Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Residual symptoms in patients with partial versus complete remission of a major depressive disorder episode: patterns of painful physical symptoms in depression
Authors Harada E, Satoi Y, Kikuchi T, Watanabe K , Alev L, Mimura M
Received 22 December 2015
Accepted for publication 14 April 2016
Published 30 June 2016 Volume 2016:12 Pages 1599—1607
DOI https://doi.org/10.2147/NDT.S102767
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Eiji Harada,1 Yoichi Satoi,2 Toshiaki Kikuchi,3 Koichiro Watanabe,3 Levent Alev,1 Masaru Mimura4
1Medical Science, Medicines Development Unit-Japan, 2Statistical Science, Medicines Development Unit-Japan, Eli Lilly Japan K.K., Kobe, Hyogo, 3Department of Neuropsychiatry, Kyorin University School of Medicine, 4Department of Neuropsychiatry, Keio University School of Medicine, Tokyo, Japan
Objective: The patterns of residual painful physical symptoms (PPS) and emotional symptoms among patients with partial remission (PR) or complete remission (CR) of a major depressive disorder (MDD) episode were compared.
Methods: This is a multicenter, cross-sectional, observational study. Patients who had originally been diagnosed with MDD, were treated with an antidepressant for 12 weeks for that episode, and achieved either PR or CR at study entry were enrolled in the study. Using the 17-item Hamilton Rating Scale for Depression (HAM-D17), PR was defined as a score of ≥8 and ≤18 and CR as a score of ≤7. Residual symptoms were assessed using the Brief Pain Inventory-Short Form (BPI-SF) and the HAM-D17.
Results: A total of 323 patients (CR =158, PR =165) were included in the study. Patients in the PR group had a higher mean (standard deviation) score in the HAM-D17 than those in the CR group (11.8 [3.1] and 4.4 [2.0], respectively). BPI-SF results showed that “at least moderate PPS” (score ≥3 on BPI-SF question 5) was significantly more prevalent among patients with PR than those with CR (37.0% vs 16.5%, respectively; odds ratio =3.04; P<0.001). Presence of pain (any severity) was also more prevalent among patients with PR than those with CR (54.5% vs 35.4%, respectively). The HAM-D17 results for individual items indicated that impaired work and activities, depressed mood, psychological and somatic anxiety, and general somatic symptoms were observed in at least 75% of patients with PR.
Conclusion: PR was associated with a higher prevalence of at least moderate PPS. Other residual symptoms commonly observed in patients with PR included typical core emotional symptoms (eg, loss of interest, depressed mood, and psychological anxiety). These results underline the importance of PPS, because PPS is clinically relevant for the patients but difficult to assess with the commonly used depression evaluation scale.
Keywords: major depressive disorder, residual symptoms, partial remission, complete remission, painful physical symptoms, pain, depression
Introduction
Major depressive disorder (MDD) encompasses a broad range of emotional and physical symptoms.1 The optimal goal in the treatment of patients with MDD should be the complete remission (CR) of symptoms and a return to the same functionality level experienced prior to the episode.2 However, it is known that approximately one-third of patients experience only partial remission (PR), experiencing insufficient improvement and persistent residual symptoms.3,4 PR is characterized by the presence of residual symptoms that heavily impact depression prognosis. Judd et al5 reported that patients who experienced MDD with residual symptoms relapsed more than three times faster than those without. Those symptoms were also found to be a predictor of nonrecovery from depression.5
A clinical pattern of residual symptoms has been reported by Paykel et al6 using the rating scale of the 17-item Hamilton Rating Scale for Depression (HAM-D17). In their study, patients often gave a positive response to the general somatic symptoms item, along with other items related to more typical depressive symptoms, such as depressed mood, impairment of work and interests, and psychological anxiety.6 However, because the general somatic symptoms item on the HAM-D17 refers to both nonpainful physical symptoms (non-PPS; eg, loss of energy and fatigability) and painful physical symptoms (PPS; eg, backaches, headache, and muscle aches) and does not distinguish one from the other, the prevalence of PPS as a residual symptom has remained unclear. From a clinical perspective, PPS in patients with depression are known to worsen patients’ prognosis. For example, the presence of pain slowed treatment progression and was predictive of an increased time to remission,7 but little emphasis has been placed on the presence of PPS as part of a pattern of residual symptoms.
