Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 9
Response by gender of HIV-1-infected subjects treated with abacavir/lamivudine plus atazanavir, with or without ritonavir, for 144 weeks
Authors Squires KE, Young B, Santiago L, Dretler RH, Walmsley SL, Zhao HH, Pakes GE, Ross LL, Shaefer MS
Received 18 March 2016
Accepted for publication 20 May 2016
Published 3 March 2017 Volume 2017:9 Pages 51—61
DOI https://doi.org/10.2147/HIV.S108756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Bassel Sawaya
Kathleen E Squires,1 Benjamin Young,2,3 Lizette Santiago,4 Robin H Dretler,5 Sharon L Walmsley,6 Henry H Zhao,7 Gary E Pakes,8 Lisa L Ross,8 Mark S Shaefer8
On behalf of the ARIES Study Team
1Thomas Jefferson University, Philadelphia, PA, 2Apex Family Medicine and Research, Denver, CO, 3International Association of Physicians in AIDS Care, Washington DC, USA; 4HOPE Clinic and Wellness Center, San Juan, Puerto Rico; 5ID Specialists of Atlanta, Decatur, GA, USA; 6University Health Network, Toronto, ON, Canada; 7GlaxoSmithKline, 8ViiV Healthcare, Research Triangle Park, NC, USA
Purpose: The 144-week results of the open-label, multicenter Atazanavir/Ritonavir Induction with Epzicom Study (ARIES) were stratified by gender to compare treatment responses.
Methods: A total of 369 HIV-infected, antiretroviral-naïve subjects receiving once-daily abacavir/lamivudine + atazanavir/ritonavir (ATV/r) whose HIV-1 RNA was <50 copies/mL by week 30 were randomized 1:1 at week 36 to maintain or discontinue ritonavir for 108 subsequent weeks. Between- and within-treatment gender-related efficacy and safety differences were analyzed.
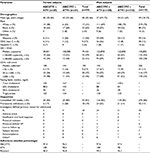
Results: Subjects were 85% male; 64% white; and had a mean age of 39 years, baseline median HIV-1 RNA of 114,815 copies/mL, and median CD4+ cell count of 198 cells/mm3. Gender (ATV [n=189]: 29 females/160 males; ATV/r [n=180]: 25 females/155 males) and most other demographics were similar between groups; more females than males were black (65% vs 25%) and fewer females had baseline HIV-1 RNA ≥100,000 copies/mL (41% vs 58%). At week 144, no significant differences between genders were observed in proportion maintaining HIV-1 RNA <50 copies/mL (ATV, 79% vs 77%; ATV/r, 60% vs 75%) or <400 copies/mL (ATV, 83% vs 84%; ATV/r, 68% vs 82%) (intent-to-treat-exposed: time to loss of virologic response analysis); median CD4+ change from baseline (ATV, +365 vs +300 cells/mm3; ATV/r, +344 vs +301 cells/mm3); proportion with treatment-related grade 2–4 adverse events (baseline to week 144: ATV, 41% vs 31%; ATV/r, 36% vs 43%; weeks 36 to 144: ATV, 14% vs 13%; ATV/r, 24% vs 23%); or proportion developing fasting lipid changes. Female and male virologic failure rates (ATV, 0 vs 5; ATV/r, 2 vs 4) and proportions completing the study were similar during the extension phase. Primary withdrawal reasons were loss to follow-up and pregnancy for females and loss to follow-up and other for males.
Conclusion: Over 144 weeks, no significant gender differences were observed in efficacy, safety, or fasting lipid changes with abacavir/lamivudine +ATV or abacavir/lamivudine +ATV/r.
