Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Respiratory syncytial virus-like nanoparticle vaccination induces long-term protection without pulmonary disease by modulating cytokines and T-cells partially through alveolar macrophages
Authors Lee Y, Ko E, Hwang HS, Lee JS, Kim K, Kwon Y, Kang S
Received 25 February 2015
Accepted for publication 29 April 2015
Published 14 July 2015 Volume 2015:10(1) Pages 4491—4505
DOI https://doi.org/10.2147/IJN.S83493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Young-Tae Lee,1,* Eun-Ju Ko,1,2,* Hye Suk Hwang,1,2 Jong Seok Lee,1,3 Ki-Hye Kim,1 Young-Man Kwon,1 Sang-Moo Kang1,2
1Center for Inflammation, Immunity and Infection, Institute for Biomedical Sciences, 2Department of Biology, Georgia State University, Atlanta, GA, USA; 3National Institute of Biological Resources, Incheon, South Korea
*These authors contributed equally to this work
Abstract: The mechanisms of protection against respiratory syncytial virus (RSV) are poorly understood. Virus-like nanoparticles expressing RSV glycoproteins (eg, a combination of fusion and glycoprotein virus-like nanoparticles [FG VLPs]) have been suggested to be a promising RSV vaccine candidate. To understand the roles of alveolar macrophages (AMs) in inducing long-term protection, mice that were 12 months earlier vaccinated with formalin-inactivated RSV (FI-RSV) or FG VLPs were treated with clodronate liposome prior to RSV infection. FI-RSV immune mice with clodronate liposome treatment showed increases in eosinophils, plasmacytoid dendritic cells, interleukin (IL)-4+ T-cell infiltration, proinflammatory cytokines, chemokines, and, in particular, mucus production upon RSV infection. In contrast to FI-RSV immune mice with severe pulmonary histopathology, FG VLP immune mice showed no overt sign of histopathology and significantly lower levels of eosinophils, T-cell infiltration, and inflammatory cytokines, but higher levels of interferon-γ, which are correlated with protection against RSV disease. FG VLP immune mice with depletion of AMs showed increases in inflammatory cytokines and chemokines, as well as eosinophils. The results in this study suggest that FG nanoparticle vaccination induces long-term protection against RSV and that AMs play a role in the RSV protection by modulating eosinophilia, mucus production, inflammatory cytokines, and T-cell infiltration.
Keywords: alveolar macrophage, nanoparticle vaccine, VLP, FI-RSV, RSV disease
Introduction
Respiratory syncytial virus (RSV) is a leading cause of a pulmonary disease in children. In the 1960s, formalin-inactivated RSV (FI-RSV) vaccine was tested in clinical trials, but it caused severe vaccine-enhanced respiratory diseases leading to high hospitalizations and deaths of two children during the RSV epidemic winter season.1 The cause of the vaccine-enhanced disease was not fully understood, but the symptoms were known to be caused by T helper (Th)2-biased immune responses and eosinophil infiltration in lungs after RSV infection. Since the tragic failure of FI-RSV vaccine trials, there is no licensed RSV vaccine.1–3
RSV fusion (F) and attachment glycoproteins (G) are major antigenic surface proteins and contain neutralizing antibody and T-cell epitopes. Virus-like nanoparticles (VLPs) are genetically engineered particles expressing immunogenic proteins.4 VLPs mimic the structure of a virus but lack viral genomes. Newcastle disease virus-based or recombinant baculovirus-expressed VLPs containing RSV F and/or G glycoproteins, or mixed RSV F and G VLPs (FG VLPs), were shown to elicit antigen-specific antibody production and provide protection against RSV infection in mouse models.5–9 Compared to FI-RSV, immunization with RSV VLP vaccines induced a higher IgG2a/IgG1 antibody ratio, which refers to Th1 immune responses.5,6 Nanoparticles of FG VLPs were shown to be in a range of 60–120 nm-size particles and produced in insect cells using the recombinant baculovirus expression system.5
Alveolar macrophages (AMs) are the first defense line of innate immune cells in the respiratory tract. AMs are a major resident cell in alveoli and interstitial spaces of the lung.10 AMs can eliminate the foreign antigens by phagocytosis and microbicidal enzymes, and also regulate inflammatory responses by secreting proinflammatory cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α.11,12 AMs are known to be required to induce protective immune responses against bacteria, fungi, and viruses.13 Dendritic cells (DCs) together with AMs are major antigen-presenting cells in the lungs. Respiratory DCs play a crucial role in inducing adaptive immune responses.14 Monocyte-derived DCs are phenotypically immature and poor activators of naïve T-cells, although they are able to transport antigens from the lungs to the draining lymph node.15 In addition, AMs have anti-inflammatory activity to inhibit antigen presentation of DCs.16 However, the roles of AMs in the RSV protection and disease after vaccination largely remain unknown.
