Back to Journals » Research and Reports in Neonatology » Volume 5
Response to bronchodilators in very preterm infants with evolving bronchopulmonary dysplasia
Authors Morrow DK, Schilling D, McEvoy C
Received 23 September 2015
Accepted for publication 9 November 2015
Published 2 December 2015 Volume 2015:5 Pages 113—117
DOI https://doi.org/10.2147/RRN.S96961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Schelonka
Daniel K Morrow, Diane Schilling, Cindy T McEvoy
Department of Pediatrics, Oregon Health and Science University, Portland, OR, USA
Background: There are few effective and safe medications to treat very low birth weight (VLBW) infants with evolving bronchopulmonary dysplasia. Bronchodilators are often given to patients who have clinical signs of reactive airway disease, but there is not enough information regarding their effectiveness within this population.
Objective: To quantify the pulmonary function response to bronchodilator therapy in a population of VLBW infants with evolving bronchopulmonary dysplasia.
Materials and methods: This is a retrospective analysis of an ongoing large database of pulmonary function tests (PFTs) in premature infants. We reviewed the pre- and post-bronchodilator PFTs ordered by a physician due to concern for reactive airway disease. Inclusion criteria: Birth weight (BW) <1,500 g; >14 days of age; admission diagnosis of respiratory distress syndrome; requiring ongoing oxygen, continuous positive airway pressure, or ventilator support at the time of PFT. PFTs were done prior to albuterol therapy and repeated 30 minutes after the therapy was given. PFTs included the measurement of passive respiratory mechanics with the single breath occlusion technique, including passive respiratory system compliance, resistance, and tidal volume.
Results: Forty VLBW infants (mean gestation of 27.4 weeks; mean BW of 848 g) were identified as having PFTs. Twenty-nine of these patients had a BW of ≤1,000 g. The patients were studied at a mean corrected gestational age of 34.9 weeks. Twenty-nine of 40 patients were extubated at the time of the PFT. Of these patients, 21 (52.5%) had a decrease in respiratory system resistance of ≥10%. From the other 19 patients, five (12.5%) had a decrease of 0% to <10% in respiratory system resistance, and 14(35%) showed no response to therapy. There was no significant difference in respiratory system compliance between the groups.
Keywords: Albuterol, pulmonary function
Introduction
Each year more than 15,000 cases of bronchopulmonary dysplasia (BPD) are diagnosed, with the majority of cases occurring in very low birth weight (VLBW) infants (<1,500 g).1 With the standard use of prenatal steroids, postnatal surfactant, and gentler modes of ventilation, the gestational age of viable infants has decreased, and therefore, the incidence of BPD has not changed.2,3 There are a number of factors that predispose infants to develop BPD, including gestational age, antenatal and postnatal infections, nutrition, and the need for mechanical ventilation.4 There are also ongoing investigations evaluating the role of genetics as well as epigenetics in the risk for developing BPD.5–7
Inhaled bronchodilators are often given to infants at risk for BPD who demonstrate clinical symptoms of reactive airway disease, with rapid fluctuations in oxygen saturation. Another common practice is to treat patients who appear to benefit from a trial of therapy. To date, neither of these practices have been validated. More importantly, there is minimal data on bronchodilator response in patients with modern-day BPD. A Cochrane review was done in 2012 looking at the prevention and treatment of BPD using bronchodilators.8 In this meta-analysis, Denjean et al9 was the only study that evaluated relevant clinical outcomes, in this case death or BPD. Study patients received salbutamol, placebo, or salbutamol in combination with beclometasone (inhaled steroid). The goal of the study was prevention of BPD and the therapy was continued for 28 days, starting at 10 days of life. There was no decrease in the incidence of BPD and no effect on mortality. The authors also quantified days on oxygen and days of mechanical ventilation and again found no benefit of bronchodilators.
There have not been any bronchodilator treatment studies in the neonatal intensive care unit that targeted infants with BPD. It is, however, known that up to 50% of infants with BPD will develop wheezing in the first few years of life that is often treated with a bronchodilator for symptomatic or clinical benefit.10 Furthermore, there are a number of studies utilizing PFT data to assess lung function in early childhood in previously preterm infants.11–13 The use of bronchodilators in early childhood has shown short-term improvements in lung function, but both short- and long-term clinical benefits warrant further study.14
Though the study done by Denjean et al did not demonstrate a decrease in the incidence of BPD with bronchodilators, it is feasible that only a subset of infants with evolving BPD actually respond to the therapy. This may be secondary to pre- and postnatal factors as well as genetic predisposition. We hypothesize that there is a subset of neonates who demonstrate airway reactivity that can be screened for using pulmonary function test (PFT) data. In the current study, we utilized an ongoing clinical PFT database to generate preliminary data regarding the response to bronchodilator therapy in a population of VLBW infants with evolving BPD. We also compared available clinical characteristics of those who showed a positive response to bronchodilators with those who did not. The goal of the study was to demonstrate proof of concept that there are subsets of patients who may respond to bronchodilators that can be identified using PFT data. This would serve as pilot data for a randomized controlled trial that would assess relevant clinical outcomes in patients who had demonstrated a PFT response to bronchodilators prior to being randomized.