Keywords: ARIES, HIV-infected, gender, virologic efficacy
Introduction
From a worldwide perspective, women currently comprise more than half of all people living with HIV/AIDS.1 For women in their reproductive years (15–49 years of age), HIV/AIDS is the leading cause of death.1 The HIV/AIDS epidemic in women in sub-Saharan Africa, the Caribbean, Latin America, Asia, and Eastern Europe is rapidly outpacing the occurrence of this disease in men. In the US, the proportion of AIDS diagnoses reported among women has more than tripled since 1985.2 Women now account for one in four new HIV diagnoses and AIDS-related deaths in the US.2 From a racial/ethnicity perspective, the rate of new HIV infections in the US is disproportionately higher by 20- and fourfold among African American and Hispanic women, respectively, compared with the rate observed in white women.2
Despite this surge in female HIV infection cases since the AIDS epidemic began, women remain largely underrepresented in clinical studies and, as a consequence, antiretroviral treatment guidelines continue to be based on data from clinical trials that enrolled predominantly male subjects.3 This issue of underrepresentation of women was particularly well illustrated by the findings of a large meta-analysis of 43 pivotal, randomized, registrational clinical trials conducted from 2000 through 2008, which showed that women accounted for only 20% of the 22,411 HIV-positive study subjects evaluated.4
Numerous studies have reported that HIV-infected women have disease characteristics, antiretroviral drug pharmacokinetics, and pharmacodynamic responses to HIV treatment that may vary from those seen in men.5–11 HIV-positive women tend to have higher CD4+ cell count levels than men throughout the course of their infection,8–10 and their viral loads average 0.25 log10 lower than men’s viral loads at specific CD4+ cell count cut-offs.5 Women tend to have lower gastrointestinal motility than men (due to higher female estrogen blood levels), which can slow absorption of antiretroviral agents.7 Women have also been reported to have lower expression of P-glycoprotein, a key ATP-dependent efflux pump transporter protein present in the intestinal epithelium, leading to enhanced gut absorption of protease inhibitors (PIs).11 Studies investigating possible gender-related effects on hepatic enzyme cytochrome P450 3A4 activity have yielded varying results.7,11 Women have shown greater ritonavir-associated pharmaco-enhancement of PI activity than men.11 A woman’s generally smaller body size, higher body fat, and lower average plasma volume can lead to higher plasma concentrations of antiretroviral drugs, while a woman’s lower average organ blood flow may result in prolonged drug effects in the liver and kidney compared with what is seen in men.7
Given the potential for gender to affect response, analysis of response by gender in clinical trials of HIV-infected patients has emerged as a potentially important component in research evaluating antiretroviral regimens. Some, but not all, studies have reported no gender-related differences in virological and immunological response to combination antiretroviral therapy, although data are conflicting.4,12–20 To better define any effect that gender may have had on the efficacy or safety of atazanavir (ATV)/abacavir (ABC)/lamivudine (3TC)-based antiretroviral treatment over a 144-week period, a gender stratification analysis was performed in Atazanavir/Ritonavir Induction with Epzicom Study (ARIES; EPZ108859).
Materials and methods
Study design and participants
ARIES was a randomized, open-label, noninferiority, multicenter study that was conducted at 66 outpatient HIV clinics in the US and Canada from March 2007 to July 2010. The ARIES (EPZ108859) ClinicalTrials.gov registration number is NCT00440947. ARIES enrolled antiretroviral-naïve HIV-infected subjects who were ≥18 years of age, had a screening viral load ≥1,000 copies/mL, an appropriate screening viral genotype, and any CD4+ cell count. The details of the study design have been published previously.21–23 Subjects considered naïve to antiretroviral therapy could have received no more than 14 days of prior treatment with nucleoside reverse transcriptase inhibitors with no prior treatment with PIs or non-nucleoside reverse transcriptase inhibitors. Enrolled subjects were negative for the HLA-B*5701 allele and hepatitis B surface antigen. Subjects were excluded if they had medical conditions that investigators considered severe enough to compromise safety, including diabetes mellitus, congestive heart failure, cardiomyopathy, or other cardiac dysfunction.
Research ethics
The study was conducted in accordance with good clinical practice and the ethical principles of the Declaration of Helsinki. The institutional review board/independent ethics committee at each study site (Table S1) approved the protocol, amendments, and informed consent forms before initiation. All subjects provided written informed consent to participate in the study. The trial is registered with ClinicalTrials.gov, number NCT00440947.
Procedures
Subjects were initiated on a 36-week regimen (prerandomization phase) of a once-daily fixed-dose combination of ABC/3TC 600/300 mg (Epzicom®, ViiV Healthcare, Research Triangle Park, NC, USA) plus ATV 300 mg (Reyataz®, Bristol-Myers Squibb, New York, NY, USA) and ritonavir 100 mg (Norvir®, Abbott Laboratories, Abbott Park, IL, USA). Subjects with a confirmed viral load <50 copies/mL by week 30 were randomized 1:1 at week 36 (randomization phase) to either continue ABC/3TC + ATV/ritonavir (ATV/r) 300 mg/100 mg od for 48 weeks or switch to ABC/3TC plus unboosted ATV 400 mg od (the “simplification” treatment group). Subjects who remained in the study through week 84 were eligible to participate in an optional extension phase, irrespective of whether their viral load was <50 copies/mL at week 84. Subjects who participated in the extension phase maintained their current treatment regimen through week 144. Subjects were required to withdraw from the study if they experienced confirmed HIV-1 RNA rebound ≥400 copies/mL after achieving virologic suppression to <400 copies/mL and their confirmatory viral load result was ≥2,000 copies/mL.
