessed (Table 3). Significant positive correlations were observed in the teriparatide-treated group between baseline OCN, absolute OCN change at week 12/24, percentage OCN change, and percentage change in LS-BMD at week 24 (P<0.05). Absolute changes in OCN at week 12 and LS-BMD at week 24 showed the highest correlation (r=0.24, P<0.001) (Table 3). In the calcitonin-treated group, there was no significantly positive correlation between OCN and LS-BMD at week 24.
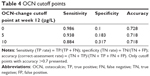
Since the absolute change in OCN at week 12 was highly correlated with LS-BMD response at week 24, a series of cutoff points were tested, and three (0, 5, and 10 μg/L) with accuracy >0.7 are listed (Table 4). The sensitivity of each selected cutoff point was >0.88. Specificity was highest (0.317) among the three listed candidate cutoff points when using a week 12 OCN absolute-change cutoff value of 10 μg/L.
Proportions of patients achieving more than 10 μg/L absolute OCN change from baseline in the teriparatide- and calcitonin-treated groups were 81% and 6% at week 12, respectively (P<0.001). Proportions of patients with LS-BMD increase ≥3% at week 24 from baseline were 71% and 35% in the teriparatide- and calcitonin-treated groups, respectively (P<0.001). Proportions of patients meeting both criteria were 63% for the teriparatide group and 1% for the calcitonin-treated group (P<0.001) (Table 5).
Subgroup analyses of efficacy and safety
Subgroup analyses were performed to assess the difference in OCN and LS-BMD changes between the two treatment arms in each subgroup of osteoporosis patients. Teriparatide treatment resulted in a significant increase on LS-BMD and OCN within all the subgroups compared with calcitonin treatment (P<0.05). Within each subgroup, patients on teriparatide experienced more TEAEs compared with the calcitonin-treated group, and the OR estimate was 1.17–2.86. Subgroup analyses of efficacy and safety of teriparatide vs calcitonin by baseline age, baseline LS-BMD, and baseline fracture times are summarized in Table 6.
Discussion
This is the first analysis showing OCN changes in Chinese osteoporotic patients using teriparatide. OCN is a protein produced by osteoblasts and incorporated into the bone matrix. It is also released into the circulation from the matrix during bone resorption. There have been discussions on whether OCN is a marker of bone formation or an aspecific bone-turnover marker.14 OCN is positively related to risk of hip fracture, and may be a prediction factor of fracture independently of BMD.15 In this analysis, we explored the correlation between OCN change and LS-BMD in a teriparatide-treated group, which was statistically significant positively. This result may provide support for using OCN as a marker of bone formation. A combination of OCN and LS-BMD assessment in risk evaluation of osteoporosis has been propagated by researchers outside China.16,17 This post hoc analysis is the first of its kind to analyze the correlation between OCN and BMD changes for Chinese patients with osteoporosis. Absolute OCN change at week 12 had the most significant correlation with percentage change in LS-BMD for patients in the teriparatide group (r=0.24, P<0.001) (Table 3). The correlation weakened at week 24 (r=0.16, P=0.02) and was negligibly negative for patients taking calcitonin. A cutoff OCN value of 10 μg/L showed relatively high accuracy (71.8%), sensitivity (88.4%), and specificity (31.7%) in predicting the change of LS-BMD at week 24 (Table 4). As such, 10 μg/L was applied in further exploratory analyses as a cutoff value. Proportions of patients with an increase in OCN >10 μg/L at week 12 and an increase in LS-BMD ≥3% at week 24 were 63% in the teriparatide group patients and 1% in the calcitonin group (P<0.001). OCN is the main noncollagenous bone protein, and its expression is stimulated by calcitriol and inhibited by glucocorticoids.18,19 Lasco et al reported treatment with teriparatide increased plasmatic and urinary levels of cortisol after 6 and 12 months in their study.20 The change reached significance after 12 months compared with baseline and control. In vitro and in vivo experimental data suggest that teriparatide and teriparatide-related peptide increase secretion of both aldosterone and cortisol from human adrenocortical cells. A direct secretagogue effect of teriparatide on adrenals in osteoporotic postmenopausal women has also been reported.20 It is possible that OCN secretion is inhibited by adrenal hormones, especially glucocorticoids, thus weakening the correlation with BMD after 12 weeks. Niimi et al reported using an 80 μg/L increase in PINP as a predictor of a 10% increase in LS-BMD with teriparatide therapy for 12 months, and two-thirds of patients were in line with this judgment.21 Our results show that the early response to OCN for subjects on teriparatide can predict the late response of LS-BMD, which has a high degree of consistency with PINP.
In addition, changes in LS-BMD, OCN, and TEAE occurrence between teriparatide and calcitonin treatment were assessed to investigate efficacy and safety within subgroups. Changes in LS-BMD and OCN between teriparatide and calcitonin within defined subgroups were significant. TEAE rates within each subgroup were similar to the overall analysis. The response to teriparatide is independent of age, initial BMD, and prevalent vertebral fractures.22 The published results may also extrapolate to Chinese patients, based on our research data that teriparatide is well tolerated and efficacious in Chinese patients with different baseline characteristics.