Here, we investigated the potential effects of AMs on long-term protection, Th1 and Th2 cytokines, modulating innate and adaptive cellular phenotypes, and vaccination-induced inflammatory disease in mice that were a year earlier immunized with FG VLPs or FI-RSV. Clodronate liposomes (CLs) have been used to deplete tissue macrophages. CL is an apoptosis-inducing agent and uses phagocytic properties of macrophages that uptake CL.17,18 In contrast to FI-RSV, FG VLPs conferred long-term protection against RSV without causing vaccine-enhanced RSV disease by appropriately modulating granulocytes, cytokines, and T-cells toward Th1-type immune responses. Depletion of AMs resulted in differential effects on DCs, eosinophils, inflammatory cytokines, and T-cells as well as mucus production in the lungs of mice with different immunization history upon RSV infection, suggesting important roles of AMs in modulating innate and adaptive immunity.
Materials and methods
Preparation of F and G VLPs and FI-RSV
The VLPs containing RSV F or G were produced using Spodoptera frugiperda SF9 insect cells (ATCC CRL-1711) as previously described.5 Briefly, SF9 insect cells maintained in SF900-II serum-free medium were coinfected5 with recombinant baculoviruses expressing influenza matrix core protein (M1) and full-length RSV F or RSV G surface proteins. After 2 days of culture, RSV F and G VLPs were purified from the culture supernatants using ultracentrifugation and found to have spherical sizes of 60–120 nm and to express RSV F and G proteins, respectively.5 HEp-2 cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA). RSV A2 strain was used for FI-RSV preparation and RSV challenge. RSV was grown in HEp-2 cells, harvested, and inactivated with formalin for 3 days at 37°C. FI-RSV was collected by ultracentrifugation and its inactivation confirmed by an immune-plaque assay.5 FI-RSV was adsorbed to aluminum hydroxide adjuvants (alum, 4 mg/mL) and used for immunization of mice.
Mice and immunization
Balb/c mice were purchased from Harlan (Indianapolis, IN, USA) and maintained in the Georgia State University (GSU) (Atlanta, GA, USA) animal facility under the guidelines of the GSU Institutional Animal Care and Use (IACUC) protocol. Female Balb/c mice aged 3 weeks (n=10 per group) were primed with FI-RSV (1 μg) or FG VLPs (10 μg of each VLP), and then boosted with the same dose 4 weeks later.
RSV-specific serum enzyme-linked immunosorbent assay
To determine RSV-specific serum antibodies at 1 month and 12 months after boost immunization, enzyme-linked immunosorbent assay (ELISA) was performed using an FI-RSV (400 ng/well) antigen as previously described.5,19
Clodronate treatment and RSV challenge
Specific tissue macrophages can be depleted by choosing an appropriate administrative route.18,20 AMs can be selectively depleted in lungs through intranasal administration of the CL.21 Before RSV infection, naïve, FI-RSV-immunized, and FG VLP-immunized mice were treated intranasally with 100 μL of phosphate-buffered saline (PBS) or CL to deplete AMs in the respiratory tract and lung. After 4 hours, RSV A2 strain (2×106 plaque-forming units) was used to challenge PBS-treated and CL-treated mice.
Preparation of bronchoalveolar lavage fluid and lung samples after challenge
At day 5 post-RSV challenge, the mice were euthanized to collect bronchoalveolar lavage fluid (BALF) and lung samples. BALF samples were obtained by infusing 1 mL of PBS into the lungs via trachea using an 18-gauge and 1¼-inch catheter (Exelint International Co., Saint Petersburg, FL, USA). BALF and bronchoalveolar lavage (BAL) cells were separated after centrifugation. The collected lungs were homogenized and centrifuged to get lung extracts and to produce single cell suspension. The homogenized lung tissues were digested with 150 U/mL collagenase (Thermo Fisher Scientific) in RPMI 1640 medium supplemented with 1 mM MgCl2, 1 mM CaCl2, and 5% fetal calf serum at 37°C for 90 minutes. The whole lung cell suspensions were treated on 44%/67% Percoll gradients at 2,800 rpm for 15 minutes, and the immune cell-enriched layer was harvested, washed, and used for further experiments.22
Immune-plaque assay
To determine viral loads in lung extracts from naïve and immunized mice after RSV challenge infection, an immune-plaque assay was performed as described before.5 Briefly, the lung extracts day 5 post-challenge were serially diluted and applied to the confluent HEp-2 cell monolayer. After 2 hours’ incubation, the lung extracts were removed and the infected HEp-2 cells were cultured for 3 to 5 days until plaque formation by RSV. Then, the RSV plaques were detected by anti-RSV F monoclonal antibody (EMD Millipore, Billerica, MA, USA) and 3,3′-diaminobenzidine substrate.