HIV-1 RNA, CD4+ cell counts, adverse events, and treatment adherence (evaluated by pill count of each regimen component) were assessed at baseline and weeks 2, 4, 8, 12, and every 12 weeks thereafter through 144 weeks. Adverse events were monitored and graded using the 2004 Division of AIDS Toxicity Grading Scale. Fasting lipids were evaluated at baseline and at weeks 84 and 144. Median fasting lipid concentrations were compared with those at the cut points (maximum concentrations considered within normal limits) established by the US Department of Health and Human Services National Cholesterol Education program (NCEP) guidelines.24
Statistical analysis
Gender stratification within and between treatment groups was applied to demographic data, study disposition results, protocol-defined virologic failures, proportions of subjects with HIV-1 RNA <50 and <400 copies/mL, CD4+ cell counts, fasting lipid results, and drug-related grade 2–4 adverse events. Descriptive statistical analyses were applied to demographic information.
The primary population for efficacy analyses was the intent-to-treat exposed (ITT-E) population, which included all enrolled subjects exposed to ≥1 dose of study medication. Time to loss of virologic response (TLOVR) analysis was applied to virologic suppression measurements. The significance of differences between genders for achievement of HIV-1 RNA <50 and <400 copies/mL and incidence of adverse events were assessed by the Cochran–Mantel–Haenszel test stratified by treatment regimen. The Fisher’s exact test was used to compare binary outcomes between genders within each treatment group, and the Wilcoxon rank-sum test was used to compare the change from baseline in CD4+ cell count and fasting lipid values between genders by treatment group. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Study demographics and disposition
A total of 515 subjects participated in the 36-week prerandomization phase of ARIES; 419 of these subjects met randomization criteria to enter the 84-week randomization phase, while 369 subjects elected to participate in the 144-week extension phase. The percentage of female subjects remained similar (15%–17% during the pre- and randomization and extension phases) throughout the study.21–23
As the overall proportion of female subjects remained fairly stable throughout the study, an in-depth analysis of study demographics and disposition stratified by gender was performed for the extension phase. The overall baseline demographics were generally similar when stratified by gender, except that, proportionally, more female than male subjects were black (65% vs 25%; Table 1). These proportions were consistent with those seen in the 36-week prerandomization phase (66% vs 26%) and the 84-week randomization phase (65% vs 26%). Proportionally, fewer female than male subjects had a baseline HIV-1 RNA ≥100,000 copies/mL (41% vs 58%; Table 1), which was consistent with the proportion observed at week 36 (40% vs 59%) and week 84 (39% vs 56%). Baseline median HIV-1 RNA, median CD4+ cell count, mean age, and percentage of subjects with Hispanic ethnicity, Centers for Disease Control and Prevention classification C, or concurrent hepatitis C were generally similar between male and female subjects in each of the treatment groups.
By treatment group, the proportions of female and male subjects who completed 144 weeks of the study were similar (83% and 85%, respectively, in the ATV group, and 80% and 86%, respectively, in the ATV/r group; Table 1). However, the primary reasons given by the investigators for subjects discontinuing the study varied somewhat by gender. For the ten female subjects who left the study between week 84 and 144, five (50%) were lost to follow up and three (30%) withdrew for pregnancy; for the 45 male subjects who withdrew, the most common reasons were lost to follow-up (29%) and “other” (typically site or sponsor reasons, 29%; Table 1). The extension phase discontinuation findings were similar to those reported during the prerandomization phase in that proportionally more male subjects withdrew due to being lost to follow-up (24% vs 13%). Proportionally, more male than female subjects withdrew due to adverse events (24% vs 13%) in the prerandomization phase, and, proportionally, fewer male than female subjects withdrew due to insufficient viral load response (10% vs 33%) or noncompliance (10% vs 20%).
Disposition results in male and female subjects during the randomization phase were comparable to those observed during the extension phase with respect to discontinuations for noncompliance and protocol violations, although proportionally more male than female subjects withdrew during the randomization phase for adverse events (20% vs 10%), and fewer male subjects withdrew because of protocol-defined virologic failure (3% vs 10%) or loss to follow-up (23% vs 30%).
During the extension phase, pill count assessments at each study visit showed that median adherence to ABC/3TC, ATV, and ritonavir exceeded 96% for both genders over the course of the study (Table 1).