Therefore, we aimed to assess the pattern of residual PPS, hypothesizing that PPS of at least moderate severity would be more prevalent among patients with PR than patients with CR.
Methods
Study design
This was a multicenter, cross-sectional, observational study conducted at 27 psychiatry and psychosomatic outpatient clinics in Japan. Patients with PR or CR after 12 weeks of treatment were enrolled and underwent pair-wise matching by age and sex: two strata for sex (male and female) and three strata for age (20–39, 40–65, and ≥66 years). The number of patients with PR and CR in each age and sex stratum was as equal as possible.
Study population
Patients ≥20 years old who had originally been diagnosed with MDD without psychotic features, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision,1 were treated with an antidepressant for 12 weeks (±3 weeks) for that episode, and achieved either PR or CR at study entry were eligible for enrollment in the study. The diagnosis of the MDD episode was made by the investigators in normal clinical settings. PR was defined as an HAM-D17 score of ≥8 and ≤18,6 and CR was defined as an HAM-D17 score of ≤7.8 The antidepressant treatment regimen prior to and after enrollment was at the sole discretion of the physician and the patient, according to usual standard of care. Patients were excluded if they had a previous diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder; had a current diagnosis of dysthymic disorder or adjustment disorder; or had an HAM-D17 score ≥19.
Ethics
This study was reviewed and approved by the applicable institutional ethical review boards and was conducted in accordance with applicable laws and regulations of Japan, as appropriate. All patients provided written informed consent after they were given an explanation of the study and prior to enrollment and data collection. The confidential nature of patient information was maintained. The investigators or appointed personnel entered data in electronic data collection application, and the investigators validated the data for correctness with a written signature.
Study assessments
The Brief Pain Inventory-Short Form (BPI-SF) questionnaire was used to assess PPS. Question (Q)1 reveals the presence of pain of any severity (referred to as “any pain” or “any severity of pain” hereafter) and Q2 identifies the location of pain (body site). Q3 through Q6 assess the intensity of worst, least, average, and current pain, respectively. A score of ≥3 on Q5 (average pain) is considered at least moderate severity of pain (referred to as “at least moderate PPS” hereafter).9 Q8 evaluates how much relief pain treatments or medications have provided. Q9 assesses how much pain interferes with daily activities in various forms. For Q3–Q6 and Q9, two populations were analyzed: all patients (including the patients who reported no pain; ie, those who answered “no” on Q1) and the pain-positive subgroup (only the patients who reported any pain; ie, those who answered “yes” on Q1).
The HAM-D17 is a 17-item, clinician-rated scale used to assess the severity of depression and its improvement.8,10 Item score ranges in this scale are 0–2 or 0–4. Residual symptoms at the time of enrollment were regarded as present when the score on each item of the HAM-D17 was ≥1. In addition, residual symptoms were grouped into mild (score of 1) and moderate or higher (scores of 2–4), as defined in the study by Paykel et al.6 The Structured Interview Guide for the Hamilton Depression Rating Scale was also used in this study.11–13
The Social and Occupational Functioning Assessment Scale (SOFAS), a 100-point single-item scale, was used to indicate the individual’s level of social and occupational functioning across a continuum ranging from a state of optimal functioning to a state of grossly impaired functioning.14 An SOFAS score ≥80 was defined as normal levels of functionality.15
Clinicians assessed and categorized patients’ treatment adherence by asking whether the patients were taking the medicine: 1) exactly as prescribed (100%); 2) most of the time (76%–99%); 3) slightly less than prescribed (50%–75%); or 4) significantly less than prescribed (<50%). No data regarding precise individual antidepressant dosing were collected. Sociodemographic parameters were also collected.
Statistical analysis
All patients who provided consent to release information and who fulfilled the study entry criteria were included in all of the analyses. A logistic regression model was used for the endpoint analysis. The model included a prevalence of at least moderate PPS as a response variable; group (CR and PR), sex (female and male), and age (category by 10 years) were treated as explanatory variables. In addition, comparisons using Fisher’s exact test were also made between the groups, overall and by sites where at least moderate PPS were experienced. Each component of the BPI-SF was also summarized and compared between the CR and PR subgroups. Each item of the HAM-D17 was compared using a two-sample t-test. Due to the observational nature of this study, covariate adjustments were made to control for biases and confounding factors in the logistic regression model. Although age and sex were used as matching factors, they were also included in the analysis model to decrease the data variation and the potential bias due to the imbalance of these factors between groups. All of the statistical tests were based on a two-sided significance level of 0.05, unless otherwise specified.