Flow cytometry
To analyze detailed cell subsets in respiratory tract after CL treatment and RSV challenge, the BAL and lung cells were stained with fluorescence-labeled anti-mouse CD45, CD11b, CD11c, CD3, CD4, CD8, B220, F4/80, and Siglec F antibodies in the presence of anti-mouse CD16/32 antibodies. The surface marker expressions of the stained cells were acquired by BD Fortessa (BD Biosciences, San Jose, CA, USA) and analyzed by FlowJo (Tree Star Inc., Ashland, OR, USA).
Cytokine ELISA
Cytokine production of the RSV-challenged mice was determined by ELISA. BALF and lung extracts from the RSV-challenged mice were analyzed by mouse interleukin (IL)-5, IL-6, IL-13, TNF-α, and IFN-γ ELISA Ready-Set-Go® kits (eBioscience, San Diego, CA, USA) and mouse eotaxin and macrophage inflammatory protein-1 beta (MIP-1β) duoset® (R&D Systems Inc., Minneapolis, MN, USA) following the manufacturer’s protocol.
Cytokine enzyme-linked immunospot
Lung cells were obtained from mice euthanized at day 5 post-challenge as described above. The lung cells (5×105 cells/well) were seeded and cultured in anti-mouse IL-4- or IFN-γ-coated plates. The cells were treated with RSV F92–106 (ELQLLMQSTPPTNNR) or RSV G183–195 (WAICKRIPNKKPG) for 3 days to stimulate antigen-specific cytokine-producing cells. The number of IL-4- or IFN-γ-secreting cell spots was counted using an ELIspot Bioreader 5000 (BioSys, Miami, FL, USA).
Lung histopathology
For histological analysis, intact lungs from the challenged mice were harvested at day 5 post-challenge. The lungs were fixed in 10% neutral formalin and processed with ethanol and xylene as described previously.23 The lung sections were stained with hematoxylin and eosin for evaluation of the lung inflammation and periodic acid–Schiff (PAS) stain for mucus production. Photographs were acquired under a microscope (Zeiss Axiovert 100; Carl Zeiss Meditec AG, Jena, Germany) at a magnification of 20, using an attached digital camera (Canon 30D; Canon, Japan). For lung histopathology assessment, blinded scoring in bronchiole, vessels, and interstitial spaces was performed. The scores were in a range of 0 (no inflammation) to 3 (severe inflammation) following the criteria of the scoring system.24 PAS-positive areas were calculated using the Photoshop program.
Statistical analysis
Experimental results are presented as mean ± standard error of the mean. Data were analyzed using Prism software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis was performed by unpaired two-tailed Student’s t-test. Probability values less than 0.05 were considered to be statistically significant.
Results
FG VLPs confer long-lasting protection against RSV
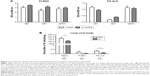
It is desirable to induce long-lasting protective antibody responses after vaccination. The groups of mice were primed and boost-immunized with FI-RSV or FG VLPs at a 4-week interval, and RSV-specific antibody responses were determined. Antibody levels were similar at 1 and 12 months post-immunization, suggesting that long-lasting RSV-specific antibodies were induced by FI-RSV or FG VLP immunization (Figure 1A). To determine the protective efficacy of FG VLP vaccination, naïve and immunized mice were challenged with live RSV. Viral titers were observed at higher levels in the lungs of unimmunized mice after RSV infection compared to FI-RSV- or FG VLP-immunized mice that lowered lung viral loads by over 1,000-fold (Figure 1B). Therefore, immunization of mice with FI-RSV or FG VLP induced high efficacy of protection by effectively clearing lung viral loads even at 12 months after vaccination.
AMs were found to be dominantly present in the airways from naïve mice (Figure S1), which is consistent with a previous study.25 To determine the possible roles of AMs in protective immunity and RSV disease in mice with vaccination, CL was intranasally given to mice that were previously immunized with FI-RSV or FG VLP 1 year before. Depletion of AMs (CD11c+CD11b−F4/80+) was confirmed by flow cytometry analysis (Figure S1A).21,26,27 AMs in the airways were significantly reduced in naïve and FG VLP immune mice, and, particularly, FI-RSV immune mice showed the lowest level of AMs after RSV infection even without CL treatment (Figure S1B). CL treatment resulted in further decreases in the percentages of AMs in the airways (Figure S1B).