Virologic outcomes
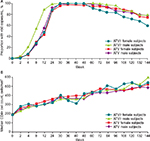
Change in the proportion of subjects with HIV-1 RNA <50 copies/mL (ITT-E, TLOVR analysis) over the course of the extension phase is shown in Figure 1A. In the ATV group, the proportion of female and male subjects achieving HIV-1 RNA <50 copies/mL at week 144 was similar: 79% (23/29) versus 77% (123/160). Although a lower proportion of female subjects (60%; 15/25) than of male subjects (75%; 117/155) achieved this milestone in the ATV/r group, these differences were not statistically significant between genders either across treatment groups or within treatment groups.
Similarly, 83% of female subjects (24/29) versus 84% of male subjects (135/160) in the ATV group achieved HIV-1 RNA <400 copies/mL in the week 144 ITT-E, TLOVR analysis. A lower proportion of female subjects (68%; 17/25) than male subjects (82%; 127/155) achieved viral suppression in the ATV/r group; again, these differences were also not statistically significant between gender either across treatment groups or within treatment groups.
In comparing the virological results (ITT-E, TLOVR analysis) of subjects in the prerandomization and randomization phases with the results of subjects completing the extension phase, female and male subjects generally had similar findings in all phases. The proportion of subjects with HIV-1 RNA <50 copies/mL at week 36 was lower in the prerandomization population (female subjects, 76% [65/86]; male subjects, 80% [345/429] in the ATV/r group) than in the randomization population (female subjects: 94% [32/34] in the ATV group, 100% [32/32] in the ATV/r group; male subjects: 99% [175/176] in the ATV group, 98% [174/177] in the ATV/r group) and/or in the extension phase population (female subjects: 100% [29/29, 25/25] in both groups; male subjects: 99% [159/160] in the ATV group, 98% [152/155] in the ATV/r group). Similarly, the proportion of subjects with HIV-1 RNA <50 copies/mL at week 84 was modestly lower in the randomization population (female subjects: 85% [29/34] in the ATV group, 66% [21/32] in the ATV/r group; male subjects: 86% [152/176] in the ATV group, 84% [144/177] in the ATV/r group) than in the extension phase population (female subjects: 97% [28/29] in the ATV group, 84% [21/25] in the ATV/r group; male subjects: 93% [149/160] in the ATV group, 94% [146/155] in the ATV/r group).
The proportion of female and male subjects who experienced protocol-defined confirmed virologic failure during the extension phase was similar: 4% of female subjects (n=2, both in the ATV/r group) versus 3% of male subjects (n=9; five in the ATV group and four in the ATV/r group).
CD4+ cell count changes
CD4+ cell count rose comparably in female and male subjects in each treatment group (observed analysis; Figure 1B). In the ATV/r group, the median CD4+ count in female subjects was 194 cells/mm3 at baseline and 538 cells/mm3 at week 144, with an increase from baseline to week 144 of 344 cells/mm3. In male subjects, these respective values were 205 and 520 cells/mm3, with an increase from baseline of 301 cells/mm3. In the ATV group, the median CD4+ count in female subjects was 190 cells/mm3 at baseline and 595.5 cells/mm3 at week 144, with an increase from baseline to week 144 of 365 cells/mm3. In male subjects, these respective values were 193, 484, and +300 cells/mm3. For both treatment groups, none of these changes from baseline values analyzed by gender were statistically significant.
Adverse events
When adverse events were compared by gender adjusting for treatment group, no significant differences were observed between female and male subjects (Table 2). In addition, there were no significant differences between genders within treatment groups.
Lipid changes
At baseline, male and female subjects in each treatment group generally had similar median fasting levels of total cholesterol (ATV/r group: 149.5 vs 167 mg/dL; ATV group: 151 vs 154 mg/dL), low-density lipoprotein (LDL) cholesterol (ATV/r group: 83 vs 103 mg/dL; ATV group: 88 vs 88.5 mg/dL), high-density lipoprotein (HDL) cholesterol (ATV/r group: 38 vs 42 mg/dL; ATV group: 36 vs 42 mg/dL), and triglycerides (ATV/r group: 123 vs 124 mg/dL; ATV group: 130 vs 123 mg/dL). Male and female subjects continued to have similar median values at week 144 in both treatment groups for fasting total cholesterol (ATV/r group: 181.5 vs 191.5 mg/dL; ATV group: 180.5 vs 188 mg/dL), LDL cholesterol (ATV/r group: 97 vs 108 mg/dL; ATV group: 96.5 vs 117 mg/dL), HDL cholesterol (ATV/r group: 50 vs 56 mg/dL; ATV group: 48 vs 57 mg/dL), and triglycerides (ATV/r group: 151 vs 158 mg/dL; ATV group: 131 vs 90 mg/dL). Week 144 median fasting total and LDL cholesterol levels remained below NCEP cut-offs (200 and 130 mg/dL, respectively) for both genders in each treatment group. Increases in median HDL cholesterol levels at week 144 were observed for both genders in each treatment group (above the NCEP minimum cut-off of 40 mg/dL). Week 144 median triglyceride levels were above the NCEP cut-off (150 mg/dL) for male and female subjects in the ATV/r group, but below this cut-off for both genders in the ATV group.