Materials and methods
This was a retrospective analysis of an ongoing database of PFTs in premature and newborn infants performed in the neonatal intensive care unit at the Oregon Health and Science University. The study protocol was reviewed and approved by the Oregon Health and Science University Institutional Review Board and the need for consent was waived due to the retrospective nature of the study. We reviewed the pre- and post-bronchodilator PFTs ordered by a physician due to concern for reactive airway disease in a premature patient with evolving BPD. Inclusion criteria included: birth weight <1,500 g; >14 days of age; admission diagnosis of respiratory distress syndrome; requiring oxygen, continuous positive airway pressure, or ventilator support at the time of PFT. Exclusion criteria included patients with congenital heart disease or congenital airway anomalies.
All of the testing was done by a single respiratory therapist following a standard PFT protocol (see “Measurements” section). Infants were studied in the supine position while quietly asleep. No sedation was used during the study period. PFTs were done prior to two puffs of albuterol therapy and repeated 30 minutes after the therapy was given. A responder was defined as an infant who demonstrated a greater than 10% decrease in respiratory system resistance (Rrs, cmH2O/mL/s) after the bronchodilator treatment. This criteria is based on the 2002 National Heart, Lung, and Blood Institute expert panel recommendations for the diagnosis and management of asthma.15 We then assessed responders for associated changes in compliance (Crs, mL/cmH2O/kg) and tidal volume (mL/kg).
Measurements
PFTs were measured with computerized infant pulmonary function cart (SensorMedics 2600; SensorMedics Inc., Yorba Linda, CA, USA). The measurements were done by connecting the pneumotachograph to the endotracheal tube if the infant was intubated or with the infants breathing through a face mask that was connected to a three-way valve if they were extubated. Passive respiratory system compliance (Crs) was obtained with the single-breath occlusion technique. The airway was briefly occluded at end inspiration until an airway pressure plateau was observed and the Hering–Breuer reflex was invoked. The linear portion of the passive flow volume curve was identified and a regression line was drawn to obtain the best fit. From the intercepts on the flow and volume axes, Crs and Rrs were calculated. Acceptance criteria included: 1) stable end expiratory baseline; 2) plateau pressure lasting >100 ms; 3) plateau pressure varying by ±0.125 cmH2O or less; 4) acceptable flow volume curve by visual inspection, with linear data segment identified; and 5) at least ten breaths accepted with a coefficient of variation of <20%.16–19
Pre- and post-bronchodilator measured variables were compared using a paired, two-tailed t-test (SPSS version 21, IBM Corporation, Armonk, NY, USA). Significance was set at P<0.05.
Results
Forty VLBW infants (mean gestation of 26.6 weeks; mean birth weight of 881 g) were identified as having pre- and post-bronchodilator PFTs. Seventy-five percent of the patients studied were male and 93% were receiving supplemental oxygen at the time of testing (Table 1). The patients were studied at a mean corrected gestational age of 34.9 weeks. The study group as a whole did not demonstrate a significant difference in Rrs following administration of a bronchodilator. The individual resistance is shown for all 40 patients (Figure 1). Within the cohort, 21 (52.5%) patients had a decrease in Rrs of ≥10% (P<0.05) (Table 2). From the other 19 patients, five (12.5%) had a decrease of 0% to <10% in Rrs, and 14 (35%) showed no response to therapy. The individual change in Rrs for patients who were responders is shown in Figure 2. There was not a consistent change in the Crs and tidal volume associated with a decrease in resistance. Of note, the infants who did not respond to bronchodilators, based on change in Rrs, were two times more likely to have received antenatal steroids and tended to have a lower baseline Rrs than those who did respond.
  | Table 1 Patient characteristics |
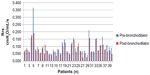  | Figure 1 Pre- and post-albuterol Rrs for all patients within the cohort. |
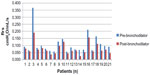  | Figure 2 Pre- and post-albuterol Rrs for only the responders. |
  | Table 2 Patient characteristics |
Discussion
Our study showed that approximately half of VLBW infants with evolving BPD in our contemporary cohort had a greater than 10% decrease in Rrs after bronchodilator therapy. It also appears that infants who were exposed to prenatal steroids are less likely to respond to bronchodilators based on PFT data alone. Due to the retrospective nature of this study, there are a number of limitations to the current dataset. There were insufficient data to determine changes in respiratory rate or supplemental oxygen need before and after the bronchodilator treatment for individual patients. These data will be important in future studies to correlate lung mechanics in response to bronchodilator therapy.