Lung viral loads from the groups of mice were determined in the presence or absence of CL administration 5 days post-challenge. CL treatment of naïve mice resulted in a further decrease (approximately 30%) in viral loads from the lungs compared to PBS-treated mice (Figure 1B). A pattern of reducing viral loads was observed in CL-treated FI-RSV mice, but there was no significance between PBS- and CL-treated FI-RSV mice. FG VLP mice with CL treatment showed a slightly further reduction in viral loads from the lungs compared to nontreated mice. Taken together, these data indicate that vaccination with FI-RSV or FG VLPs induces long-lasting humoral immunity primarily responsible for controlling lung viral loads. Levels of AMs were significantly reduced in naïve and FG VLP mice, and, in particular, the FI-RSV group showed the lowest AMs upon RSV infection. CL treatment resulted in further depleting AMs in the airways.
Differential mobilization of pulmonary DCs after clodronate treatment
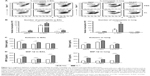
To further understand the effect of depleting AM population in modulating pulmonary DCs, we analyzed distinct subsets of DCs including CD11b+ DCs (CD11b+CD11c+F4/80−CD45+) and plasmacytoid DCs (pDCs) (B220+CD11c+F4/80−CD45+).28,29 After RSV infection, pDCs in the airways were observed at similar levels in the naïve and FG VLP groups regardless of CL treatment, but CL-treated FI-RSV mice showed substantially increased numbers of pDCs than those in PBS-treated FI-RSV mice (Figure 2A). The pDC numbers in the lungs were lower in the FI-RSV group compared to the naïve-infected mice (Figure 2B). Similar numbers of pDCs were observed in the lungs between the groups of mice with and without CL administration. CD11b+ DCs were observed at similar levels in the BAL or lungs of the naïve, FI-RSV, and FG VLP groups regardless of CL treatment (Figure 2C and D). These data suggest that AMs may have an inverse correlation with pDCs in the airways of FI-RSV immune mice, but not in naïve and FG VLP immune mice.
Airway AM depletion increases eosinophil recruitment and chemokine production
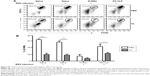
To understand whether CL treatment influenced eosinophilia in the lungs during RSV infection, eosinophil infiltration was analyzed by using the surface markers (CD11b+Siglec F+CD11c−). Approximately 10- to 50-fold higher numbers of eosinophils were detected in the BAL and lungs of FI-RSV immune mice than those in naïve or FG VLP immune mice day 5 post-RSV challenge (Figure 3A and B). Interestingly, CL treatment resulted in increases in numbers of eosinophils in the BAL and lungs of FI-RSV immune mice (Figure 3A and B). Eosinophils were increased to a moderate level in the BAL of naïve and FG VLP mice with CL treatment (Figure 3A). Importantly, we found that CL treatment resulted in significant increases in the levels of eotaxin and MIP-1β from the lungs and BAL samples of all groups of mice except the BAL eotaxin from the FG group (Figure 3C and D). These results suggest that AMs may contribute to modulating the production of chemoattractants and eosinophil recruitment in FI-RSV immune mice, although chemokine levels alone do not explain the enormous infiltration of eosinophils into the lungs of FI-RSV immune mice.
FG VLP immunization induces IFN-γ but not proinflammatory cytokines after RSV infection
It has been demonstrated that imbalanced cytokines resulted in vaccine-enhanced RSV disease.30 Proinflammatory and antiviral cytokines were analyzed in the BALF and lung extracts from each group of mice upon RSV infection. Th1 cytokine IFN-γ in BAL and lung samples was detected at the lowest levels in the FI-RSV group (Figure 4A). In contrast, significantly higher levels of IFN-γ in BALF (Figure 4A) and in lungs (Figure 4A) were observed in FG VLP immune mice compared to those in the FI-RSV group. Naïve mice also exhibited moderate levels of IFN-γ in BAL and lung samples (Figure 4A). CL treatment resulted in an increase in IFN-γ production from the lungs of FG VLP immune mice and in BAL IFN-γ levels from RSV-infected naïve mice (Figure 4A). TNF-α proinflammatory cytokine levels were observed at relatively higher levels in naïve RSV-infection mice (Figure 4B). The levels of TNF-α were found to be increased in both BAL and lung samples from all groups of mice with CL treatment (Figure 4B).