Median changes in fasting lipid levels in male and female subjects from week 36 (when all subjects had been receiving ATV/r, irrespective of which treatment group they were ultimately randomized into) to week 144 were as follows: for those randomized into the ATV treatment group, the change in total cholesterol was −9 versus −22 mg/dL; in HDL cholesterol, +3 versus +4 mg/dL; in LDL cholesterol, −4 versus −7 mg/dL; and in triglycerides, −42 versus −33 mg/dL. For those randomized into the ATV/r group, the change in total cholesterol was +4 versus −2 mg/dL; in HDL cholesterol, +4 versus −1 mg/dL; in LDL cholesterol, 0 versus −6.5 mg/dL; and in triglycerides, −13 versus +2 mg/dL. None of the differences between genders in these lipid values were statistically significant within the treatment groups. Between week 36 and 144, lipid-lowering agents were used by a higher proportion of female than of male subjects in both the ATV (21% [n=6] vs 11% [n=18]) and the ATV/r groups (28% [n=7] vs 14% [n=22]).
Discussion
Our gender stratification analysis of the 144-week results of ARIES showed no significant differences between treatment-naïve female and male subjects in virologic and CD4+ cell count response to ABC/3TC + ATV with or without ritonavir. Similar proportions of female and male subjects achieved HIV-1 RNA <50 and <400 copies/mL across the study phases. Slight differences in response rates were not unexpected because the subject populations were different at the three study phases (515, 419, and 369 subjects). As previously mentioned, only those subjects who had achieved virologic suppression prior to week 36 were eligible to participate in the randomization phase and participation in the extension phase was voluntary. The study dropout rates and adherence rates remained similar across genders.
The overall similarity in rates of adherence and discontinuation across genders in ARIES contrasts with some clinical trials that have reported notable differences in virologic response between female and male subjects. Such differences appeared to be largely driven by higher dropout rates for female subjects, especially dropouts due to adverse events and consequent poor adherence. When efficacy results have been censored for discontinuations other than virologic failure, female subjects have generally shown as good a virologic response as male subjects.5,25–27 In a large meta-analysis conducted by the US Food and Drug Administration that evaluated response of 20,328 HIV-infected subjects from 40 randomized clinical trials across 18 new drug applications, the overall percentage of female and male subjects achieving HIV-1 RNA <50 copies/mL at 48 weeks did not differ statistically, although five studies in antiretroviral-naïve subjects that favored male subjects and three that favored female subjects were found.4
Response to the ABC/3TC + ATV/r regimen has also undergone gender stratification analysis in the ACTG A5202 study.15 A5202 applied a more restrictive definition of virologic failure than ARIES and reported more virologic failures among the female than male subjects while they were receiving ABC/3TC + ATV/r over the 192-week randomization period (hazard ratio, 1.72 in multivariable analysis), although adherence was similar between genders. The investigators hypothesized that the higher virologic failure was due to lower adherence or tolerability; however, no significant differences in adherence, safety, or tolerability were observed, although female subjects receiving ABC/3TC + ATV/r had a higher incidence of grade 3 to 4 gastrointestinal safety events than did male subjects (13% vs 7%; A5202 did not report grade 2 adverse events). In ARIES, adherence rates, as assessed by pill count, were >96% for both genders, and the percentage of grade 2 to 4 treatment-related adverse events was similar between genders.