Results
Patient characteristics
A total of 323 patients (CR =158 [48.9%], PR =165 [51.1%]) were included in the study. Mean patient age was 46.2 years, and 52.0% of patients were women (Tables 1 and 2). Mean age at the onset of the first depressive episode was 41.5 years, with a mean of 1.5 previous MDD episodes and a mean total duration of 33.8 weeks for the current MDD episode. Physical comorbidities with the potential to cause PPS were present in 9.9% of patients. Sociodemographic variables, including age, sex, educational background, and current work status, were similar between groups (Table 1). Most clinical variables were also similar between groups, but patients in the PR group had, by definition, a higher mean (standard deviation) score in the HAM-D17 than those in the CR group (11.8 [3.1] and 4.4 [2.0], respectively). In addition, the mean (standard deviation) SOFAS score was higher for the CR group than the PR group (75.5 [10.7] and 58.9 [11.3], respectively). Similarly, the proportion of patients achieving SOFAS score of ≥80 was higher for the CR group than the PR group (43% and 3%, respectively).
  | Table 1 Sociodemographic characteristics |
Treatment pattern and adherence
All patients had received acute antidepressant treatment for approximately 3 months prior to this study. Most patients were treated with selective serotonin reuptake inhibitors (45.5%) or selective serotonin and noradrenalin reuptake inhibitors (36.5%); the distribution of antidepressant classes was similar between patients with CR and PR (Table 2). Overall, 78.6% of patients took antidepressants “exactly as prescribed (100%)” and 18.9% of patients did so “most of the time (76%–99%)”. Differences in treatment compliance between groups were not statistically significant (Table 2). Concomitant medication use is also presented in Table 2. Patients with PR received more nonsteroidal anti-inflammatory drugs (26.7% vs 12.7%, respectively) and benzodiazepine treatment (84.2% vs 67.1%, respectively) compared to patients with CR. Approximately 50% of patients in each group received psychotherapy (eg, cognitive behavioral therapy, group psychotherapy, psychoanalysis) (Table 2).
Individual residual symptoms
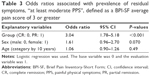
The prevalence of at least moderate PPS in the PR group was significantly higher than that in the CR group (37.0% vs 16.5%, respectively; P<0.001). As described above, logistic regression analysis included the prevalence of at least moderate PPS as a response variable and group (CR and PR), sex, and age as explanatory variables. Using logistic regression, the prevalence of at least moderate PPS in the PR group was significantly higher than that in CR group (odds ratio =3.04; 95% confidence interval [CI] =1.78–5.18; P<0.001) (Table 3). The prevalence of PPS among female patients was numerically higher than among male patients, but this was not statistically significant (odds ratio =1.61; 95% CI =0.96–2.70). There appeared to be no significant effect of age (Table 3).
BPI-SF results are shown in Table S1. Overall, 45.2% of patients experienced pain symptoms, with head (10.8%), neck (7.1%), shoulder (5.6%), lower back (5.3%), and back (4.0%) being the most commonly reported locations of pain. Patients with PR were more likely to experience pain than patients with CR (54.5% vs 35.4%, respectively), although the body locations that hurt most were similar between groups. Pain sites that were seen in more than 10% of patients with PR were the head (15.2%) and neck (10.3%), which were more than twice that of the CR group (Table S1).
The degree of worst, least, average, and current pain was higher in the PR group than in the CR group, and a smaller difference was seen in the pain-positive subgroup. Daily activities of the PR group were generally more disrupted than those of the CR group in both the all-patients population and the pain-positive subgroup. Among the pain-positive subgroups, interference with daily activities for the PR group was especially high for enjoyment of life, mood, and normal work.
The mean and distribution of scores on each item of the HAM-D17 are shown in Table S2. Patients with CR had lower scores across all items of the HAM-D17, compared to patients with PR. The same trend also applied to the frequency of residual symptoms for each HAM-D17 item. The symptoms present were typical core symptoms of depression. The most frequently observed residual symptoms (more than 75%) among patients with PR were work and activities (97.6%), depressed mood (95.8%), psychological anxiety (86.7%), somatic anxiety (81.8%), and general somatic symptoms (78.2%). The same order of frequency was observed for moderate or higher scores (scores of 2–4) for each item: work and activities (64.2%), depressed mood (64.2%), psychological anxiety (35.2%), somatic anxiety (26.1%), and general somatic symptoms (18.2%). The remaining symptoms were present, to at least a mild degree, in 20% of the patients with PR, with the exceptions of loss of weight and insight. The same top five residual symptoms were also common in patients with CR: work and activities (63.9%), depressed mood (53.2%), general somatic symptoms (46.2%), psychological anxiety (43.0%), and somatic anxiety (41.1%).