Noticeably, high levels of Th2 cytokines such as IL-5, IL-6, and IL-13 were observed in the lung extracts from FI-RSV immune mice but not from FG VLP immune mice (Figure 4C–E). High levels of Th2 cytokines (IL-5, IL-6, IL-13) were detected in BAL samples from naïve-infected mice (Figure 4C–E). Also, FI-RSV immune mice treated with CL showed significantly increased levels of Th2 cytokines including IL-5, IL-6, and IL-13 in the airways of BAL fluids (Figure 4C–E). In contrast, FG VLP immune mice did not show increases in IL-5 and IL-13 cytokines regardless of CL treatment (Figure 4C and E). Meanwhile, IL-6 production in the lungs was enhanced in all groups of mice with CL treatment (Figure 4D). Interestingly, CL treatment of naïve mice resulted in a pattern of lowering airway Th2 (IL-5, IL-6, IL-13) cytokines upon RSV infection (Figure 4C–E), which is different from an increasing pattern in FI-RSV mice. These data suggest that airway AMs play a differential role in modulating inflammatory and antiviral cytokines in the BAL airways and lungs upon RSV infection. More importantly, the type of vaccine appears to be a primary determinant of cytokine profiles induced in the airways and lungs upon RSV infection.
CL treatment of FI-RSV immune mice increases T-cell recruitment but reduces IFN-γ T-cell responses
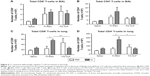
A trend of inducing higher numbers of CD8+ and CD4+ T-cells was observed in the BAL from the FI-RSV group with CL treatment (Figure 5A and B). High levels of BAL and lung CD8+ T-cells were shown in the FG group (Figure 5A and C). As a result of CL treatment, the FI-RSV group displayed a significant increase in numbers of lung CD8+ and CD4+ T-cells (Figure 5C and D).
To further characterize whether CL treatment altered RSV antigen-specific T-cell responses, the cells from the lungs 5 days after RSV challenge were in vitro stimulated with the synthetic F92–106 (ELQLLMQSTPPTNNR) and G183–195 (WAICKRIPNKKPG) peptides which are RSV-specific CD8+ and CD4+ T-cells, respectively.31 The cytokine-positive spots were counted using an enzyme-linked immunospot reader. The FI-RSV immune group showed the highest levels of IL-4-positive spots with stimulation of F or G peptides, whereas IL-4 spot numbers were similarly low in the lungs of naïve and FG VLP immune mice (Figure 6A). There was no significant change in the numbers of IL-4-positive spots with CL treatment. In addition, higher numbers of IFN-γ-positive spots after stimulation with G peptide were detected in the FI-RSV group compared to those in the naïve and FG VLP groups (Figure 6B). Also, F peptide-stimulated IFN-γ spots were relatively high in the lungs from FI-RSV immune mice, but there were no statistical significances compared to other groups. Noticeably, CL-treated FI-RSV mice showed dramatically reduced numbers of IFN-γ-positive spots in both F and G peptide-stimulated lung cells (Figure 6B). These data suggest that AMs in FI-RSV immune mice may modulate inflammatory adaptive responses as well as antigen-specific T-cell responses in lungs.
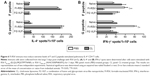
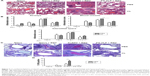
FI-RSV but not FG VLP causes severe pulmonary mucus production and inflammation
Pulmonary histopathology was analyzed to assess the safety concerns of FG VLP vaccines and the possible effects of CL treatment in vaccinated mice upon RSV infection 1 year later. The FI-RSV group showed severe leukocyte infiltrates compared to the FG VLP group (Figure 7A). Also, naïve aged mice, upon RSV infection, showed severe inflammatory histology around the blood vessels and interstitial spaces (naïve + RSV with PBS, Figure 7A and B). Importantly, FG VLP immune mice did not display such obvious pulmonary histopathology as evidenced by significantly less cellular infiltrates (FG VLP + RSV with PBS, Figure 7A). The highest levels of histological inflammation were observed in the airways, blood vessels, and interstitial spaces from FI-RSV immune mice compared to those from the naïve and FG VLP groups (Figure 7B). CL treatment did not induce lung inflammation in naïve uninfected mice (naïve, Figure 7). CL treatment of FI-RSV immune mice did not show apparent changes in histopathology probably due to the full-blown severe histopathology, but showed a trend of further increasing inflammation scores to the maximum (FI-RSV + RSV, Figure 7B). Naïve mice with CL treatment showed a trend of lowering infiltrates and lung inflammation scores (naïve + RSV with CL, Figure 7A and B). In contrast, FG VLP immune mice with CL treatment showed a moderate trend of increasing infiltrates and lung inflammation scores (FG VLP + RSV with CL, Figure 7A and B).