A5202 investigators also evaluated ATV pharmacokinetics in both male and female subjects. Female subjects generally had slower rates of clearance of ATV than male subjects; however, fast ATV clearance in female subjects was associated with an increased risk of virologic failure, presumably due to low drug exposure.16 In another study (KLEAN) in which grade 2 to 4 treatment-related adverse events were reported for ABC/3TC paired with boosted PIs other than ATV/r (fosamprenavir/ritonavir [FPV/r] and lopinavir/ritonavir [LPV/r]), the overall rate of these adverse events was similar between female and male subjects (FPV/r: 35% vs 38%; LPV/r: 36% vs 33%).18
Gender stratification analyses of efficacy have also been performed in other antiretroviral-naïve studies where ABC/3TC was combined with other PIs. In APV30002, similar proportions of female and male subjects receiving ABC/3TC in combination with nelfinavir achieved HIV-1 RNA <400 copies/mL at 48 weeks (69% vs 67%), while those receiving ABC/3TC plus unboosted FPV had a marginally smaller proportion of female subjects (63% vs 71%) who reached virologic suppression.17 In KLEAN,18 female subjects treated with ABC/3TC plus FPV/r had a lower rate of attaining HIV-1 RNA <400 copies/mL (64%) than did male subjects (75%) at week 48, as did those treated with ABC/3TC plus LPV/r (67% vs 73%). In the HEAT study, 46% of female subjects receiving ABC/3TC plus LPV/r achieved HIV-1 RNA <50 copies/mL (at week 96 compared with 62% of male subjects), although female subjects experienced greater median CD4+ cell count increases (+307 vs +245 cells/mm3 in male subjects).19 In CASTLE, where ATV/r was combined with tenofovir/emtricitabine, female subjects discontinued treatment more frequently than male subjects (22% vs 15%) and, at week 96, a lower proportion of female subjects achieved HIV-1 RNA <50 copies/mL compared with male subjects (63% vs 77%), although the increase in CD4+ cell count from baseline was virtually identical (+265 vs +269 cells/mm3) across genders.20 In contrast to our study, the high discontinuation rate in female subjects in CASTLE was driven primarily by lack of efficacy, adverse events, and poor adherence.
Our study is the first to have done a gender analysis of changes in fasting lipid levels in subjects treated with ABC/3TC + ATV with or without ritonavir. In general, the increases in HDL cholesterol, combined with the reductions or lack of notable changes in total cholesterol, should lead to a more favorable cardiovascular profile. However, in the case of the ATV/r group (but not the unboosted ATV group), any optimism must be tempered by the fact that, over the 144-week period, median triglyceride levels rose above the NCEP cut-off point in both female and male subjects, and this is likely due to the inclusion of ritonavir in the regimen.28 Nevertheless, among the ritonavir-boosted PIs, ATV/r has a relatively low lipogenicity, as evidenced by fewer lipid increases than LPV/r, and only small lipid elevations have been reported in direct comparisons in treatment-naïve subjects.29,30 Conversely, unboosted ATV, relative to most other PIs, has been documented to have little effect on lipids.31
In addition to the gender stratification findings reported above, we previously published a gender analysis of Framingham 10-year cardiovascular risk scores observed in subjects enrolled in ARIES.32 This analysis showed that there was little change in these scores over 144 weeks in the study population in general and no notable differences between female and male subjects in score changes.
Our study enrolled subjects from the US and Canada, and the demographics by race for the female participants closely reflected the demographics of HIV-infected female subjects in the US during the time period this study was conducted.33 Although our study was limited by its open-label design, its design did allow for statistical analysis of differences between genders for virologic endpoints, CD4+ cell count changes, and incidence of total treatment-related grade 2–4 adverse events. However, if our study had included a higher proportion of female subjects, additional clinically meaningful comparisons by gender between the two treatment groups may have been possible. Due to the lower number of female subjects enrolled in this study, these analyses may have been unable to detect some gender-related differences. In our study, three female subjects withdrew due to pregnancy. It is important to emphasize that having to leave a study because of pregnancy does not reflect on treatment efficacy, tolerability, or adherence, and this should be considered when between-study analyses are done in HIV clinical trials evaluating results by gender.
Conclusion
In conclusion, over 144 weeks, no significant gender differences were observed in clinical efficacy, safety, or changes in fasting lipid levels with ABC/3TC + ATV with or without ritonavir. Larger-scale clinical trials may need to be considered to evaluate by gender the endpoints we examined as well as others that were not within the scope of our study.