Several limitations deserve mention. First, the treatment period was 24 weeks, and OCN change at week 4 was not tested. However, we found a similar increasing trend in bone-formation biomarkers at week 12 to other research.23 Second, asymptomatic vertebral compression changes were not collected. As there were no new clinical fractures in the teriparatide group and only one subject had a phalangeal fracture, the relationship between fracture and changes in BMD or OCN was not analyzed. In addition, no other biomarkers (such as PINP, bone ALP, C-terminal telopeptide) were evaluated.
In conclusion, an increase in OCN >10 μg/L at week 12 might be clinically relevant in predicting the efficacy of daily subcutaneous injection of 20 μg teriparatide in increasing LS-BMD level at week 24. Within each subgroup, teriparatide was tolerable and significantly increased both LS-BMD and OCN. TEAEs within all subgroups were consistent with the overall analyses. These results may support the observation that teriparatide stimulates bone formation in patients with osteoporosis and that OCN is a promising biomarker to monitor the response to teriparatide therapy.
Acknowledgments
All research in this article was sponsored by Eli Lilly and Company. We thank Liqun Gu, Xin Wang, and Xiaoyue Wang (Eli Lilly and Company) for medical, statistical, data integrity, and editorial review of this manuscript.
Disclosure
Yun Chen, Yu Chen, Bin Zhang, and Fan Bai are employees of Eli Lilly and Company. Yu Chen owns stock in Eli Lilly and Company. The other authors report no conflicts of interest in this work.
References
Li N, Ou P, Zhu H, Yang D, Zheng P. Prevalence rate of osteoporosis in the mid-aged and elderly in selected parts of China. Chin Med J (Engl). 2002;115(5):773–775. | ||
Ross PD. Osteoporosis: frequency, consequences, and risk factors. Arch Intern Med. 1996;156(13):1399–1411. | ||
Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos Int. 2015;26(7):1929–1937. | ||
Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87(10):4528–4535. | ||
Buxton EC, Yao W, Lane NE. Changes in serum receptor activator of nuclear factor-κB ligand, osteoprotegerin, and interleukin-6 levels in patients with glucocorticoid-induced osteoporosis treated with human parathyroid hormone (1–34). J Clin Endocrinol Metab. 2004;89(7):3332–3336. | ||
Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18(1):9–17. | ||
Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. | ||
Lumachi F, Orlando R, Fallo F, Basso SM. Relationship between bone formation markers bone alkaline phosphatase, osteocalcin and amino-terminal propeptide of type I collagen and bone mineral density in elderly men: preliminary results. In Vivo. 2012;26(6):1041–1044. | ||
Niimi R, Kono T, Nishihara A, et al. An algorithm using the early changes in PINP to predict the future BMD response for patients treated with daily teriparatide. Osteoporos Int. 2014;25(1):377–384. | ||
Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011;48(4):798–803. | ||
Shimizu T, Takahata M, Kameda Y, et al. Vitamin K-dependent carboxylation of osteocalcin affects the efficacy of teriparatide (PTH1–34) for skeletal repair. Bone. 2014;64:95–101. | ||
Dai K, Chen D, Zhang Z. Comparison of the effects of subcutaneous teriparatide and intranasal calcitonin in treating established osteoporosis in postmenopausal Chinese women. Chin J Osteoporos Bone Miner Res. 2011;4:23–31. | ||
Lu Y, Ye K, Mathur AK, Hui S, Fuerst TP, Genant HK. Comparative calibration without a gold standard. Stat Med. 1997;16(16):1889–1905. | ||
Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem. 2000;37(Pt 4):432–446. | ||
Dai Z, Wang R, Ang LW, Yuan JM, Koh WP. Bone turnover biomarkers and risk of osteoporotic hip fracture in an Asian population. Bone. 2016;83:171–177. | ||
Kalaiselvi VS, Prabhu K, Ramesh M, Venkatesan V. The association of serum osteocalcin with the bone mineral density in post menopausal women. J Clin Diagn Res. 2013;7(5):814–816. | ||
Susanto LT. Serum osteocalcin and bone mineral density in postmenopausal women. Univ Med. 2016;30(3):155–161. | ||
Lisa L, Neradilova M, Tomasova N, Soutorova M, Zimak J. Osteocalcin in congenital adrenal hyperplasia. Bone. 1995;16(1):57–59. | ||
Maresova KB, Pavelka K, Stepan JJ. Acute effects of glucocorticoids on serum markers of osteoclasts, osteoblasts, and osteocytes. Calcif Tissue Int. 2013;92(4):354–361. | ||
Lasco A, Catalano A, Morabito N, et al. Adrenal effects of teriparatide in the treatment of severe postmenopausal osteoporosis. Osteoporos Int. 2011;22(1):299–303. | ||
Niimi R, Kono T, Nishihara A, et al. Determinants associated with bone mineral density increase in response to daily teriparatide treatment in patients with osteoporosis. Bone. 2014;66:26–30. | ||
Marcus R, Wang O, Satterwhite J, Mitlak B. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18(1):18–23. | ||
Krege JH, Lane NE, Harris JM, Miller PD. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int. 2014;25(9):2159–2171. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.