Significant mucus production was observed in PAS-stained lung sections from FI-RSV immune mice and a low level of PAS-positive mucus was also detected in lung samples from naïve mice upon RSV infection (Figure 7C and D). In contrast, PAS-positive mucus production was not observed in FG VLP mice after RSV infection. More importantly, CL treatment of FI-RSV-immunized mice resulted in a significant increase in PAS positivity, suggesting more pulmonary mucus production (Figure 7C and D). CL treatment of naïve mice resulted in lowering mucus production upon RSV infection (naïve + RSV with CL, Figure 7C and D). These data suggest that FG VLP immunization does not cause pulmonary inflammation and infiltrates even 1 year after vaccination and that AMs play a role in regulating pulmonary inflammation and mucus production during RSV infection.
Discussion
Long-term protection against RSV by vaccination is highly desirable, since the elderly are also a target population for RSV disease in addition to young children. However, a safety concern of vaccine-enhanced RSV disease has been a challenge in developing effective and safe RSV vaccines. In this study, at 12 months after vaccination of mice, we found that FI-RSV and FG VLP immunization induced long-lasting RSV-specific antibody responses and lowered lung viral loads close to a detection limit upon RSV infection. It supports that lung viral clearance is largely dependent on RSV antibodies induced by vaccination. The effective control of lung viral loads in FI-RSV immune mice is consistent with that of previous studies.6,7,9,19,23 Herein, we addressed long-term vaccine safety concerns of FG VLP vaccines and investigated innate and adaptive cellular immune components that are likely responsible for RSV protection or pulmonary inflammation upon RSV infection.
AMs were found to be a major phenotypic cell present in the airways in uninfected naïve mice as well as RSV-infected naïve and FG VLP mice. However, AMs were significantly reduced in FI-RSV immune mice after RSV infection. In addition, the cellularity of AMs was differentially regulated between in the airways and in lung tissues. Interestingly, AM cellularity in the lungs (data not shown) was substantially reduced in both naïve and FI-RSV immune mice that showed severe pulmonary inflammation, indicating that AMs might play an important role in RSV pathogenesis. Therefore, the possible roles of AMs in the RSV protection or disease were investigated in vaccinated mice by applying intranasal CL administration. CL has been shown to deplete macrophage phenotypic cells.18 Percentages of AMs in the airways were substantially reduced in naïve, FI-RSV, and FG VLP mice as a result of CL treatment prior to RSV infection (Figure S1). Based on this observation, we could determine the possible roles of airway AMs in modulating DCs, eosinophils, chemokines, Th1 and Th2 cytokines, RSV-specific CD4+ and CD8+ T-cells, and histopathology among different RSV-vaccinated groups. Although there were differential effects of CL treatment depleting AMs, the type of RSV vaccine was found to be the most critical factor in determining the outcome of host immune responses and protection.
The effects of CL treatment prior to RSV infection were found to be differential among naïve, FI-RSV, and FG VLP immune mice. CL treatment caused two- to threefold increases in eosinophils in the airways and lungs from FI-RSV immune mice that showed the highest levels of eosinophil infiltration. There were fourfold increases in the numbers of B220+ pDCs in the airways of FI-RSV immune mice with CL treatment but not in naïve or FG VLP immune mice. Meanwhile, limited increases in eosinophils were observed in naïve or FG VLP mice as a result of CL treatment. Also, cellularity in CD4+ and CD8+ T-cells was significantly increased in the lungs of FI-RSV immune mice as a result of CL treatment but not in other groups of mice. This is in line with a previous finding that antigen presentation is required for T-cell migration into the lungs.22 In contrast, RSV-specific CD4+ and CD8+ T-cells producing IFN-γ were substantially reduced in the lungs of FI-RSV immune mice after CL treatment. Thus, it is also possible that reduced IFN-γ-producing cells and increases in eosinophils, pDCs, and CD4+ and CD8+ T-cell cellularity in the lungs might have contributed to further causing the mucus production and severe lung histopathology in FI-RSV immune mice. Eosinophils and IL-4-producing lung T-cells are likely the most significant cellular factors contributing to severe histopathology in FI-RSV immune mice in addition to the induction of other Th2-type cytokines regardless of CL pretreatment.