Acknowledgments
We wish to thank the subjects, study coordinators, and investigators who participated in this study and made this analysis possible. ARIES US investigators included B Akil, J Applebaum, N Bellos, D Berger, I Brar, C Brinson, F Carpio-Cedraro, P Cook, M Cuenca, E DeJesus, R Dretler, J Duggan, R Elion, T File, J Gathe, E Godofsky, R Greenberg, R Hao, K Henry, AM Khalsa, J Kort, P Kumar, P Lackey, A LaMarca, C Lucasti, C McDonald, P McLeroth, I Melendez-Rivera, A Mestre, J Morales-Ramirez, R Nahass, C Newman, W O’Brien, P O’Keefe, E Oldfield, H Olivet, T Overton, D Pearce, M Ramgopal, B Rashbaum, F Rhame, G Richmond, J Rodriguez, P Salvato, A Sanchez, J Sarria, L Santiago, K Sathasivam, P Sax, S Schneider, R Scott, A Scribner, G Sepulveda-Arzola, G Simon, D Siraj, J Slim, L Sloan, C Small, K Squires, K Tashima, P Tebas, M Thompson, J Torres, V Trivedi, T Vanig, D Ward, W Weinberg, M Weinert, and B Young. ARIES Canadian Investigators included JG Baril, D Murphy, M Potter, A Rachlis, G Smith, and S Walmsley. Contributors from GlaxoSmithKline included E Blackmon, N Figliola, V Garay, S LaBelle, D Margolis, D Percival, D Raimonde, M Schultz, B Wine, and all the GSK monitors. Bristol-Myers Squibb generously donated study medication.
Funding for this work was provided by ViiV Healthcare. The sponsor developed the study design with input from prospective investigators. All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. The authors wish to acknowledge the following individual for editorial assistance during the development of this manuscript: Meredith MacPherson.
Author contributions
Substantial contributions to study conception and design were made by KES, BY, LLR, HHZ, and MSS. Substantial contributions to the analysis and interpretation of the data were made by HHZ, KES, LLR, BY, SLW, GEP, and MSS. Substantial contributions to acquisition of patient clinical study data were made by KES, BY, LS, RHD, and SLW. All authors had full access to the data and vouch for the accuracy and completeness of the data and analyses. The manuscript was written and approved by all of the authors, each of whom contributed to the drafts and revisions.
Disclosure
KES has received consultancy fees and/or research funding from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Koronis, Merck, Pfizer, Schering-Plough, Tibotec, and Tobira. BY has received consultancy fees, speaking honoraria, and/or research funding from Gilead Sciences, GlaxoSmithKline, Merck, Monogram Biosciences, Pfizer, and ViiV Healthcare. LS has received consultancy fees, speaking honoraria, and/or research funding from Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Pfizer, and ViiV Healthcare. RHD is employed by ID Specialists of Atlanta, GA, USA. SLW is employed by University Health Network, Toronto, ON, Canada and has served on advisory boards and spoken at CME events for AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck, and ViiV Healthcare. HHZ is employed by GlaxoSmithKline. LLR, GEP, and MSS are employed by ViiV Healthcare. The authors report no other conflicts of interest in this work.
References
amfAR, The Foundation of AIDS Research. Statistics: Women and HIV/AIDS. Available from: http://www.amfar.org/about_hiv_and_aids/facts_and_stats/statistics__women_and_hiv_aids/. Accessed November 28, 2015. | ||
Centers for Disease Control and Prevention. National Women and Girls HIV/AIDS Awareness Day. Available from: http://www.cdc.gov/features/womengirlshivaids/. Accessed November 28, 2015. | ||
Squires KE. Gender differences in the diagnosis and treatment of HIV. Gend Med. 2007;4(4):294–307. | ||
Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS. 2012;26(8):444–453. | ||
Belden KA, Squires KE. HIV infection in women: do sex and gender matter? Curr Infect Dis Rep. 2008;10(5):423–431. | ||
Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther. 2005;3(2):213–227. | ||
Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. | ||
Delmas MC, Jadand C, De Vincenzi I, et al. Gender difference in CD4+ cell counts persist after HIV-1 infection. AIDS. 1997;11(8):1071–1073. | ||
Prins M, Meyer L, Hessol NA. Sex and the course of HIV infection in the pre- and highly active antiretroviral therapy eras. AIDS. 2005;19(4):357–370. | ||
Prins M, Robertson JR, Brettle RP, et al. Do gender differences in CD4 cell counts matter? AIDS. 1999;13(17):2361–2364. | ||
Ofotokun I, Chuck SK, Hitti JE. Antiretroviral pharmacokinetic profile: a review of sex differences. Gend Med. 2007;4(2):106–119. | ||
Floridia M, Giuliano M, Palmisano L, Vella S. Gender differences in the treatment of HIV infection. Pharmacol Res. 2008;58(3–4):173–182. | ||