Discussion
The results presented here demonstrate that at least moderate residual PPS are more prevalent among patients with PR than those with CR after antidepressant treatment. Furthermore, our results revealed the details of residual PPS, which are clinically relevant to patients, but are difficult to assess in the commonly used depression scale. For example, HAM-D item 13 “general somatic symptoms” assesses both non-PPS and PPS and does not distinguish one from the other. The frequencies of general somatic symptoms (indicated by a HAM-D item 13 score ≥1), any pain (indicated by BPI-SF Q1 answer of yes), and at least moderate pain (indicated by a BPI-SF Q5 score ≥3) were 78.2%, 54.5%, and 37.0%, respectively, in the PR group and 46.2%, 35.4%, and 16.5%, respectively, in the CR group. Therefore, we may assume that among those with residual somatic symptoms, approximately three-quarters of patients have some pain, and this is consistent across patients with PR and CR. Similarly, regarding the severity of pain, about two-thirds of the pain symptoms among patients with PR and approximately half of the pain symptoms among patients with CR were of at least moderate severity. These results suggest that both frequency and severity of pain are higher among patients with PR compared to those with CR. This clinical perspective will help clinicians to accurately and thoroughly assess and treat residual physical symptoms from which patients with MDD suffer.
This study reinforces and advances our understanding of the nature of residual symptoms. We have shown that core emotional symptoms in MDD, such as impaired work and activities, depressed mood, and psychological anxiety, were highly prevalent among patients with PR and to a lesser extent among patients with CR. In addition, this study has shown that physical symptoms, represented by general somatic symptoms, may have been overlooked by doctors and patients, but were actually common residual symptoms, following the core emotional symptoms in prevalence.
Paykel et al6 reported in their study that residual symptoms were a combination of typical core depressive symptoms with both emotional and physical symptoms. Our study supports this finding, providing more detailed information on PPS as a pattern of residual symptoms. Regarding the location of pain, the head, neck, and shoulder pain were very common, and this is consistent with other studies.16,17 Furthermore, the fact that patients with PR exhibited more head and neck pain may indicate an increased risk of migraine and tension-type headaches as a secondary effect of MDD.18,19
Limitations
There are several limitations to our study. Firstly, because this was an observational study, one cannot infer causality between PR and the presence of PPS, but rather only an association between the two. Secondly, at the time of enrollment, patients had been treated for 12 weeks and no information was collected at treatment initiation. Therefore, it was difficult to match the patients with PR and CR according to the severity of the depressive symptoms, backgrounds, or PPS at the initiation of treatment. Thirdly, only patients who continued treatment for 12 weeks were recruited for the study; therefore, those who discontinued early were not included. However, due to the naturalistic design of the study, both cohorts reflected the reality of a typical clinical setting. In addition, to minimize the effect of confounders, patients were matched for age and sex. Similarity in social and clinical backgrounds, including the duration of current MDD episode, may have helped to ensure similarity in MDD episode severity at onset. Because patients were included in the study during the course of an MDD episode, information regarding the BPI-SF, HAM-D17, SOFAS, and other relevant information at the onset of the episode was not collected. Fourthly, we did not assess symptom improvement from the beginning of treatment (ie, before our study). We assessed it only at baseline (ie, approximately 12 weeks after drug initiation). Only the baseline HAM-D17 scores were used to define CR/PR. However, the diagnosis of MDD was made by the investigator according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria, though not necessarily using a structured interview guide. Lastly, we have not analyzed each antidepressant’s impact on PPS, since our objective was to provide details on residual symptoms using a noninterventional, cross-sectional design. Assessment of efficacy of antidepressants on PPS was out of the scope of the study.
Conclusion
In summary, MDD presents with various residual symptoms, including typical core depressive symptoms and PPS. Our results contribute to a better understanding of the pattern of residual PPS in patients with depression. Our findings underline the importance of PPS, because PPS is clinically relevant for the patients but difficult to assess in the commonly used depression evaluation.