Types of cytokines are known to be involved in vaccine-enhanced RSV disease.2,32 The highest levels of IL-5 and IL-13 were induced in the lungs from FI-RSV but not in FG VLP immune mice upon RSV infection, which have a correlation with severe pulmonary histopathology. In addition, extremely high levels of IL-4-producing lung cells in FI-RSV but not in FG VLP immune mice were detected independent of CL treatment after RSV challenge. High levels of IL-5 and IL-6 cytokines in the airways and lungs were detected in live RSV previously infected mice with CL treatment upon RSV reinfection, which seems to be associated with enhanced pulmonary inflammation (data not shown). In support of this, the FI-RSV group showed increases in the levels of Th2 cytokines including IL-5, IL-6, and IL-13 as well as eosinophils in the airways, supporting the evidence that these Th2 cytokines were contributing to further mucus secretion in FI-RSV but not in FG VLP immune mice. The cause of FI-RSV vaccine-enhanced disease is not fully understood yet, but is assumed to be caused by multiple parameters including FI-RSV vaccine-induced Th2-biased immune responses (IL-4, IL-5, IL-13), neutrophils, and eosinophil infiltration in lungs after RSV infection.33,34 The general features of FI-RSV vaccine-enhanced disease as represented by pulmonary histopathology in humans could be recapitulated in mice. However, detailed pathological features of RSV disease appear to be different in mice and humans. Naïve mice are less permissive to RSV infection and disease and require high RSV challenge doses for significant lung viral loads.35 Severe histopathology and eosinophilia in FI-RSV immune mice despite lung viral clearance were obvious in mice, but it is not known whether these same pathological parameters happen in humans. Regarding these aspects, it is important to note that, in a murine model, eosinophils and lung viral loads were not required for FI-RSV vaccine-enhanced disease.2,36,37 Meanwhile, it was noted that, as a result of CL treatment, levels of TNF-α were increased in the lungs from all RSV-infected mice. Also, BAL IFN-γ and lung IFN-γ levels were increased in naïve, FI-RSV, and FG VLP mice as a result of CL treatment, which might contribute to moderately decreasing lung viral loads. In line with this, IFN-γ receptor signaling was found to be critical in controlling dengue virus replication.38
Infection of unimmunized naïve mice with RSV usually does not induce severe lung histopathology since mice are not highly permissive to RSV.6,8,23 In this study, we observed a substantial degree of lung histopathology in naïve mice after RSV infection probably due to the nature of 1-year-old aged mice and high lung viral loads. FI-RSV and FG VLP immune mice as well as live RSV previously infected mice (data not shown) were found to be highly effective in controlling lung viral loads by lowering 500- to 1,000-fold. Levels of Th2 cytokines were not high, but there were some cell infiltrates such as pDCs, CD11b+ DCs, eosinophils, and CD4+ and CD8+ T-cells and inflammatory cytokines including IL-6, TNF-α, and IFN-γ in the lungs of RSV-infected naïve mice compared to those in uninfected naïve mice. Thus, high lung viral loads, and cellular infiltrates and inflammatory cytokines, may contribute to pulmonary RSV disease in naïve mice upon RSV infections at older ages. CL treatment of naïve mice significantly reduced airway AMs, which resulted in moderate increases in eosinophils, MIP-1β chemokine, BAL IFN-γ, and TNF-α and lowering Th2 cytokines. These changes by CL treatment likely contribute to lung viral control and lessening pulmonary inflammation. In contrast, a moderate increase in histopathology and/or mucus production was observed in FI-RSV and FG VLP mice with CL treatment. These observations indicate that the effects of CL treatment or possible roles of AMs can be different depending on the preexisting immune statuses of mice prior to RSV infection.
Conclusion
FG VLP immunization of mice can induce long-lasting immunity to RSV infection without causing an overt sign of pulmonary inflammation. After RSV infection, AMs were substantially reduced in the airways of FI-RSV immune mice. In contrast, a high level of AMs was maintained in the lungs from FG VLP immune mice similar to that from naïve mice after RSV infection. CL treatment of FG VLP immune mice partially depleted AMs and resulted in an increase in TNF-α, IFN-γ, IL-6, and chemokines, which might be associated with moderate histopathology. In contrast to FG VLP, FI-RSV vaccination induced high levels of eosinophils, IL-4-producing cells, Th2 cytokines (IL-5 and IL-13), and mucus production and low levels of IFN-γ, all of which contribute to pulmonary RSV disease. FI-RSV immune mice with CL showed further increases in BAL B220+ pDCs, lung eosinophils and chemokines, BAL Th2 cytokines (IL-5, IL-6, IL-13) and TNF-α, and lung CD4+ and CD8+ T-cells and decreases in RSV F- and G-specific IFN-γ-secreting cell spots, revealing possible host immune parameters responsible for FI-RSV vaccine-enhanced RSV disease. The results in this study provide evidence that FG VLP immunization confers protection against RSV even 1 year later without vaccine-associated RSV disease and that AMs play a regulatory role in the RSV disease including eosinophilia, mucus production, inflammatory cytokines, and T-cell infiltration.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grants AI105170, AI093772, and AI119366, to Sang-Moo Kang.