Despite the stated limitations, it is intriguing that there may be a subset of patients who benefit from bronchodilator therapy. Inflammation of the airways is clearly associated with reactive airway disease in a susceptible host.20 BPD is also associated with lung inflammation, thought to be secondary to both pre- and postnatal factors, and it is likely that a subset of BPD patients have concomitant airway reactivity.21,22 It was unexpected but interesting that in the current study, the patients who received prenatal steroids did not have the same response to bronchodilators. Based on this finding, one could speculate that prenatal steroids alter inflammatory cascades, thus decreasing the likelihood of airway reactivity. An alternative explanation is that a lack of prenatal steroid exposure is simply a marker of fetal distress, illness severity, and need to deliver urgently. In other words, the reason for early delivery, preeclampsia, or chorioamnionitis may put them at higher risk for airway reactivity. It should be noted that the patients who responded to bronchodilators demonstrated a trend toward higher baseline airway resistance.
There is increasing evidence that prenatal factors including, but not limited to, antenatal steroids, infection, smoking, obesity, and even a genetic predisposition have the potential to alter the BPD phenotype of a premature infant. It is likely that within a cohort of patients, there is significant heterogeneity regarding the pathophysiology causing hypoxemia leading to a diagnosis of BPD. Historically, therapies have been evaluated for their potential to benefit a randomized population of BPD patients. This approach assumes that all patients have the same potential to respond to a therapy instead of recognizing that alternative disease phenotypes exist.
The challenge to treating BPD is that the pathophysiology of the current disease is poorly understood. Many of the infants who die from prematurity may not represent the pulmonary histopathology of the surviving population of infants with BPD. Furthermore, BPD is diagnosed based on the need for oxygen for at least 28 days and/or the need for oxygen at 36 weeks corrected gestational age.23 The need for oxygen during this time does not lend insight into the cause of hypoxemia.
Conclusion
The goal of the current study was to generate preliminary data and further display the potential individualized nature of BPD. We were able to show that PFT data can be used to identify patients who respond to bronchodilator therapy and that exposure to prenatal steroids may decrease airway responsiveness. In future, it will be important to correlate PFT data with relevant clinical response and outcomes. As therapies to prevent and treat BPD are investigated, it is necessary to develop screening tools to characterize the variety of probable BPD phenotypes that exist and evaluate therapies within the context of these phenotypes.
Disclosure
The authors report no conflicts of interest in this work.
References
Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. 2013;37:132–137. | |
Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:305–313. | |
Kobaly K, Schluchter M, Minich N, et al. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121:73–81. | |
Jobe AH. Antenatal factors and the development of bronchopulmonary dysplasia. Semin Neonatol. 2003;8:9–17. | |
Somaschini M, Castiglioni E, Volonteri C, Cursi M, Ferrari M, Carrera P. Genetic predisposing factors bronchopulmonary dysplasia: preliminary data from a multicentre study. J Matren Fetal Neonatal Med. 2012;Suppl 4:127–130. | |
Wang H, St Julien KR, Stevenson DK, et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 2013;132:290–297. | |
Li J, Yu KH, Oehlert J, et al. Exome sequencing of neonatal blood spots and the identification of genes implicated in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:589–596. | |
Ng G, da Silva O, Ohlsson A. Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2012;6:CD003214. | |
Denjean A, Paris-Llado J, Zupan V, et al. Inhaled salbutamol and beclomethasone for preventing broncho-pulmonary dysplasia: a randomised double-blind study. Eur J Pediatr. 1998;157:926–931. | |
Hibbs AM, Walsh MC, Martin RJ, et al. One-year respiratory outcomes of preterm infants enrolled in the nitric oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153:525–529. | |
Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182:237–245. | |
Ronkainen E, Dunder T, Peltoniemi O, Kaukola T, Marttila R, Hallman M. New BPD predicts lung function at school age: follow-up study and meta-analysis. Pediatr Pulmonol. 2015;50:1090–1908. | |
Vom Hove M, Prenzel F, Uhlig HH, Robel-Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr. 2014;164:40–45. | |
Kotecha SJ, Edwards MO, Watkins WJ, Lowe J, Henderson AJ, Kotecha S. Effect of bronchodilators on forced expiratory volume in 1 s in preterm-born participants aged 5 and over: a systematic review. Neonatology. 2015;107:231–240. | |
National Asthma E, Prevention P. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics – 2002. J Allergy Clin Immunol. 2002;110:S141–S219. | |
Gappa M, Colin AA, Goetz I, Stocks J. Passive respiratory mechanics: the occlusion techniques. Eur Respir J. 2001;17:141–148. | |
McEvoy C, Schilling D, Spitale P, Peters D, O’Malley J, Durand M. Decreased respiratory compliance in infants less than or equal to 32 weeks’ gestation, delivered more than 7 days after antenatal steroid therapy. Pediatrics. 2008;121:e1032–e1038. | |
Morris MG, Gustafsson P, Tepper R, Gappa M, Stocks J. The bias flow nitrogen washout technique for measuring the functional residual capacity in infants. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Eur Respir J. 2001;17:529–536. | |
McEvoy C, Schilling D, Peters D, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. Am J Obstet Gynecol. 2010;202:544. e1–e9. | |
Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol. 1992;90:32–42. | |
Bhandari V. Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100:189–201. | |
Ericson JE, Laughon MM. Chorioamnionitis: implications for the neonate. Clin Perinatol. 2015;42:155–165. | |
Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