Ko NY, Lai YY, Liu HY, et al. Gender differences in HIV manifestations at presentation to care and continuity of care among HIV-infected persons in Taiwan. AIDS Care. 2011;23(10):1254–1263. | ||
Nicastri E, Angeletti C, Palmisano L, et al. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS. 2005;19(6):577–583. | ||
Smith KY, Tierney C, Mollan K, et al. Outcomes by sex following treatment initiation with atazanavir plus ritonavir or efavirenz with abacavir/lamivudine or tenofovir/emtricitabine. Clin Infect Dis. 2014;58(4):555–563. | ||
Venuto CS, Mollan K, Ma Q, et al. Sex differences in atazanavir pharmacokinetics and associations with time to clinical events: AIDS Clinical Trials Group Study A5202. J Antimicrob Chemother. 2014;69(12):3300–3310. | ||
ViiV Healthcare. Result summary for APV30002. Available from: http://www.viiv-clinicalstudyregister.com/result_detail.jsp?protocolId=APV30002&compound=fosamprenavir. Accessed November 28, 2015. | ||
DeJesus E, Gathe J, Katlama C, et al. Virologic response and tolerability by sex and race in subjects receiving fosamprenavir/ritonavir (FPV/r) BID and lopinavir/ritonavir (LPV/r) BID, each in combination with abacavir/lamivudine QD (the KLEAN study). Abstract presented at: 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 22–25, 2007; Sydney, Australia. | ||
Smith KY, Kumar P, Patel P, et al. Differences in virologic response among African-Americans and females regardless of therapy in the HEAT study. Abstract presented at: 5th International AIDS Society Conference on HIV Treatment Pathogenesis and Prevention; July 19–22, 2009; Cape Town, South Africa. | ||
Squires KE, Johnson M, Yang R, et al. Comparative gender analysis of the efficacy and safety of atazanavir/ritonavir and lopinavir/ritonavir at 96 weeks in the CASTLE study. J Antimicrob Chemother. 2011;66(2):363–370. | ||
Squires KE, Young B, DeJesus E, et al. Safety and efficacy of a 36-week induction regimen of abacavir/lamivudine and ritonavir-boosted atazanavir in HIV-infected patients. HIV Clin Trials. 2010;11(2):69–79. | ||
Squires KE, Young B, DeJesus E, et al. Similar efficacy and tolerability of atazanavir compared with atazanavir/ritonavir, each with abacavir/lamivudine after initial suppression with abacavir/lamivudine plus ritonavir-boosted atazanavir in HIV-infected patients. AIDS. 2010;24(13):2019–2027. | ||
Squires KE, Young B, DeJesus E, et al. ARIES 144-week results: durable virologic suppression in HIV-infected patients simplified to unboosted atazanavir/abacavir/lamivudine. HIV Clin Trials. 2012;13(5):233–244. | ||
National Institutes of Health. ATP III Guidelines at-a-Glance Quick Desk Reference. Available from: https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf. Accessed November 28, 2015. | ||
Andrade-Villanueva J, Herrera G, Chiliade P, et al. ARTEMIS: week 48 safety and efficacy of darunavir/r by gender, age and race. Terapeut Farmacol Toxicol Clin. 2008;12(3):367–371. | ||
Walmsley SL, Squires K, Weiss L, et al. Multidrug-experienced HIV-1-infected women demonstrated similar virological and immunological responses to tipranavir/ritonavir compared with men. AIDS. 2009;23(3):429–431. | ||
Moore AL, Kirk O, Johnson AM, et al. Virologic, immunologic, and clinical response to highly active antiretroviral therapy: the gender issue revisited. J Acquir Immune Defic Syndr. 2003;32(4):452–461. | ||
Collot-Teixeira S, De Lorenzo F, Waters L, et al. Impact of different low-dose ritonavir regimens on lipids, CD36, and adipophilin expression. Clin Pharmacol Ther. 2009;85(4):375–378. | ||
Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48-week efficacy and safety results of the CASTLE study. Lancet. 2008;372(9639):646–655. | ||
Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses. 2012;28(10):1184–1195. | ||
Malvestutto CD, Aberg JA. Management of dyslipidemia in HIV-infected patients. Clin Lipidol. 2011;6(4):447–462. | ||
Young B, Squires KE, Ross LL, et al. Inflammatory biomarker changes and their correlation with Framingham cardiovascular risk and lipid changes in antiretroviral-naive HIV-infected patients treated for 144 weeks with abacavir/lamivudine/atazanavir with or without ritonavir in ARIES. AIDS Res Hum Retroviruses. 2013;29(2):350–358. | ||
Centers for Disease Control and Prevention (CDC). HIV surveillance–United States, 1981–2008. MMWR Morb Mortal Wkly Rep. 2011;60(21):689–693. |
Supplementary material
  | Table S1 List of institutional review boards/independent ethics committee at each study site that approved the protocol Abbreviation: IRB, institutional review board. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.