Disclosure
The authors report no conflicts of interest in this work.
References
Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89(4):405–421. | ||
Johnson TR, Parker RA, Johnson JE, Graham BS. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J Immunol. 2003;170(4):2037–2045. | ||
Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res. 2007;39(1–3):225–239. | ||
Zeltins A. Construction and characterization of virus-like particles: a review. Mol Biotechnol. 2013;53(1):92–107. | ||
Quan FS, Kim Y, Lee S, et al. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis. 2011;204(7):987–995. | ||
McGinnes LW, Gravel KA, Finberg RW, et al. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol. 2011;85(1):366–377. | ||
Lee S, Quan FS, Kwon Y, et al. Additive protection induced by mixed virus-like particles presenting respiratory syncytial virus fusion or attachment glycoproteins. Antiviral Res. 2014;111:129–135. | ||
Murawski MR, McGinnes LW, Finberg RW, et al. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010;84(2):1110–1123. | ||
Ko EJ, Kwon YM, Lee JS, et al. Virus-like nanoparticle and DNA vaccination confers protection against respiratory syncytial virus by modulating innate and adaptive immune cells. Nanomedicine. 2015;11(1):99–108. | ||
Bowden DH. The alveolar macrophage. Environ Health Perspect. 1984;55:327–341. | ||
Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141(2):471–501. | ||
Rubins JB. Alveolar macrophages: wielding the double-edged sword of inflammation. Am J Respir Crit Care Med. 2003;167(2):103–104. | ||
Schneider C, Nobs SP, Heer AK, et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10(4):e1004053. | ||
Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12(4):295–305. | ||
Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4(1):e4204. | ||
Holt PG, Oliver J, Bilyk N, et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177(2):397–407. | ||
van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193(1):93–99. | ||
Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174(1–2):83–93. | ||
Lee JS, Kwon YM, Hwang HS, et al. Baculovirus-expressed virus-like particle vaccine in combination with DNA encoding the fusion protein confers protection against respiratory syncytial virus. Vaccine. 2014;32(44):5866–5874. | ||
van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. | ||
Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170(2):499–509. | ||
Lee YT, Suarez-Ramirez JE, Wu T, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. 2011;85(9):4085–4094. | ||
Hwang HS, Kwon YM, Lee JS, et al. Co-immunization with virus-like particle and DNA vaccines induces protection against respiratory syncytial virus infection and bronchiolitis. Antiviral Res. 2014;110:115–123. | ||
Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol. 2009;37(2):249–255. | ||
Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2(5):403–411. | ||
Hall JD, Woolard MD, Gunn BM, et al. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun. 2008;76(12):5843–5852. | ||
van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15(5):178–185. | ||
Sung SS, Fu SM, Rose CE Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176(4):2161–2172. | ||
Beaty SR, Rose CE Jr, Sung SS. Diverse and potent chemokine production by lung CD11b high dendritic cells in homeostasis and in allergic lung inflammation. J Immunol. 2007;178(3):1882–1895. | ||
Bueno SM, González PA, Riedel CA, Carreño LJ, Vásquez AE, Kalergis AM. Local cytokine response upon respiratory syncytial virus infection. Immunol Lett. 2011;136(2):122–129. | ||
Varga SM, Wissinger EL, Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J Immunol. 2000;165(11):6487–6495. | ||
Johnson TR, Graham BS. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J Virol. 1999;73(10):8485–8495. | ||
Bueno SM, González PA, Pacheco R, et al. Host immunity during RSV pathogenesis. Int Immunopharmacol. 2008;8(10):1320–1329. | ||
Bataki EL, Evans GS, Everard ML. Respiratory syncytial virus and neutrophil activation. Clin Exp Immunol. 2005;140(3):470–477. | ||
Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301(2):L148–L156. | ||
Castilow EM, Meyerholz DK, Varga SM. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol. 2008;180(4):2376–2384. | ||
Castilow EM, Legge KL, Varga SM. Cutting edge: eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J Immunol. 2008;181(10):6692–6696. | ||
Prestwood TR, Morar MM, Zellweger RM, et al. Gamma interferon (IFN-γ) receptor restricts systemic dengue virus replication and prevents paralysis in IFN-α/β receptor-deficient mice. J Virol. 2012;86(23):12561–12570. |
Supplementary material
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.