Acknowledgments
The authors are deeply grateful to the primary investigators, subinvestigators, and staff at the 27 study sites and to all of the patients who participated in this study. The authors would also like to thank Dr Hirofumi Tokuoka, a full-time employee of Eli Lilly Japan K.K., for his assistance with this project and Ms Aki Yoshikawa, a full-time employee of Eli Lilly Japan K.K., for her writing, editorial, and logistical support. Medical writing assistance was provided by the Medical Writing Department of CMIC Co., Ltd., funded by Eli Lilly Japan K.K. Additional medical writing support was provided by Rodney Moore, PhD (Inventiv Health Clinical, LLC), and data analysis support was provided by CMIC Co., Ltd., both funded by Eli Lilly Japan, K.K.
This study was sponsored by Eli Lilly Japan K.K. and conducted by CMIC Co., Ltd. under the contract with Eli Lilly Japan K.K.
Author contributions
All authors participated in the interpretation of the data, drafting, critical revision, and approval of the final version of the manuscript.
Disclosure
EH contributed to this work as a former full-time employee of Eli Lilly Japan K.K.. The opinions expressed in this work are solely his and do not represent his current affiliation, that is, the Japanese Ministry of Health, Labour and Welfare.
YS and LA are employees of Eli Lilly Japan K.K.
TK has received speaker fees from Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Jansen Pharmaceutical K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., and Yoshitomiyakuhin Corporation.
KW has received grants from Astellas Pharma Inc., Eisai Co., Ltd., MSD K.K., Otsuka Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Meiji Seika Pharma Co., Ltd.; consultancy fees from Otsuka Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., and Mochida Pharmaceutical Co., Ltd.; and speaker fees and payment for manuscript preparation from Astellas Pharma Inc., MSD K.K., Otsuka Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Shionogi & Co., Ltd., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., Pfizer Japan Inc., Meiji Seika Pharma Co., Ltd., and Janssen Pharmaceutical K.K.
MM has received grants and/or speaker’s honoraria from Asahi Kasei Corp., Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., Meiji Seika Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., MSD K.K., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., Shionogi & Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation and Yoshitomiyakuhin Corporation within the past three years. The authors have no other conflicts of interest in this work.
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. | ||
The Japanese Society of Mood Disorders (JSMD). Treatment Guideline II: Major Depressive Disorder. Ver. 1. Tokyo: The Japanese Society of Mood Disorders; 2012. | ||
Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):179–200. | ||
Ramana R, Paykel ES, Cooper Z, Hayhurst H, Saxty M, Surtees PG. Remission and relapse in major depression: a two-year prospective follow-up study. Psychol Med. 1995;25(6):1161–1170. | ||
Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2–3):97–108. | ||
Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171–1180. | ||
Karp JF, Scott J, Houck P, Reynolds CF 3rd, Kupfer DJ, Frank E. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591–597. | ||
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. | ||
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. | ||
Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. | ||
Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742–747. | ||
Williams JB. Standardizing the Hamilton Depression Rating Scale: past, present, and future. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II6–II12. | ||
Nakane Y, Williams JBW. Structured interview guide for the Hamilton Depression Rating Scale (SIGH-D) Japanese version. Rinsyo Seishin Yakuri. 2003;6(10):1353–1368. | ||
Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992(9);149:1148–1156. | ||
Romera I, Perez V, Menchon JM, Delgado-Cohen H, Polavieja P, Gilaberte I. Social and occupational functioning impairment in patients in partial versus complete remission of a major depressive disorder episode. A six-month prospective epidemiological study. Eur Psychiatry. 2010;25:58–65. | ||
Shimodera S, Kawamura A, Furukawa TA. Physical pain associated with depression: results of a survey in Japanese patients and physicians. Compr Psychiatry. 2012;53(6):843–849. | ||
Lee P, Zhang M, Hong JP, et al. Frequency of painful physical symptoms with major depressive disorder in Asia: relationship with disease severity and quality of life. J Clin Psychiatry. 2009;70(1):83–91. | ||
Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KM. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology. 2003;60:1308–1312. | ||
Puca F, Genco S, Prudenzano MP, et al. Psychiatric comorbidity and psychosocial stress in patients with tension-type headache from headache centers in Italy. The Italian Collaborative Group for the study of psychopathological factors in primary headaches. Cephalalgia. 1999;19(3):159–164. |
Supplementary materials
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.